Elta Diah Pasmanasari1 and Jeanne Adiwinata Pawitan 2, 3 ,4*
and Jeanne Adiwinata Pawitan 2, 3 ,4*
1Doctoral Program for Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia.
2Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia.
3Stem Cell Medical Technology Integrated Service Unit, Dr. Cipto Mangunkusumo General Hospital/Faculty of Medicine Universitas Indonesia, Indonesia.
4Stem Cell and Tissue Engineering Research Center, Indonesia Medical Education and Research Institute (IMERI), Faculty of Medicine Universitas Indonesia, Indonesia.
Corresponding Author E-mail: jeanneadiwip@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2136
Abstract
Parkinson disease (PD) is a neurodegenerative disease that causes the loss of dopaminergic neurons in the brain. The imbalance in dopamine production causes motoric disorder that can produce specific electrical signal that can be detected by electromyography. Some methods were developed to diagnose PD and the use of a questionnaire and clinical observation was widely used to diagnose the disease. The limitation of the methods includes the fact that there are some differences in assessment results from clinicians due to the need of experience. The use of electromyography hopefully can obtain an objective assessment that can be easily used by clinicians. Some studies showed differences between normal muscle electric-activity compared to PD related abnormal muscle electric activity. Some methods were developed to use electromyography as a tool to diagnose PD related motoric symptoms, such as rigidity, gait abnormality and tremor. The use of electric signals, which are produce in muscle contraction, as markers to diagnose PD, as well as to monitor complications and the effect of therapy hopefully can be developed. In this review article, we will discuss about the use of electromyography signals that are related to PD. Therefore we will explain about basics of electromyography, the use of electromyography signals to detect tremor and gait abnormalities in PD, the use of electromyography for monitoring PD patients.
Keywords
Dysphagia; Electromyography; Gait; Parkinson’s disease; Tremor
Download this article as:| Copy the following to cite this article: Pasmanasari E. D, Pawitan J. A. The Potential of Electromyography Signals as Markers to Detect and Monitor Parkinson’s Disease. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Pasmanasari E. D, Pawitan J. A. The Potential of Electromyography Signals as Markers to Detect and Monitor Parkinson’s Disease. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/30bHU3O |
Introduction
Parkinson’s Disease (PD) is a neurodegenerative disease due to the lack of production of some neurotransmitters such as dopamine that causes tremor, rigidity, bradykinesia and postural instability as the main (cardinal) symptoms.1 Further, there is an imbalance in dopamine receptor activity in extrapyramidal pathways, both direct and indirect pathways. Dopamine receptor in direct pathways is D1 receptor that causes excitatory effect, while in indirect pathways the receptor is D2 receptor that produces inhibitory effect. The imbalance between D1 and D2 produces different symptoms in each PD patient, which is not all PD patients show all four cardinal symptoms.2, 3 PD currently has no significant tool to diagnose it, and definite diagnosis is through post mortem pathology examination. Recently questionnaires based on clinical symptoms (motor and non-motor symptom) were developed, but the use of the questionnaire needs clinical observations from experts to do clinical judgments based on the questionnaires.3 In addition, a more objective clinical judgments based on abnormal muscle activity in electromyography (EMG), which can be found in PD patients due the clinical symptoms like rigidity, bradykinesia and tremor, were developed. Therefore, tool-based markers are needed to provide objective, quantitative, continuous, and real assessment of PD, not only to diagnose, but also to help patients monitor the effects of therapeutics on their symptoms and help clinicians to monitor the progressivity of the disease.4
Electromyography is a procedure to measure electrical activity in muscle contraction. EMG is able to distinct clinical pathologies in muscle that is represented by the appearance of muscle activity and sound of muscle activity. Muscle contraction will produce a signal that can be seen on a monitor, and a specific sound will appear especially when the EMG needle is inserted at the muscle. There is a difference in signal activities and the sounds that were produced in muscle contraction between healthy and abnormal muscle.5 The signal is produced in neuromuscular activation based on muscle contraction; and an abnormality in the contraction of a muscle caused by injury, nerve damage, or muscular or neurological disorder can be identified through EMG signals, and can be observed during voluntary muscle contraction.6
Due the alteration in muscle activity in PD patients due to lack of dopamine in central nervous system, the EMG signal in PD patients showed different pattern than normal person.6, 7 Therefore the aim of this review was to discuss basics of electromyography, the use of EMG for PD detection and monitoring that were related with the abnormality of gait, tremor, swallowing and motor abnormality in PD patient, and additional approaches for PD diagnosis.
Basics of Electromyography
Electromyography is a detection and recording method of biomedical signals from electrical activity of musculoskeletal contraction.8, 9 In a contraction of skeletal muscle, there’s a process of depolarization and repolarization at muscle fiber membrane that is known as action potential. The action potentials of a skeletal muscle that are summed up is called Motor Unit Action Potential (MUAP).8 The EMG technique is recorded by inserting a needle at the ‘belly’ of a muscle and recording the action potential of some area that needle covers.5 This needle using method is inconvenient for patient. Therefore, methods that used electrodes that were attached on the surface of the muscle (known as surface EMG) were developed. This method has a limitation that only surface muscles can be detected or recorded. Some materials of surface electrodes were developed to overcome the limitation, and the use of wet gel electrodes was recommended to get better conduction and impedance.8 The position of the electrodes have an important role to get better signals, so guidelines were developed to locate the electrode; and the standard guideline that is widely used is SENIAM (surface EMG for non-invasive assessment of muscles).8, 10
There are many clinical uses of EMG signals such as for diagnosis of neuromuscular pathologies. EMG are used in many research laboratories in relation to biomechanics, motor control, neuromuscular physiology, movement disorder, postural therapy and physical therapy.9
Emg Signals as Potential Markers for Pd Detection and Monitoring
Electromyography signals that are produced from body movements can be used to identify abnormality of body movement. Abnormal body movements in a certain disease can produce abnormality in electromyography signal that can be distinguished from that of normal person. In PD patients the abnormalities such as increased muscle tone, abnormal posture, gait and tremor were found. Many studies tried to compare the difference in EMG signal between PD patients and normal persons.9, 11, 12 Therefore, EMG can be used to detect the presence of PD and monitor the progress of disease and treatment.
EMG to measure abnormal gait in PD patients
Some studies had been held to distinguish abnormal gait in PD using EMG signals. Keloth et al reviewed the difference between normal gait in normal person and abnormal gait in PD patients and obtained the parameters to distinguish between both of them.11
Clinicians or neurologists usually asses gait abnormality in PD with Unified Parkinson’s Disease Rating Scale (UPDRS) score that needs an experience in clinical observations.13 Further, the information that is needed to assess UPDRS score can be obtained from the patient, caregiver or both. The information includes motor and non motor functions that are divided into four parts, and the scoring is divided into 5 stages (normal-slight-mild-moderate-severe).14
Therefore, the use of EMG signals was developed to asses gait in PD patients (Figure 1).11 Some inconvenience in the use of EMG signals as a marker might occur due to its noisy characteristics, such as noise from other muscle contraction, artifact, or from equipment itself. To overcome the disadvantage, scientists tried to reduce some unwanted noise from EMG signal.13, 15 Thus, to reduce unwanted noise, smoothing algorithms, such as moving average method, to yield a proper amplitude parameter were applied. Moving average is one of the methods to remove baseline fluctuation that consists of four steps, i.e. select the data, select the smoothing methods, select the span and select the degree.8, 16
Putri et al analyzed the abnormal gait that was assessed by EMG, using artificial neural network (ANN), and measured the voluntary contraction of tibialis anterior, gastrocnemius lateral and medial muscles. In the studies, the EMG electrodes were attached at the belly of the muscles that physiologically produced most electrical signal. Using ANN classification for signal processing, they showed that the accuracy of the device to diagnose PD was 97%, which means that the method has a benefit as a future diagnostic tool for motoric symptoms in PD patients.7, 17 The ANN classification, which was used consisted of three layers, i.e. input, hidden and output layer. Input layer is the feature of calculation result, hidden layer consists of log-sigmoid transfer, and the output layer is a hyperbolic tangent sigmoid transfer function.7
A review from Singh et al discussed various EMG signal processing techniques, such as simple enveloping, EMG onset/offset detection, EMG signal decomposition into MUAP, and some algorithm of EMG signal analysis. Simple enveloping is an EMG signal processing technique that includes preprocessing, signal filtering, rectification, smoothing, standardization, statistical testing and computational algorithms. Some scientists suggested to use band-pass filters for the filtering of frequency within 10-500Hz.These EMG signal processing may help to detect alterations in EMG patterns between a healthy person and a person with an abnormal gait (including a PD patient). The various EMG signal processing techniques are considered helpful to analyze EMG signals that usually show a complex pattern, thus for diagnosis and monitoring of some diseases, including PD.6
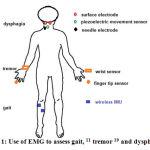 |
Figure 1: Use of EMG to assess gait, 11 tremor 19 and dysphagia 23 |
EMG to measure tremor in PD patient
Tremor in a PD patient is different from other tremors. PD’s tremor occurs when a body part of a PD patient is relaxed, and the tremor is usually called as resting tremor. Such tremor has intermediate amplitude, the frequency is approximately 3-7 Hz, and can be modulated by cognitive and physical activity.4, 18, 19 EMG signal can be used to differentiate tremor in PD from other tremors, as well as for quantification, monitoring, evaluation and detection of the tremor itself.12, 19 At first there was a controversy to use EMG to diagnose tremor in PD patients, because the use of needles was very unpleasant and raised the risk of infection. Therefore, analysis of tremor using surface EMG (sEMG) was introduced, and the promising results showed that it could discriminate tremor in PD from a healthy person.12 A study showed that EMG signals of patients with PD contained more tonic background activity and rhythmic burst activations than healthy controls. EMG signal morphology was studied by using sample histograms during isometric contraction of biceps brachii muscle with varying loads to observe differences between diseases.3
Jeon et al used wearable devices that were attached to fingertips and wrists to measure EMG signals and to evaluate tremor from PD patients (Figure 1), and compared it with the UPDRS scoring, which was evaluated by two neurologists. The result showed that scoring from the device was similar to the clinicians’ scoring. The EMG signal pattern can be used to diagnose tremor in PD, as well as to evaluate the severity of tremor in PD.19
A study by Moghadam et al showed the difference of the intensity of tremor in PD patients, which the severity of tremor was measured using EMG, between Extensor Carpi Radials Brevis (ECRB) Muscle and Flexor Carpi Ulnaris (FCU) muscle. The new algorithm, which is called Fast Orthogonal Search (FOS), was used in the study, and hopefully can be developed to determine the severity of tremors that can be related to the dose of medication. The result of the study showed that the accuracy, sensitivity, and specificity of the algorithm to detect tremor severity were 99.26%, 97.17%, and 99.72%, respectively.20
Some limitations were found in the use of EMG for PD detection due to various environment exposures that might have effects on the result of the examination, e.g. thermal condition like cold that might increase the severity of tremor, or otherwise heat that might increase muscle rigidity.12
EMG to measure dysphagia and motor symptoms in PD
Neurogenic dysphagia often occurs in PD patient in early or late period of PD. The swallowing difficulty in PD range between 10-100% and can increased the risk of death due to infection.21, 22 Ertekin used electrophysiology method to identify dysphagia in PD at oropharyngeal phase that were recorded from suprahyoid muscle under the chin using surface electrode. The method was called submental EMG (Figure 1). The purpose of this method is to understand the pathogenesis of oropharyngeal swallowing disorder in patient, to identify spontaneous swallowing, and to diagnose and monitor objectively.23
A monitoring method for motor symptom in PD using EMG signals was developed later by Boroojerdi et al that combined an accelerator (a tool to detect the movement of the body) and EMG device to detect motor fluctuation in PD through wearable bio-censor patches. This study compared the result from the sensor-patch (called NIMBLE) connected EMG device and from clinical observation using a questionnaire. The study proved that the use the NIMBLE sensor-patch could predict the motor-symptom score that obtained from clinical observation.24
Additional Approaches For Pd Diagnosis
Recent studies showed that PD is not only related to dopamine pathology, but also to other neurotransmitters, such as norephineprine, serotonin, GABA and glutamate. The non dopamine pathology of PD generates non motoric symptoms of PD that are considered as prodromal symptoms. The prodromal symptoms can be variable, such as depression, behavior and cognitive disorder, olfactory dis-function, or sleep disorder.25-27
Recent studies used non motoric symptoms for early diagnosis of PD. Electroencephalography (EEG) can be used to measure the alteration of sleep wave in healthy patients and to predict a sleep disorder in a PD patient. Some neuroimaging examinations, including magnetic resonance imaging (MRI), transcranial Doppler ultrasonography, positron emission tomography (PET), single-photon emission computed tomography (SPECT), morphometric MRI studies, functional MRI and perfusion imaging can be used to distinguish idiopathic PD from other Parkinsonian disorders.15, 28
Some biomarker were studied for early diagnosis to give early treatment for PD patients, such as orexin, 8-Hydroxy-20-Deoxyguanosine, α-synuclein, apolipoprotein A and many other neurochemicals.28 Neuroimaging and neurochemicals are considered to be promising markers to develop objective diagnostic tools.15, 28
The UK Parkinson Disease society brain bank for diagnosis of Parkinson Disease developed a questionnaire and a criteria to diagnose PD that consist of main motor symptoms, exclusion criteria and supportive criteria for PD, whose accuracy was approximately 90%. The limitation for this questionnaire is that it only consists of motor symptoms, while the non-motor symptoms as prodromal symptom was not assessed. However, it is likely to be used by clinicians, because it is more familiar.1, 29
In conclusion, many studies showed the accuracy of EMG signal compared to scoring systems that were widely used by clinician (especially neurologist) and showed high accuracy, specificity and sensitivity. EMG is a potential marker to distinguish abnormal tremor and gait in PD, and hopefully can be used to diagnose or monitor PD symptoms that are more objective and have a quantitative results compared to scoring systems.
Acknowledgement
This work was supported by a research grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Hibah Penelitian Pengembangan 2019, contract no.NKB-1804/ UN2.R3.1/ HKP.05.00/ 2019
Conflict of interest
The authors declare that they have no competing interest.
Funding Source
Research grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Hibah Penelitian Pengembangan 2019, contract no.NKB-1804/UN2.R3.1/HKP.05.00/2019.
References
- Jankovic J. Parkinson’s disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79 (4):368-3
CrossRef - Haines DE, and Mihailoff G. The Telencephalon. In: Fundamental neuroscience for basic and clinical applications. 5th Ed. Philadelphia; Elsevier:2018, p. 225-242.
CrossRef - Robichaud J.A, Pfann K.D, Leurgans S, Vaillancourt D.E, Comella C.L, and Corcos D. Variability of EMG patterns: A potential neurophysiological marker of Parkinson’s disease? Clin Neurophysiol, 2009;120(2):390-397.
CrossRef - Romero L.E, Chatterjee P, and Armentano R. An Iot approach for integration of computational intelligence and wearable sensors for Parkinson’s disease diagnosis and monitoring. Health and Technology. 2016;6(3):167-172.
CrossRef - Mills K. The basics of electromyography. J Neurol Neurosurg Psychiatry 2005;76 Suppl 2:ii32-35.
CrossRef - Singh R.E, Iqbal K, White G, and Holtz J. A review of EMG techniques for detection of gait disorders. In: Aceves-Fernandez MA (Editor). Artificial intelligance aplication in medicine and biology. Intechopen: 2019, 1-22. doi:10.5772/intechopen/84403
- Putri F, Caesarendra W, Ariyanto M, and Pasmanasari E. Electromyography gait test for Parkinson disease recognition using artificial neural network classification in Indonesia. Momentum. 2016;12(2):23-2
- Medved V, and Cifrek M. Kinesiological Electromyography. In: Klika V (Editor). Biomechanic in application. Rijeka: Intech Open; 2011, 349-366. doi:10.5772/21282
CrossRef - Raez M.B, Hussain M.S, and Mohd-Yasin F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol Proced Online. 2006;8:11-35.
CrossRef - Stegeman D.F, and Hermens H. Standards for surface electromyography: The European Project surface EMG for non-invasive assessment of muscles (SENIAM). In: Hermens H.J, Rau G, Disselhorst-Klug C, and Freriks B. (Editors.). Surface electromyography application areas and parameters. Proceedings of the Third General SENIAM Workshop on surface electromyography; 1998, p.109-112. [Internet]. http://citeseerx. ist.psu.edu/ viewdoc/download;jsessionid=1C93E98FF7ECF2B26BB9C8D34FE3D7C1?doi=10.1.1.623.2040 &rep=rep1 & type=pdf.
- Keloth S.M, Viswanathan R, Jelfs B, Arjunan S, Raghav S, and Kumar D. Which gait parameters and walking patterns show the significant differences between Parkinson’s disease and healthy participants? Biosensors (Basel), 2019;9(2):59. doi:10.3390/bios9020059.
CrossRef - Meigal A.Y, Rissanen S.M, Tarvainen M.P, Airaksinen O, Kankaanpaa M, and Karjalainen P. Non-linear EMG parameters for differential and early diagnostics of Parkinson’s disease. Front Neurol. 2013;4:135. doi:10.3389/fneur.2013.00135
CrossRef - Ahsan R, Ibrahimy M, and Khalifa O. EMG signal classification for human computer interaction : A review. Eur J Sci Res. 2009; 33(3):480-501.
- Goetz C, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins G.T, Stern M.B, Tilley B.C, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang A.E, Lees A, Leurgans S, LeWitt P.A, Nyenhuis D, Olanow W, Rascol O, Schrag A, Teresi J.A, van Hilten J.J, and LaPelle N.. MDS-UPDRS the MDS-sponsored revision of the Unified Parkinson’s Disease Rating Scale. Milwaukee: International Parkinson and Movement Disorder Society;2019,31. [Internet]. https://www.movementdisorders.org/MDS-Files1/PDFs/Rating-Scales/MDS-UPDRS_English_FINAL_Updated_August2019.pdf
- Rizek P, Kumar N, and Jog M. An update on the diagnosis and treatment of Parkinson disease. CMAJ. 2016;188(16):1157–1165. doi:10.1503/ cmaj.151179
CrossRef - Yadav J, Singh A, and Kumar M. Comparative study of various techniques for elimination of noise in EMG signal. Int J Sci Eng Res. 2012; 3(11):1-10
- Putri F, Caesarendra W, Pasmanasari E, Ariyanto M, and Setiawan J. Parkinson disease detection based on voice and EMG pattern classification method for Indonesian case study. JEMMME. 2016;3(2):87-98.
CrossRef - Dovzhenok A, and Rubchinsky L. On the origin of tremor in Parkinson’s disease. PLoS ONE. 2012; 7(7): e41598.
CrossRef - Jeon H, Lee W, Park H, Lee HJ, Kim SK, Kim H.B, Jeon B, and Park K. Automatic classification of tremor severity in Parkinson’s disease using a wearable device. Sensors (Basel). 2017 ;17(9). pii: E2067. doi: 10.3390/s17092067.
CrossRef - Moghadam H, Kobravi H, and Homam M. Quantification of Parkinson tremor intensity based on EMG signal analysis using fast orthogonal search algorithm. Iranian Journal of Electrical and Electric Engeneering. 2017;14(2):106-1
- Luchesi K.F, De Toledo I.P, and Mourao L. Dysphagia in Parkinson’s disease: Prevalence, impact and management challenges. J Otolaryngol ENT Res. 2017;6(5):00176. doi: 10.15406/joentr.2017.06.00176
CrossRef - Umemoto G, and Furuya H. Management of dysphagia in patients with Parkinson’s disease and related disorders. Intern Med. 2020; 59:7-14.
CrossRef - Ertekin C. Electrophysiological evaluation of oropharyngeal dysphagia in Parkinson’s disease. J Mov Disord. 2014;7(2): 31-56.
CrossRef - Boroojerdi B, Ghaffari R, Mahadevan N, Markowitz M, Melton K, Morey B, Otoul C, Patel S, Phillips J, Sen-Gupta E, Stumpp O, Tatla D, Terricabras D, Claes K, Wright J.A Jr, and Sheth N. Clinical feasibility of a wearable, conformable sensor patch to monitor motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2019;61:70-7
CrossRef - Li Y, Jiao Q, Du X, Bi M, Han S, Jiao L, and Jiang H. Investigation of behavioral dysfunctions induced by monoamine depletions in a mouse model of Parkinson’s disease. Front Cell Neurosci. 2018;12:241. doi:10.3389/fncel.2018.00241
CrossRef - Błaszczyk J. Parkinson’s disease and neurodegeneration: GABA-collapse hypothesis. Front Neurosci. 2016;10:269. doi:10.3389/fnins.2016.00269
CrossRef - O’Gorman Tuura R.L, Baumann C.R, and Baumann-Vogel H. Beyond dopamine: GABA, glutamate, and the axial symptoms of Parkinson disease. Front Neurol. 2018;9:806. doi:10.3389/fneur.2018.00806
CrossRef - Emamzadeh F.N, and Surguchov A. Parkinson’s disease: Biomarkers, treatment, and risk factors. Front Neurosci. 2018;12:612. doi: 3389/fnins.2018.00612
CrossRef - Marsili L, Rizzo G, and Colosimo C. Diagnostic criteria for Parkinson’s disease: From James Parkinson to the concept of prodromal disease. Front Neurol. 2018;9:156. doi:10.3389/fneur.2018.00156
CrossRef
Abbreviation
| PD | Parkinson’s disease |
| EMG | Electromyography |
| MUAP | Motor Unit Action Potential |
| SENIAM | Surface EMG for Non-Invasive Assessment of Muscles |
| UPDRS | Inified Parkinson’s Disease Rating Scale |
| ANN | Artificial Neural Network |
| sEMG | surface EMG |
| ECRB | Extensor Carpi Radials Brevis |
| FCU | Flexor Carpi Ulnaris |
| FOS | Fast Orthogonal Search |
| EEG | Electroencephalography |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| SPECT | Single-Photon Emission Computed Tomography |







