Nidham M. Jamalludeen*
Department of Microbiology, College of Medicine, University of Basrah, Basrah, Iraq
DOI : https://dx.doi.org/10.13005/bpj/2146
Abstract
There is an evidence of high infections with community acquired Staphylococcus aureus among the healthy children. Anterior nasal colonization with S. aureus plays important role of spreading such infections with this organism. To evaluate the prevalence of S. aureus among healthy children, nasal swabs were collected from 119 children within the age from 1 month to 5 years. Parents or/and guardians combined the children were also interviewed for a questionnaire associated with the organism risk factors. Staphylococcus aureus was isolated in percentage of 14.28% out of 119 samples processed; among these 41.2% were Methicillin resistant S. aureus. The carriage of the organism was significantly noticed between the age groups that were live with big family size and were not attending preschool. In this study, the prevalence of MRSA was relatively high. Three bacteriophages specific for s. aureus were isolated as a candidate for biocontrol or treatment of the nasal carriages.
Keywords
Bacteriophages; Basrah; Nasal Carriage; Staphylococcus aureus
Download this article as:| Copy the following to cite this article: Jamalludeen N. M. Nasal Carriage of Staphylococcus Aureus in Healthy Children and its Possible Bacteriophage Isolates in Basrah, Iraq. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Jamalludeen N. M. Nasal Carriage of Staphylococcus Aureus in Healthy Children and its Possible Bacteriophage Isolates in Basrah, Iraq. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3cRjGCI |
Introduction
Staphylococcus aureus has been recognized as the main causative agent and the most common microorganism in community-acquired and hospital-acquired infections. (Askarian et al., 2009). It asymptomatically colonizes mainly the anterior nares of a human; It is also a clinically important factor in the pathogenesis and spread of S. aureus infection (Wertheim et al., 2005).The methicillin-resistant Staphylococcus aureus (MRSA) bacteria discovered in the 1960s are very serious health problems that have become more serious due to the increased occurrence of this organism that encodes the mecA gene for antibiotic resistance (Hartman and Tomasz, 1984; Sousa-Junior et al., 2009). The anterior nares in both adults and children can carry S. aureus bacteria through contaminated hands and from surfaces where these organisms can live for months (Kluytmans et al., 1997).In addition, the spread of this organism occurs particularly in close contact areas such as schools, nurseries or daycare, households that may be via contaminated hands and surfaces (Peacock et al., 2003). The incidence of community-associated MRSA infections in children, including children with no identified risk factors, has increased worldwide in the past decade.This indicates that healthy children may constitute a reservoir of MRSA in the community (Hussain et al., 2001). One possible approach to reduce methicillin-resistant Staphylococcus aureus in nasal carriage is phage bio control program, which is described as the application of phage to reduce or selectively eliminate pathogen-susceptible organisms from certain environments (Kutter et al., 2010).Phage therapy may be considered as an alternative to antibiotic therapy or biological microorganism control due to its high phage specificity and effectiveness against multidrug-resistant bacteria (Rakhuba et al., 2010; Golkar et al., 2014). Therefore, this study aimed to investigate the prevalence of a nasal carriage of Staphylococcus aureus in healthy children in the nursery in Basrah, Iraq. Isolation of a group of phages effective against a wide range of MRSA isolates, characterization of these phages according to their morphological characteristics, and detection of the presence of genes encoding unwanted toxins in these isolated phages.
Materials and Methods
Bacteriologic Media and chemicals
Blood agar base, Mannitol Salt Agar (M.S.A) and Mueller-Hinton agar were purchased from HiMedia Laboratories (Mumbai, India) and prepared according to the manufacturer’s instructions. Biochemical tests to determine the identity of the Staphylococci as well as Gram stain, Catalase and oxidase were also purchased from HiMedia Laboratories. Brain heart infusion agar (Salucea, Netherlands), Brain heart infusion broth (HIMEDIA, India), Agarose (Bio Basic, Canada), Peptone (DIFCO, USA), Beef extract (OXOID, England), Yeast extract (Sigma-Aldrich, Switzerland), Sodium chloride (Sigma-Aldrich, Switzerland), Sodium hydroxide (Sigma-Aldrich, Switzerland), potassium dihydrogen phosphate (Sigma-Aldrich, Switzerland), Gelatin (BDH, England), Barium chloride (Hopkins & Williams Limited, England), Gram stain (HIMEDIA, India), Catalase (HIMEDIA, India), Coagulase plasma (HIMEDIA, India), Sulfuric acid (BDH, England), Hydrochloric acid (BDH, England), tris-(hydroxymethyl)-aminomethane (PH7.5) (Riedel-deHaën, Germany), Chloroform (Sigma-Aldrich, Switzerland), Glycerol (Chem-supply, Australia), Absolute ethanol (BDH, England), Nuclease free water (Promega, USA), Ethidium bromide (Fisher, USA), Bromophenol blue (Fisher, USA), TBE buffer (Promega, USA), TE buffer (Promega, USA), (Bioanalyse, USA), Cefoxitin disc (30µg) (Bioanalyse, USA), and DNA ladder marker (100-10000) bp (KAPA, USA).
Study Design
The study was conducted in pediatric outpatient clinics of two hospitals (Ibn-Gazwan and Teaching Hospitals, Basrah, Iraq), between the period of March till December, 2019. Healthy children between the ages of 1 month and 5 years who visit these hospitals for a routine vaccination program were included in the study to determine nasal carriage of S. aureus. Only the healthy children were selected in this study.Ethical approval was obtained from the Board of the Iraqi Health and Higher Education Committee before sample collection begins.A questionnaire was filled-in for a total of 119 patients and the parents were interviewed for this purpose. The questionnaire contained patient information associated with their ages and other questions might assist in sampling process are shown in (Table 1).
Table 1: Staphylococcus aureus associated with nasal carriage from children between 1 month to 5 years old in Basrah, Iraq and factors analysis.
|
Factor |
Totals (%) |
Staphylococcus aureus |
P value |
|
| Positive (%) | Negative (%) | |||
| Sex:
Boy Girl |
59 (49.6%) 60 (50.4%0 |
9 (15.3%) 8 (13.3%) |
50 (84.7%) 52 (86.7%) |
.765 |
| Age group:
1-6 months 7-12 months 13-24 months 25-60 months |
34 (28.6%) 33 (27.7%) 38 (31.9%) 14 (11.7%) |
6 (17.6%) 4 (12.1%) 2 (5.3%) 5 (35.7%) |
28 (82.4%) 29 (87.9%) 36 (94.7%) 9 (64.3%) |
.009 |
| Breastfeeding status:
Yes No |
56 (47.1%) 63 (52.9%) |
3 (5.4%) 14 (22.2%) |
53 (94.6%) 49 (77.8%) |
.009 |
| Child attends:
No school preschool |
112 (94.1%) 7 (5.9%) |
14 (12.5%) 3 (42.9%) |
98 (87.5%) 4 (57.1%) |
.026 |
| Family size:
≤4 5-10 ˃10 |
35 (29.4%) 83 (69.7%) 1 (0.8%) |
6 (17.1%) 10 (12.0%) 1 (100%) |
29 (82.9%) 73 (88.0%) 0 (0%) |
.337 |
| Education of mother:
Illiterate Up-to primary Up-to secondary Graduate or post graduate |
20 (16.8%) 32 (26.9%) 55 (46.2%) 12 (10.1%) |
3 (15.0%) 7 (21.9%) 6 (10.9%) 1 (8.3%) |
17 (85.0%) 25 (78.1%) 49 (89.1%) 11 (91.7%) |
.499 |
| Occupation:
Yes No |
13 (10.9 %) 106 (89.1) |
1 (7.7%) 16 (15.0%) |
12 (92.3%) 90 (85.0%) |
.426 |
| Antibiotic usage(last2 wks.):
Yes No |
98 (82.4%) 21 (17.6%) |
11 (11.2%) 6 (28.6%) |
87 (88.8%) 15 (71.4%) |
.039 |
| Hospitalization (last 2 wks.):
Yes No |
2 (1.7%) 117 (98.3%) |
0 (0.0%) 17 (14.5%) |
2 (100%) 100 (85.5%) |
.560 |
| Hospital visit:
Yes No |
102 (85.7%) 17 (14.3%) |
15 (14.7%) 2 (11.8%) |
87 (85.3%) 15 (88.3%) |
.748 |
Swabs previously moistened with sterile saline solution were obtained from all participating children. The swab was rotated in the visible areas of both anterior nares. The swabs were immediately transferred to the Microbiology Laboratory at University of Basrah, College of Medicine for processing. Swabs were cultured on blood agar and mannitol salt agar (MSA) and incubated at 37 C for 24-48 hours. Colonies growing on blood agar and MSA were identified as S. aureus by their typical colony morphology, gram’s staining, catalase production and tube coagulase test. The isolates were screened for methicillin resistance by using cefoxitin disk screening test as mentioned by Baily and Scott (2013). The zone of inhibition diameter was measured and the results were interpreted according to CLSI criteria (CLSI., 2014).
Isolation of bacteriophage
Bacteriophages were isolated from raw sewage samples obtained from the treatment plant of the hospitals according to methods described by (Sambrook and Russell., 2001; Jamalludeen et al., 2007). 200 ml of fresh sewage was mixed with 20 ml of bacteriophage broth (Peptone (100g/L), Beef extract (30g/L), Yeast extract (50g/L), Sodium chloride (25g/L), Potassium dihydrogen phosphate (80g/L)), 20 ml of mixture of S. aureusisolates in broth culture [optical density at 600 nm (OD600 = 1.4)] and 20 ml of BHI broth were aseptically added to the 1 liter size flask and incubated at 37 ºC for 24 h with gently shaking. Afterincubation,themixturewascentrifuged at 4500xg (MSE, England)for 15 minute and the supernatant was transferred into a clean flask and then filtered throughasterile (0.45µm) membrane filter(chm, Spain). The phage titter was determined by serial dilution in which 100 microliter volumes of the filtrate was mixed with 100 microliter of broth culture containing S. aureusin a test tube and incubated at 37ºC for 20m in then 3ml of top agarose (7.0g/L) was added, the tube contents was then mixed and poured on to the surface of a BHI agarose plate and allowed to harden for few minutes and then incubated at 37ºC for 16 h.Next day,the plates were examined for the presence of plaques.A control tube containing bacteria and 3ml of to pagarose with out filtrate was also cultured on BHI agarose plate. Asterile Pasteur pipette with a rubber bulb was used to gently suctiona well-isolated plaque.The pipette contents were transferred into a tube containing 1ml of SM buffer (5.8g/LNaCl, 2g/LMgSO47H2O, 50ml/Lof 1MTrispH7.5,5ml/Lof2%gelatine) and 1 drop of chloroform was added to each tube. The tube was held at room temperature for 1–2h to allow the bacteriophage particles to diffuse out of the agar. The phage titre was determined by the soft agarose overlay method and finally the phages were stored at 4ºC until stocks were prepared.
Electron microscopy
Phage particles were negatively stained with 2% uranyl acetate (Sigma-Aldrich, Switzerland) on carbon-coated copper grid with standard procedure. TEM images was captured in a LEO 912AB energy filtered transmission electron microscope operated at 100 KV (Guelph Regional STEM Facility, University of Guelph, Guelph, Ontario). All three phages were classified according to the International Committee on Taxonomy of Viruses ICTV (ICTV., 2005).
Bacteriophages DNA extraction
The bacteriophage DNA was extracted using the QIAprep Spin M13 kit (QIAGEN, Germany) and according to the manufacture instruction. The presences of DNA were ensured by using the Nano drop (Optizen, Korea) and were visualized by agarose gel electro phoresis (Sambrook and Russell., 2001).
Detection of possible toxins by polymerase chain reaction (PCR)
Undesirable genes including staphylococcal enterotoxins and exfoliating toxins as well as toxic shock syndrome toxin of the isolated phages were searched by using QIAGEN multiplex PCR kit (QIAGEN, Germany) using a thermo cycler from Eppendorf (Germany) and according to the manufacture instructions. The primers were designed for this study by Eurogentec (Belgium). 100 µm stock of each primer was prepared according to the technical data sheet of each primer and kept in TE buffer at (-70) °C. Staphylococcus aureus specific genes, primers and their exact sequence as well as the size of the amplified product (bp) are listed in (table 2) (Mehrotra et al., 2000).
Table 2: Staphylococcus aureus specific genes, primers and their exact sequence as well as the predicted size of the amplified product (bp).
| Gene | Primer | Oligonucleotide sequence (5’-3’) | Size of amplified product (bp) |
| Sea | GSEAR-1
GSEAR-2 |
GGTTATCAATGTGCGGGTGG CGGCACTTTTTTCTCTTCGG | 102 |
| Seb | GSEBR-1
GSEBR-2 |
GTATGGTGGTGTAACTGAGC CCAAATAGTGACGAGTTAGG | 164 |
| Sec | GSECR-1
GSECR-2 |
AGATGAAGTAGTTGATGTGTATGG CACACTTTTAGAATCAACCG | 451 |
| Sed | GSEDR-1
GSEDR-2 |
CCAATAATAGGAGAAAATAAAAG ATTGGTATTTTTTTTCGTTC | 278 |
| See | GSEER-1
GSEER-2 |
AGGTTTTTTCACAGGTCATCC CTTTTTTTTCTTCGGTCAATC | 209 |
| Eta | GETAR-1
GETAR-2 |
GCAGGTGTTGATTTAGCATT AGATGTCCCTATTTTTGCTG | 93 |
| Etb | GETBR-1
GETBR-2 |
ACAAGCAAAAGAATACAGCG GTTTTTGGCTGCTTCTCTTG | 226 |
| Tst | GTSSTR-1 GTSSTR-2 | ACCCCTGTTCCCTTATCATC TTTTCAGTATTTGTAACGCC | 326 |
Statistical analysis
The data was entered to the SPSS V.21 software for statistical analysis. Chi-square tests were used to test for statistical significance (0.5) between the variables.
Results
One hundred nineteen children were involved; 59 were boys (49.6%) and 60 (50.4%) girls. Twenty nine percent of them were between 1 to 6 months of age, 28% were between 7 to 12 months, 32% were 13 to 24 months, and 12% were between 25 to 60 months (Table 1). A total percent of (53%) were having no breastfeeding and almost (47%) were feed with breastfeeding status. Around (94%) of the participated children were not attending school, whereas, only (6%) were attending preschool programs. Table 1 shows that (70%) of the participated children were belong to a family size composed from 5-10 family members and almost (46%) were having mother with education status for up-to secondary but most of them were not employed (89%). It was also reported that (82%) of the children were using antibiotics within the last 2 weeks and almost (86%) were visiting hospital for a purpose other than immunization programs. The prevalence of Staphylococcus aureus was 17 (14.28%) among the total of 119 samples processed for isolation of this organism (Table 1),
of these isolates 7 (41.2%) where identified as methicillin resistant Staphylococcus aureus (MRSA).Those isolates were significantly present at the children with no breastfeeding and were not attending school in a significant P-value of 0.009 and 0.26 respectively.
Bacteriophage isolation
Bacteriophage was successfully isolated from the sewage that was collected from the hospitals. Three bacteriophages (ɸNC#1, ɸNC#2, and ɸNC#3) were selected and a stock of of the bacteriophage sample was prepared and purified. All three phages produced clear large to medium sized plaques (4-6 mm in diameter) when propagated in S. aureus isolate(Figure 1). The appearance of the three phages under the transmission electron microscopy isshown in (figure 2). All three phages have icosahedral head and long thin non-contractile flexible tail with tail fibers. Based on their morphology all three phages belong to the family Siphoviridae (order Caudovirales).
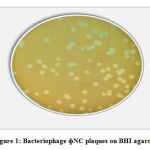 |
Figure 1: Bacteriophage ɸNC plaques on BHI agarose. |
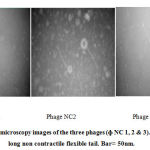 |
Figure 2: Electron microscopy images of the three phages (ɸ NC 1, 2 & 3). All phages have long non contractile flexible tail. Bar= 50nm. |
Multiplex PCR for detection of possible Staphylococcal toxins
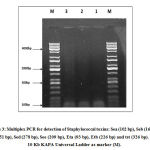
The PCR results showed that all three phages doesn’t encode for any of the staphylococcal toxins which includes staphylococcal enterotoxins (sea, seb, sec and see), exfoliating toxins (eta and etb) and the toxic shock syndrome toxin (tst) (figure 3).
Discussion
This study showed that (14.28%) of healthy children attending two hospitals clinic were carried Staphylococcus aureus; out of which 41.2% were methicillin resistant S. aureus (MRSA).A research study was shown a prevalence of 16% for S. aureus and 19% of which were MRSA among school going children in India (Ramana et al., 2009). As well, 36.4% from children study in Nashville, Tennessee were colonized with S. aureus, and 9.2% were colonized with MRSA (Creech et al., 2005). From Taiwan, Lo et al. (2007) were reported 9(13.2%) of 68 children had MRSA, and 17(25%) had S. aureus carriage. Hussain et al., (2001) were also found that 24.4% of children tested were colonized with S. aureus, and 2.5% of the participated children had MRSA. This study finding have a higher rate of MRSA at the participated children, this may because of the age group studied, however, Creech et al., (2005) was studied a group of age ranged from 5-14 years. Lowest ratio was come from a study done in Turkey (Ciftci et al., 2007), the researchers found that 28.4% of S. aureus were colonized children and only 0.3% of which were MRSA. They also reported that the education levels of the mother and father were found to be associated with the rate of nasal carriage.
Factors have been shown association with carriage of Staphylococcus aureus. Previously, Peacock et al. (2003) and Kluytmans et al. (1997) had shown age dependent with the prevalence of colonization with S. aureus. Although, Pathak et al. (2010) found no statistically significant differences in the prevalence of S. aureus with age group and only a lower prevalence was observed in the first six months of life.This current study provided a similar result. A consistent result which come in agreement with the stated criteria for clarity. There was a lower prevalence of carriage at the first months of life. Whereas, the peak of colonization has been seen at the age between 2-3 years of age.This may be due to competing colonization with other bacteria in the anterior nares by which colonization by one bacterial strain prevents colonization by another strain (Bogaert et al., 2004; Sivaraman et al., 2009).
Our study showed that children who do not go to school carry Staphylococcus aureus bacteria significantly more than children who go to school, especially with a family of 5-10 members.This might be due to poor hygienic measures and the overcrowded. Close contact may also play an important role in carriage of such organisms (Miller et al., 2009; Regev-Yochay et al., 2009).In this study, it was found that antibiotic use during the past two weeks showed a significant difference. Antibiotic use is one of the most important determinants of antibiotic resistance (Costelloe et al., 2010).Most of the MRSA isolated from children who received antibiotics as well as other S. aureus isolates. Although, Pathak et al., (2010) have found the exposure to antibiotic was not an important factor associated with S. aureus carriage. However, they found that two of the children who carry the MDR MRSA were received antibiotics in the last two weeks before taking the samples. On the other hand, it has been observed that simple hygiene measures may reduce the spread of resistant organisms in the community (Lennell et al., 2008).
An attempt was made to isolate a group of phages that have lytic activity against methicillin-resistant Staphylococcus aureus that were isolated from this study.However, phages are known to be the most abundant virus in the environment (Sandaa., 2012). They are widely spread and can appear in many different environments depending on the presence of their bacterial host such as marine and soil environments (Międzybrodzki et al., 2007; Mathur., 2011). Waste-water treatment plant was considered as the main source of phages in the current study. The isolation of Siphoviridae phages was successful from the collected samples with high phage titter ≥1010 since these phages are known for their ability to withstand adverse conditions due to their morphology (Lasobras et al., 1997;Muniesa et al., 1999).
Based on the morphological features of the phages observed by the electron microscopy all three phages belong to the Siphoviridae family with icosahedral head and long non-contractile flexible tail. The name Siphoviridae comes from the Greek word Siphon which means tube referring to the long tail (ICTV., 2005). These isolated phages are virulent against a broad range of Staphyloc occusaureus isolates as well as Staphyloc occusepidermidis isolates (Deghorain and Van Melderen., 2012; Xia and Wolz., 2014).
In order to ensure that these pahges are unable to encode virulence factors when suggesting their use in therapies or biological control; a PCR test were done to discover their empty from encoding genes. Some staphylocci bacteria are known to be able to encode some virulence factors such as staphylococcal toxin that includes staphylokinase (sak), enterotoxin A (sea), enterotoxin E (see), enterotoxin P (sep), exfoliative toxin A (eta), Panton-Valentine leukocidin (PVL), toxic shock syndrome toxin (tst), the innate immune modulators SCIN and CHIPS (Brüssow et al., 2004; Boyed., 2012). These toxins are responsible for a wide range of life-threatening illnesses such as scalded-skin syndrome, food poising and toxic shock syndrome (Boyed., 2012). The inability of these phages to encode any of the staphylococcal toxins makes them a good candidate for application as a therapeutic agent against methicillin-resistant Staphylococcus aureus as an alternative to antibiotics.
Conclusion
This study demonstrated that Staphylococcus aureus carriage was present among healthy children and methicillin-resistant S. aureus (MRSA) was present in a high proportion among Staphylococcus isolates.It shows that the children lived in a large family and did not go to any school that was carrying S. aureus more than the other groups.More surveillance studies are needed to aid the spread and to accurately assess the epidemiology of S aureus of nasal transport in different geographic regions.In addition, the three phages isolated with no toxin-coding genes indicate that Siphoviridae may be a potential alternative to antibiotics and may be used in the biological control of these organisms, but certainly more studies are needed to evaluate their activity in clinical trials.
Acknowledgment
The author thanks Mrs. Maryam Nabil for her assistance with sampling. We are grateful to Bob Harris for the assistance with the electron microscopy.
Conflicts of Interest
There are no conflicts of interest.
Funding Sourse
Nil.
References
- Askarian M1, Zeinalzadeh A, Japoni A, Alborzi A, Memish ZA. Prevalence of nasal carriage of methicillin-resistant Staphylococcus aureus and its antibiotic susceptibility pattern in healthcare workers at Namazi Hospital, Shiraz, Iran. Int J Infect Dis. 2009 Sep;13(5):e241-7
CrossRef - Bailey & Scott’s Diagnostic Microbiology, 13th Edition, 2013. Mosby.
- Bogaert D, van Belkum \A, Sluijter M, de Groot R, Rumke HC, Verbrugh HA, and HermansPW.Colonization by Streptococcus pneumonia and Staphylococcus aureus in health children. Lancet. 2004; 363 (9424):1871-1872.
CrossRef - Boyed, E. F. Bacteriophage-encoded virulence factors and phage-pathogenicity island interactions. Advance in virus research. 2012;Vol.83. Pp.91-112.
CrossRef - Brüssow, H., Canchaya, C. and Hardt, W-D. Phages and the Evolution of Bacterial Pathogens: from Genomic Rearrangements to Lysogenic Conversion. Microbiology and Molecular Biology Reviews.2004; 68(3): 560-602. doi:10.1128/MMBR.68.3.560-602.2004.
CrossRef - Ciftci IH1, Koken R, Bukulmez A, Ozdemir M, Safak B, Cetinkaya Z. Nasal carriage of Staphylococcus aureus in 4-6 age groups in healthy children in Afyonkarahisar, Turkey. Acta Paediatr. 2007: 96(7):1043-6.
CrossRef - CLSI-Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. 2014; CLSI document M100-S24, 34(1): 219.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: 2096.
CrossRef - Creech CB, Kernodle DS, Alsentzer A, Wilson C, and Edwards KM. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J. 2005: 24(7):617-21.
CrossRef - Deghorain, M. and Van Melderen, L. The Staphylococci Phages Family: An Overview. Viruses.2012; 4: 3316-3335. doi:10.3390/v4123316.
CrossRef - Golkar, Z., Bagasra, O., and Pace, D. G. Bacteriophage therapy: a potential solution for the antibiotic resistance crisis. J Infect Dev Ctries. 2014; 8(2): 129-136.
CrossRef - Hartman BJ, Tomasz, A. Low-affinity penicillin-binding protein associated with bets-lactam resistance in Staphylococcus aureus. J Bacteriol 1984; 158(2):513-516.
CrossRef - Hussain FM1, Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr Infect Dis J. 2001: 20(8):763-7.
CrossRef - ICTV-International Committee on Taxonomy of Viruses. 2005. Virus taxonomy; classification and nomenclature of viruses. Eighth report of the International Committee on Taxonomy of Viruses. Springer-Verlag/Wien, Austria, 57-70.
- Jamalludeen, N., Johnson, R. P., Friendship, R., Kropinski, A. M., Lingohr, E. J. and Gyles, C. L. Isolation and characterization of nine bacteriophages that lyse O149 enterotoxigenic Escherichia coli. Veterinary microbiology. 2007; 124: 47-57.
CrossRef - Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997: 10(3):505-520.
CrossRef - Kutter, E., De Vos, D., Gvasalia, G., Alavidze, Z., Gogokhia, L., Kuhl, S., and Abedon, S. T.Phage Therapy in Clinical Practice: Treatment of Human Infections. Current Pharmaceutical Biotechnology. 2010; 11: 69-86.
CrossRef - Lasobras, J., Muniesa, M., Lucena, F. and Jofre, J. Relationship between the morphology of bacteriophages and their persistence in the environment. Water Sci Tech. 1997; 35: 129–132.
CrossRef - Lennell A, Kuhlmann-Berenzon S, Geli P, Hedin K, Petersson C, Cars O, Mannerquist K, Burman LG, and Fredlund H. Alcohol-based hand-disinfection reduced children’s absence from Swedish day care centres. Acta Padiatr. 2008: 97(12):1672-1980.
CrossRef - Lo, W., Lin, W., Tseng, M., et al. Nasal carriage of a single clone of community-acquired methicillin-resistant Staphylococcus aureus among kindergarten attendees in northern Taiwan. BMC Infect. Dis. 2007:7: 51-56.
CrossRef - Mathur, P.Hand hygiene: Back to the basics of infection control. The Indian Journal of Medical Research. 2011; 134(5): 611-620. doi:10.4103/0971-5916.90985.
CrossRef - Mehrotra, M., Wang, G. and Johnson, W. M. Multiplex PCR for Detection of Genes for Staphylococcus aureus Enterotoxins, Exfoliative Toxins, Toxic Shock Syndrome Toxin 1, and Methicillin Resistance. J. Clin. Microbiol. 2000; 38(3): 1032–1035.
CrossRef - Międzybrodzki, R., Fortuna, W., Dąbrowska, B. W. and Górski, A.Phage therapy of staphylococcal infections (including MRSA) may be less expensive than antibiotic treatment. PostepyHig Med Dosw. 2007; 61: 461-465.
- Miller M, Cook HA, Furuya EY, Bhat M, Lee MH, Vavagiakis P, Visintainer P, Vasquez G, Larson E, Lowy FD. Staphylococcus aurues in the community: colonization versus infection. PloS One. 2009; 4(8)-e6708.
CrossRef - Muniesa, M., Lucena, F. and Jofre, J. Study of the potential relationship between the morphology of infectious somatic coliphages and their persistence in the environment. Journal of Applied Microbiology. 1999; 87(3): 402–409. doi: 10.1046/j.1365-2672.1999.00833.
CrossRef - Pathak A, Marothi Y, lyer RV, Singh B, Sharma M, Eriksson B, Macaden R, Lundborg CS. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus in healthy preschool children in Ujjain, India. BMC Pediatrics. 2010; 10:100.
CrossRef - Peacock SJ, Justice A, Griffiths D, de Silva GD, Kantzanou MN, Crook D, Sleeman K, Day NP. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J Clin Microbiol. 2003: 41(12):5718-5725.
CrossRef - Rakhuba, D. V., Kolomiets, E. I., Dey, E. S. and Novik, G. I.Bacteriophage receptors, mechanism of adsorption and penetration into host cell. Polish Journal of Microbiology.2010; 59(3):145-155.
CrossRef - Ramana KV, Mohanty SK, Wilson CG. Staphylococcus aureus colonization of anterior nares of school going children. Indian J pediatr. 2009: 76(8):813-816.
CrossRef - Regev-yochay G, Raz M, Cameli Y, Shainberg B, et al. Parental Staphylococcus aureus carriage is associated with staphylococcal carriage in young children. Pediatr Infect Dis J. 2009: 28(11):960-965.
CrossRef - Sambrook, J. and Russell, D. W. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Sandaa, R. A. 2012. Environmental viral pool. In: Schmidt T. M. and Schaechter M., editors. Topics in Ecological and Environmental Microbiology. USA. Elsevier Inc.
- Sivaraman K, Venkataraman N, Cole AM. Staphylococcus aureus nasal carriage and its contributing factors. Future Micribiol. 2009: 4: 999-1008.
CrossRef - Sousa-Junior FC, Silva-Carvalho MC, Fermandes MJ, Vieira MF, Pellegrino FL, Figueiredo AM, et al. Genotyping of methicillin-resistant Staphylococcus aureus isolates obtained in the Northeast region of Brazil. Braz J Med Bio Res. 2009; 42(10):877-81.
CrossRef - Wertheim HF, Melles Dc, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasalcarriage in Staphylococcus aureus infections.Lancet Infect Dis 2005; 5(12):751-762.
CrossRef - Xia, G. and Wolz, C. Phage of Staphylococcus aureus and their impact on host evolution. Infection, Genetics and Evolution. 2014; 21: 593-601. doi. 10.1016/j.04.022.
CrossRef