Veena R. M1 , Kalpana L2, Lavanya S. H3
, Kalpana L2, Lavanya S. H3 , Bharat Kumar V. D4 and Manasa C. R5
, Bharat Kumar V. D4 and Manasa C. R5
Department of Pharmacology, BGS Global Institute of Medical Sciences, Bangalore.
Corresponding author Email: drveenarm@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2150
Abstract
Background:To assess the knowledge, attitude andpractice (KAP) of pharmacovigilance (PV) and adverse drug reaction (ADR) reporting among nurses and to evaluate the impact of an educational intervention for improving awareness of pharmacovigilance among nurses in a tertiary care teaching hospital. Methods:A predesigned structured KAP survey questionnaire was adapted from the previous studies,modified and validated internally in the department.Nursing staff working in all the departments of BGS GIMS College were included in the study. The KAP questionnaire was used to collect the data before and after an educational intervention. Results:A total of 77 nursing staffs were involved in pre-KAP and post- KAP survey questionnaire.The pre-test response rate was 25.3% for knowledge based questions, 55% for attitude and 24.67% for practice based questions. After educational intervention, the post-test response rate was 96.6% knowledge based questions, 84.4% for attitude and 78.24 % for practice based questions. The overall scores observed between pre-test and post-test were found to be statistically significant proving the effectiveness of educational intervention and improving the knowledge of pharma covigilance among nursing staff.Post educational intervention, nursing staff strongly agreed that pharmacovigilance (PV) should be trained in detail to all health care professionals and understood with the necessity of ADR reporting to the adverse drug monitoring centre (AMC) in our hospital run by department of pharmacology. Conclusions:This study proves that knowledge, attitude and practice of pharma covigilance and adverse drug reporting in routine practice can be improved by proper orientation and medical interventions.
Keywords
Adverse Drug Reaction; Attitude Knowledge; Nursing Faculty; Pharmacovigilance; Practices
Download this article as:| Copy the following to cite this article: Veena R. M, Kalpana L, Lavanya S. H, Kumar V. D. B, Manasa C. R. Knowledge, Attitude and Practice of Pharmacovigilance Among Nursing Staff in BGS GIMS Hospital. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Veena R. M, Kalpana L, Lavanya S. H, Kumar V. D. B, Manasa C. R. Knowledge, Attitude and Practice of Pharmacovigilance Among Nursing Staff in BGS GIMS Hospital. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3bAipQf |
Introduction
Safety and efficacy are the two essential parameters that form the basis of rational drug therapy. Practically, no drug lacks adverse effects, but their use has to be associated with an acceptable risk-benefit ratio.1 ADRs are one of the leading causes of morbidity and mortality. Of all the hospital admissions, it’s likely that approximately 2.9-5.6% is due to ADRs and as many as 35% of hospitalized patients experience an ADR during their hospitalization.2,3
Following thalidomide disaster, the safety of pharmaceutical products has become as important as its efficacy. Globalization of pharmacovigilance studies was initiated by World Health Organization (WHO) through establishment of the WHO Programme for International Drug Monitoring in 1968.4 The PV programme effort within the India is coordinated by the Indian Pharmacopoeia Commission (IPC) and conducted by the Central DrugsStandard Control Organization (CDSCO). The responsibility of IPC is to keep up and develop the PV database consisting ofall suspected serious ADR to medicines observed.5
Spontaneous reporting of ADR is the cornerstone of PV programme and is an important way to improve information on drugs that have been introduced to the market with limited safety knowledge obtained from premarketing clinical trials.4 According to the current regulation, it is the responsibility of all health care professionals HCPs (medical doctors, dentists, nurses,pharmacists, and midwives) to report all serious ADRs and all suspected reactions observed with medicines.4 All the medical college hospitals are required to have ADR monitoring centre (AMC)under Pharmacology department whose responsibilities include promoting pharmacovigilance activities, reporting ADRs, and providing training and education to HCPs in their hospital.4
However, ADR reporting rates are still low by HCPs.Underreporting limits and delays initiatives that could have been taken to prevent/reduce the harmful effects of medications. 4Nurses amongst HCPs are known to have an important role in ADR reporting and constitute a potentially valuable source for spontaneous ADR reporting in the hospitals.6 Thus, the opinions and attitudes of hospital nurses on the troubles of spontaneous reporting of ADRs and the ways to resolve them are very important.
Hence, this study was conducted to assess the KAP of nursing staff in our medical college hospital and to evaluate the impact of an educational intervention for improving awareness of pharmacovigilance among nurses in our medical college hospital.
Methods
Study setting
This questionnaire based cross sectional study was conducted at BGS Global Institute of Medical Sciences, Bangalore on April 2019.
Study design and tool
The study participants consisted of all thenurses working in various departmentsat the hospital during the study periodand who gave their informed consent.Before the study was initiated, the Institutional Ethics Committee approval was obtained and conducted according to the Declaration of Helsinki guidelines. The study was carried out by the Department of Clinical Pharmacology, BGS Global Institute of Medical Sciences, which has been running the ADR Monitoring Centre (AMC) under the Pharmacovigilance Programme of India (PvPI).
Study tool
A predesigned structured KAP survey questionnaire was adapted from the previous studies,modified and validated internally in the department of pharmacology by two expertise who were trained in thefield of pharmacovigilance. The questionnaire was slightly modified to suit our hospital setup.
The questionnaire had 20 questions.Of the 20 questions, sevenquestions were knowledge based(question no: 1–7),9 questions were attitude based(question no: 9–16), and four questions were practicebased(question no:17 -20).
Pre-test was conducted and the duration of the session was for 30 min and the questionnaires were collected back. The educational intervention started with a presentation for 30 minutes which enumerated what pharmacovigilance is, its necessity, about proper reporting of ADR and filling the ADR form. The presentation was followed by hands-on training on filling up the ADR form.
At the end of the session, the post-test was conducted with the same questionnaire and was collected after 30 min. The pre-test and post-test scores were assessed and subjected to statistical analyses.
Statistical Analysis
All recorded data were entered using MS Excel software and analysed using SPSS 22 version software for determining the statistical significance. Non-parametric test for paired nominal data McNemar test was used to compare the pre and post response of the nursing staff. . Collected data were assessed by mean, percentage, and standard deviation. P value < 0.05 was considered to be statistically significant at 5% level of significance.
Results
A total of 77nursing staff participated in the study.The pre-test response rate was 25.3% for knowledge based questions, 55% for attitude and 24.67% for practice based questions. After educational intervention, the post-test response rate was 96.6% knowledge based questions, 84.4% for attitude and 78.24 % for practice based questions (Figure 1.)
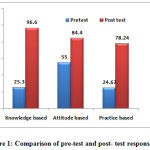 |
Figure 1: Comparison of pre-test and post- test response rate |
Knowledge analysis towards pharmacovigilance
The overall level of knowledge on ADR reporting and ADR burden were analysed.
Knowledge based questions 1 and 2 were based on the awareness of terminologies pharmacovigilance and ADR(Table 1). The percentage of correct response for the questions 1 and 2 were33.8% and 9.1% in the pre-test and 100% and 100% in the post-test respectively after the educational intervention which was statistically significant. Question 3 and 4 were about the kind and who should be reporting ADR(Table 1). Only 33.8 and 22.1% of nursing staff answered correctly in the pre-test which increased significantly to 83.1% and 97.4% in the post-test respectively(P=0.000) (Table 1).
Table 1: Knowledge Analysis toward Pharmacovigilance
| Knowledge | Questions | Pre-test n (%) | Post-test
n(%) |
McNemar
Test |
P-value |
| K1 | Have you heard the term Pharmacovigilence? | 26 (33.8) | 77 (100.0) | 49.020 | 0.000 |
| K2 | What is the full form of ADR? | 7 (9.1) | 77 (100.0) | 68.014 | 0.000 |
| K3 | What kind of ADRs should be reported? | 26 (33.8) | 64 (83.1) | 34.225 | 0.000 |
| K4 | Who according to you should report ADR? | 17 (22.1) | 75 (97.4) | 56.017 | 0.000 |
| K5 | Do you know about the ADR monitoring centre at BGS-GIMS? | 20 (26.0) | 76 (98.7) | 54.018 | 0.000 |
| K6 | Are you aware of the telephone number of the ADR monitoring centre at BGS-GIMS? | 18 (23.4) | 76 (98.7) | 56.017 | 0.000 |
| K7 | Are you aware of the Pharmacovigilence programme of India? | 22 (28.6) | 76 (98.7) | 52.019 | 0.000 |
| Overall Percentage | 25.23% | 96.6% |
Question 5, 6 and 7 assessed their knowledge regarding the presence of ADR monitoring centre at BGS, its contact number and the pharmacovigilance programme of India. In the pre-test, only 26.0%, 18% and 22% of the nursing staff gave the correct response respectively. In the post test, the percentage of correct response increased to 98.7% for the questions 5, 6, 7 which was statistically significant (P=0.000).
Attitude analysis towards pharmacovigilance
Question 1 asked the nursing staff whether reporting an ADR is a medical obligation. The pre-test and the post-test responses as “yes” were 51.9% and 97.4%. Question 2 asked them whether they are responsible for ADR reporting.The pre-test and the post-test responses as “yes” were 51.9% and 98.7%. Question 3 asked about the safety of the drugs. Only 66.2% of the nursing staff in the pre-test opined that all the drugs are not safe and score shot up to 97.4% after the post test(Table 2). Question 5, 7 and 8 was regarding the necessity of training all the health care professional for ADR reporting, whether they believe in ADR training sessions and whether they want to be part of future training sessions .The pre-test and post-test percentage for question 5 is 62.3 and 98.7% respectively, for question 7, the pre-test values are 67.5% and 96.1% and question 8 was about their willingness to be part of Pharmacovigilance training programmes in future. Only 47% wanted to be part of the future training sessions and post educational intervention, 87% of the nursing staff wanted to undergo training sessions in ADR monitoring which was statistically significant. Question 6 asked them whether they are confident in reporting ADR. 41.6% were not confident. Post educational intervention, 100 % of them were confident.Question 9 asked them about the inadequacy of ADR reporting in our hospital. 26% agreed, while in post-test, it increased to 39.0% even though not statistically significant (Table 2).
Table 2: Percentage of correct response to Attitude based questions pre and post test
| Attitude | Questions | Pre-test
N (%) |
Post-test N (%) | McNemar Test | p-value |
| A1 | Do you think ADRs should be reported? | 40 (51.9) | 75 (97.4) | 31.243 | 0.0000 |
| A2 | Do you think you are responsible for reporting ADR? | 40 (51.9) | 76 (98.7) | 34.028 | 0.0000 |
| A3 | Do you think that all the drugs are safe? | 53 (68.8) | 65 (84.4) | Exact McNemar test * | 0.0075 |
| A4 | Do you think reporting ADR will increase patient safety? | 51 (66.2) | 75 (97.4) | 18.893 | 0.0000 |
| A5 | Do you think Pharmacovigilence should be taught to every health care professional? | 48 (62.3) | 76 (98.7) |
24.300 |
0.0000 |
| A6 | Are you confident in reporting ADR? | 32 (41.6) | 77 (100.0) |
43.022 |
0.0000 |
| A7 | Do you believe ADR training sessions will be helpful? | 52 (67.5) | 74 (96.1) | Exact McNemar test * |
0.0000 |
| A8 | Would you like to part of any future training sessions in Pharmacovigilence? | 47 (61.0) | 67 (87.0) |
13.885 |
0.0000 |
| A9 | Do you think ADRs are not adequately reported? | 20 (26.0) | 30 (39.0) |
3.115 |
0.0075 |
| Overall Percentage | 55% | 84.4% |
Practice analysis towards pharmacovigilance
Question 1 asked them whether they have come across any ADR. The pre-test response was 28.6% and post-test was 67.5%. Question 2 asked them whether they had reported ADR that they had come across. Only 26 % said yes. The response increased to 54.5% post educational intervention (Table 3).
Table 3: Percentage of correct response to Practice based questions pre and post test
| Practice | Questions | Pre test n (%) | Post test
n (%) |
McNemar
Test |
p-value |
| P1 | Have you ever come across an ADR? | 22 (28.6) | 52 (67.5) | 19.114 | 0.000 |
| P2 | Have you ever reported an ADR? | 20 (26.0) | 42 (54.5) | 12.971 | 0.000 |
| P3 | Have you seen an ADR reporting form | 18 (23.4) | 76 (98.7) | 56.017 | 0.000 |
| P4 | Have you undergone any training session on ADR reporting? | 16 (20.8) | 71 (92.2) | 47.803 | 0.000 |
| Overall Response | 24.67% | 78.24% |
Question 3 was whether they have seen ADR reporting form .Only 23.4% said yes. The percentage increased to 98.7 in the post test. Question 4 asked them whether they had been trained in reporting ADR in the past. Only 20.8% of the staff said yes(Table 3). The percentage of correct answers increased after post educational intervention indicating that they were aware, seen, reported and trained also but were unaware of the same at that time.
The comparison between pre-test and post-test scores for knowledge analysis toward pharmacovigilance, attitude analysis and practice-based analysis scores were statistically significant, which clearly propels the idea that though the nurses have understood the science of pharmacovigilance to a certain level but wereunable to bridge the gap between the knowledge, attitude and its practical application.
Discussion
The spontaneous reporting of ADR, especially bythe nurse who come across the patientsinitially, is very much vital for the success of thePvPI program. Given their unique position in drug administration and recording side effects, nurses are well-placed to monitor the patients’ response to drugs.
The knowledge, attitude and practiceof nursing staff towards ADR reporting were evaluated using seven knowledge-related, nine attitudes related and four practice based questions. In our study, the overall response rate of knowledge related, attitude related and practice based questions were 25.23%, 36.3% and 24.67% in the pre-test. The overall response rate in the knowledge related, attitude related and practice based questions in the post test were 96.6%, 78.8% and 78.24% respectively. The scores improved significantly after the educational intervention.
After the educational intervention in our study, there was a significant improvement in knowledge related to pharmacovigilance among nurses such as the location of AMC, National Coordinating Centre, the purpose of monitoring ADRs and who and what are the ADR reported.
The fact that majority of respondents 98.7% agreed that reporting of ADR is necessary and more than 80% (Table 2) wanted to be part of future pharmacovigilance training programmes, the pharmacovigilance should be taught in detail to all HCPs accentuating the importance of pharmacovigilance.
InPractice based questions, only 23.4 and 26.0% of the nursing staff had seen ADR form and reported the side effects in the ADR form respectively. The percentage increased to 92.2 and 98.7 in the post test. Only 20.8% had been trained in reporting ADR in the past. The percentage of correct answers increased after post educational intervention indicating that they were aware, they had seen, reported and also trained but were unaware of the same at that time as the importance of pharmacovigilance was not stressed.
There definitely is a gap between the ADR experienced and ADR reported by healthcare professional that should not be ignored. Nurses are often the source in alerting the responsible physician about possible ADRs. There is thus a logical reason to involve nurses and encourage them to contribute in ADR reporting system.There is a need to conduct similar follow up studies among various health-care professionals to improve strategies and make the National Pharmacovigilance Program of India a great success.
Conclusions
This study proves that knowledge, attitude and practice of pharmacovigilance and adverse drug reporting in routine practice can be improved by proper orientation and medical interventions.
Strengths and Limitations
To the best of our knowledge, very few studies have been done to assess KAP of pharmacovigilance among the nursing staff or students in Karnataka. Furthermore, comparing the scores in the pre-test and the post-test is the definite proof that aneducational intervention can be very much helpful in the betterment of KAP of pharmacovigilance. The major limitation of our study was essentially the sample size and it could have been applied to a wider nursing community.
Acknowledgment
We wish to thank the management of BGS Global Institute of Medical Sciences and Teaching Institute for permitting us to conduct the awareness program among the Nurses in our BGS GIMS hospital. We also thank and acknowledge faculty of nursing, faculty from the Department of Pharmacology,BGS GIMS who participated in the study actively. We extend our special thanks to our respected Dean and Principal, Medical Superintendent, RMO, Nursing Superintendent and our statistician for their support.
Conflict of interest
There are no conflict of interest.
Funding Sourse
There are no funding of Sourse.
Ethical approval
The study was approved by the Institutional Ethics Committee
References
- SupratimDatta and ShramanaSengupta An evaluation of knowledge, attitude, and practice of adverse drug reaction reporting in a tertiary care teaching hospital of Sikkim. PerspectClin Res. 2015 Oct-Dec; 6(4): 200–206.
CrossRef - Baniasadi S, Fahimi F, Shalviri G. Developing an adverse drug reaction reporting system at a teaching hospital. Basic ClinPharmacolToxicol. 2008 Apr; 102(4):408-11.
CrossRef - SomayehHanafi, Hassan Torkamandi, Alireza Hayatshahi, Kheirollah Gholami, and Mohammadreza Javadi. Knowledge, attitudes and practice of nurse regarding adverse drug reaction reporting. Iran J Nurs Midwifery Res. 2012 Jan-Feb; 17(1): 21–25.
- MüberraDevrimGüner&PerihanElifEkmekci .Healthcare professionals’ pharmacovigilance knowledge and adverse drug reaction reporting behavior and factors determining the reporting rates. Journal of Drug Assessment. 2019; 8(1):13-20
CrossRef - Nimesh S, Chaudhary A, Sharma A and Dev K. Pharmacovigilance Programme of India: A Review. Acta Scientific Pharmaceutical Sciences.2019; 3 (9):
CrossRef - Hall M, McCormack P, Arthurs N, Feely J.The spontaneous reporting of adverse drug reactions by nurses.Br J ClinPharmacol. 1995 Aug; 40(2):173-5
CrossRef







