E Abinaya , S Saradha
, S Saradha , K. R Ilamathi
, K. R Ilamathi , A Ruckmani
, A Ruckmani and R Arunkumar*
and R Arunkumar*
Department of Pharmacology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India.
Corresponding Author E-mail: drarunvp@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2096
Abstract
Introduction: Many clinical trials are ongoing in India to evaluate the efficacy and safety of various interventions in COVID-19. It is mandatory that the clinical trials be registered in the Clinical Trials Registry-India (CTRI) before enrollment of study participants. The present study was carried out with the objective of collecting, compiling and analyzing various types of trial data such as study design, interventions, outcomes and study sites.
Methods: The clinical trial data were collected from the CTRI web portal using the key word “COVID-19” on 14 July 2020. The CTRI data output of every study registered till 14 July 2020 was stored as PDF document and the data were transcribed into a validated excel sheet based on the pre-defined methods and categories and analyzed.
Results and Conclusion: A total of 293 clinical studies have been registered in CTRI as on 14 July 2020. Among them, 188 (64.16%) are interventional and 105 (35.83%) are observational studies. The interventions being evaluated are modern medications including drugs and biologicals, AYUSH formulations, Nutraceuticals, Yoga and Naturopathy. Most of the interventions are already in clinical use for non-COVID indications and undergoing repurposing evaluations for COVID-19 in the clinical studies. Trials with AYUSH formulations constitute more than half of the interventional studies (51.06%) while modern medications in 31.09% of the studies. 119 trials (63.3%) of the interventional studies are randomized studies. Large numbers of trials are conducted in the states where the incidence of COVID-19 is high. 146 interventional studies out of 188 are expected to be completed within 6 months and the outcomes of these studies may provide valid information on the potential treatments in COVID-19.
Keywords
COVID-19; CTRI; Clinical Trials; SARS Cov2
Download this article as:| Copy the following to cite this article: Abinaya E, Saradha S, Ilamathi K. R, Ruckmani A, Arunkumar R. Description and Analysis of Characteristics of COVID-19 Clinical Trials Registered in the Clinical Trials Registry-India (CTRI). Biomed Pharmacol J 2021;14(1) |
| Copy the following to cite this URL: Abinaya E, Saradha S, Ilamathi K. R, Ruckmani A, Arunkumar R. Description and Analysis of Characteristics of COVID-19 Clinical Trials Registered in the Clinical Trials Registry-India (CTRI). Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/2YL6J5U |
Introduction
World Health Organization (WHO), in its status report on COVID-19, reported that 12,768,307 patients have been infected by Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS-CoV-2) and the mortality is 566, 654 in a span of approximately 7 months, starting from Dec 31, 2019 to July 13, 2020 [1]. In India also, there is a significant disease burden as indicated by the total number of active cases, 4,26,167, total number of cases cured or discharged, 7,82,607 and the total number of deaths, 29,861 as on 23/07/2020 [2].
Presently, there is no specific or effective drug that can produce good clinical recovery in patients with COVID-19, though different countries and regulatory agencies recommend various treatment options, which may benefit the patients. In India, symptomatic treatment is provided with antipyretics, antibiotics, cough medications and later with oxygen support, corticosteroids, antiviral agents, anticoagulants and ventilator support depending upon the clinical condition and severity of the disease. Remdesivir, Convalescent Plasma and Tocilizumab are recommended as off-label investigative therapies in a select subset of patients. Similarly, Hydroxychloroquine (HCQ) is indicated for treatment of COVID-19 based on its in-vitro anti viral activity against SARS-CoV-2 and evidences obtained in a few clinical studies [3].
Several clinical studies and clinical trials are ongoing in India and other countries in order to find out a suitable medication that can offer clinically meaningful prognosis in patients suffering from COVID-19. As per the guidelines and various regulatory notifications, clinical trials have to be registered in trial databases, before they are initiated. In the United States of America (USA), National Institute of Health’s (NIH), the National Library of Medicine maintains www.clincialtrials.gov, a database to register clinical studies, both private and public funded and conducted in the USA or in any part of the world. The purpose of this database is to provide summary information of the studies conducted in the USA and elsewhere to the patients, their family members and the general public [4].
WHO maintains International Clinical Trials Registry Platform (ICTRP) through which it ensures that the relevant and complete information about the clinical studies / trials is publicly available. This platform provides information related to clinical trial design, study conduct and administrative aspects of the studies. It will help the stakeholders involved in healthcare decision making to have up-to-date information on the clinical trial scenario across the globe [5]. Similarly, countries such as Australia, Brazil, China, Korea, India, Cuba, European Union, Germany, Iran, Japan, Thailand and Srilanka have been maintaining their own clinical trial registries.
In India, the registry is termed as “The Clinical Trials Registry- India” (CTRI). It is hosted at ICMR’s National Institute of Medical Statistics and it is now mandatory that any trial conducted in India, National or International has to be registered before enrollment of the first patient. Registration of a trial in CTRI allows sharing of information and assures the public that such trials are conducted in a transparent manner. Every researcher has to register the studies involving “drugs, surgical procedures, preventive measures, lifestyle modifications, devices, educational or behavioral treatment, rehabilitation strategies as well as trials being conducted in the purview of the Department of AYUSH (http://indianmedicine.nic.in/)”. Currently, CTRI has initiated the process for registration of studies related to COVID-19, 24×7, considering the urgency involved in conducting such trials in this pandemic situation [6].
The physicians and pharmaceutical companies have been researching on various medications in COVID-19. The medications can be broadly classified into new drugs that are specifically developed for COVID-19 and old medications that were previously approved for other indications and are presently repurposed for COVID-19. Besides, AYUSH formulations are also tested in COVID-19.
We aimed to find out the details of various drugs and interventions which are evaluated in COVID-19 as well as various aspects of clinical studies conducted in COVID-19 including study design, study interventions, outcomes and distribution of study sites.
Materials and Methods
The present study is a desktop research, conducted with the clinical trial data collected from the CTRI website (http://ctri.nic.in/Clinicaltrials/advancesearchmain.php) using one single key word ‘COVID-19’. The study was exempted from the review of Institutional Human Ethics committee as it involved only database research and analysis.
Before starting the actual study (data collection, transcription and analysis) a pilot study of trial run or validation run involving COVID-19 trials was carried out. During this process, 10 studies from CTRI web-portal were randomly selected by running the search with the same key word “COVID-19”. The data elements of 10 trials were carefully studied and based on the findings of the preliminary analysis, 17 data elements were chosen for the actual study (Table 1). The pilot study also helped in categorizing the data elements into meaningful classification that can be analyzed descriptively. The method of categorizing various data elements is as follows.
Table 1: COVID-19 Clinical Trial Data elements.
| 1 | Date of registration |
| 2 | Sponsor details and Nature of funding |
| 3 | Countries of recruitment |
| 4 | Study sites |
| 5 | Phase of development |
| 6 | Regulatory clearance |
| 7 | Trial or study type |
| 8 | Study design |
| 9 | Study outcomes |
| 10 | Disease condition studied |
| 11 | Test and control interventions and drugs |
| 12 | Inclusion and exclusion criteria |
| 13 | Sample size |
| 14 | Method of randomisation |
| 15 | Blinding and concealment of treatment |
| 16 | Trial duration |
| 17 | Study status |
Date of trial registration
This data was used to define the number of trials registered date wise and month wise.
Sponsor details and Nature of funding
The CTRI portal categorized this information into several types and the same was partially adopted in this study. The categories included were i) Pharmaceutical Industry- Indian, ii) Pharmaceutical Industry- Global, iii) Government Medical College, iv) Government Funded, v) Private Medical College, vi) Private hospital / clinic, vii) Research Institutions, viii) Self-funded, ix) WHO and ICMR and x) Others.
Countries of recruitment
This information was categorized as i) India alone, ii) India and other countries and iii) other countries alone.
Study sites
It was categorized into two, the state wise distribution of the studies registered in India and whether the studies are single centered or multi centered.
Phase of clinical trials / studies
The categorization of phase of clinical studies was adopted from the CTRI portal without any modification and it included 8 categories such as i) Phase I, ii) Phase I / II, iii) Phase II, iv) Phase II / III, v) Phase III, vi) Phase III / IV, vii) Phase IV and viii) Not Applicable
Regulatory clearance
It was based on whether Drugs Controller General of India’s (DCGI’s) approval was obtained or not applicable.
Trial or study type
Interventional or observational nature of the clinical studies was considered.
Study design
The interventional studies were categorized as randomized, non-randomized, single arm studies and other types. The observational studies were categorized as cross sectional, cohort, case-control, follow-up, retrospective, survey, clinical-observational and other types of studies.
Disease conditions studied
The disease conditions, for which the studies were proposed, were classified as per International Classification of Disease – 10 (ICD-10). COVID-19 is classified under the code “B972/ Corona virus as the cause of disease classified elsewhere”. The data were classified whether the studies were under code B972 or any other ICD codes or others.
Test and control interventions and drugs
The interventions were classified based on whether they were drugs, biologicals, vaccines, AYUSH formulations, nutraceuticals, yoga and naturopathy or others.
Inclusion and exclusion criteria
Age of the subjects enrolled and severity of COVID-19 were considered for data collection from the inclusion and exclusion criteria. Age was categorized into multiple groups based on the observed frequencies and severity was categorized as mild, moderate or severe.
Sample size
Sample size was categorized based on the number of subjects proposed to be enrolled in the studies, as follows, i) 1 – 100, ii) 101 – 200, iii) 201 – 500, iv) 501 – 1000, v) 1001 – 5000, vi) 5001 – 10000 and vii) > 10000.
Method of randomization
The types of randomization were adopted from CTRI portal without any modification such as i) Adaptive randomization, ii) Coin toss, lottery, toss of dice, shuffling cards, iii) Computer generated randomization, iv) Permuted block randomization, v) Random number table, vi) Stratified randomization and vii) Others
Blinding and concealment of treatment
Blinding was categorized by partially adopting the method defined in CTRI portal whereas concealment of treatment was categorized according to the classification given in CTRI portal without any modification.
Trial duration
The trial duration was classified into 6 types, i) less than 1 month, ii) 1 to 2 months, iii) more than 2 months to 6 months, iv) more than 6 months to 2 years, v) more than 2 years and vi) not available.
Study status
The categories proposed in the CTRI portal was taken without any modification such as closed to recruitment, completed, open to recruitment, ongoing, not yet recruiting and not applicable.
Study outcomes
They were classified into primary and secondary and both of them were sub-classified into clinical, viral, imaging, biomarker, mortality and other outcomes.
Using the methodology defined above, the CTRI database search was done on 14/07/2020 and all the clinical studies registered till 14/07/2020 were included in the analysis. The CTRI data output of every study was stored as PDF document and the data were transcribed into a validated excel sheet based on the pre-defined methods and categories and analyzed.
The name “The Clinical Trials Registry- India (CTRI)” gives a superficial impression that the clinical studies registered in the database are clinical trials. But both interventional and observational studies are registered in CTRI and not all interventional studies can be strictly considered as clinical trials in regulatory sense. Hence in this manuscript, the terms “clinical studies” and “clinical trials” will overlap and the studies are primarily classified as observational and interventional studies.
Results and Analysis
There were 293 studies inclusive of both clinical trials and clinical studies registered with the CTRI portal for the key word “COVID-19” till 14/07/2020. Among these, 9 are multinational trials and the remaining 284, single country trials. Three ‘single country trials’ are registered from Bangladesh and the remaining all (281) are Indian trials. The three Bangladeshi studies are non-interventional in nature, proposed by Mr K M Amran Hossain, Lecturer of Physiotherapy in Bangladesh Health Professions Institute, Dhaka. The multinational trials include 5 interventional studies and 4 observational studies. The observational studies are sponsored by University of Birmingham, UK (2 studies), Society of Critical Care Medicine Discovery, Critical Care Research Network, USA (1 study) and Dept. of Mental Health and Behavioral sciences, Max Super Specialty Hospital, Delhi (1 study). The interventional studies are sponsored by Acerta Pharma BV, Vicore Pharma AB-Sweden, University of Birmingham-UK, WHO-ICMR and Welcare Hospital, Gujarat, India. The interventions evaluated in the multinational trials are Lopinavir/Ritonavir, Hydroxychloroquine (HCQ),
Interferon, Chloroquine, Acalabrutinib, Remdesivir, Investigational new drug C21 and Sodium bicarbonate impregnated steam inhalation. Most of the studies are conducted in single centres accounting 224 out of 293 (76.45%) and the remaining 69 trials (23.54%) are multi centre studies.
The first trial (CTRI/2020/03/024402) was registered on 31/03/2020 for Hydroxy chloroquine (HCQ). The objective of the study was to evaluate the prophylactic efficacy of HCQ in preventing COVID-19 infection among healthy volunteers who were having moderate to high risk of exposure to infected patients. It was proposed by Dr Remesh Bhasi, Head of the Dept. of Rheumatology in Aster Malabar Institute of Medical Sciences, Kozhikode, Kerala and included 500 participants. It was a Phase III randomized controlled trial investigating two regimens of HCQ, one, the ICMR recommended dosage regimen, ie 400 mg BD on day 1 and followed by 400 mg weekly once for 7 weeks and the other, investigator proposed regimen, ie 300 mg OD for 7 days followed by 300 mg weekly once for 7 weeks. The investigator has not updated whether the recruitment of participants started and the status shown in the CTRI portal is “Not yet recruiting”.
Since the registration of first trial on 31/03/2020, the number of trials gradually increased day by day to reach 293, on 14/07/2020. 35 trials were registered in April, 107 in May, 106 in June and 44 in July till 14/07/2020. The maximum number of trials registered on a single day was 10 trials on 29/06/2020, followed by 9 trials on 13/07/2020 and 8 trials on four different days, 3 days in the month of May, ie 25/5/2020, 29/05/2020 and 31/05/2020 and 1 day in the month of June, ie 02/07/2020. The month wise data of trials registered and the day-to-day data of trials registered are given in Figure 1 and 2.
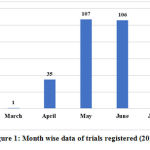 |
Figure 1: Month wise data of trials registered (2020) |
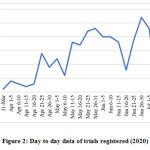 |
Figure 2: Day to day data of trials registered (2020) |
Study sites
Clinical trials and studies for COVID-19 were, so far, registered for 23 states and Union territories (UT). Delhi has the maximum number of trials, 76, followed by Maharashtra with 73 trials. States of Karnataka, Tamilnadu and Uttar Pradesh have respectively 41, 40 and 32 trials. The other states and UTs have trials ranging from 1 to 28. The states such as Arunachal Pradesh, Himachal Pradesh, Jharkhand, Manipur, Mizoram, Nagaland, Sikkim, Tripura and Union Territories such as Andaman and Nicobar, Ladakh, Lakshadweep, Dadra and Nagar Haweli do not have any trials registered. The distribution of trials across the states and UTs is given in Figure 3.
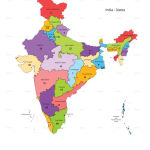 |
Figure 3: State wise distribution of COVID-19 trials |
Types of clinical studies and study design
Among the total of 293 trials, 188 are interventional and 105 observational. 63.29% of the interventional studies (119 out of 188) are randomized controlled trials and the remaining are either non-randomized, single arm or other types of studies. 47.61% of observational studies (50 out of 105) are cross sectional studies, 18.09% studies are cohort studies (19 out of 105) and the remaining studies follow various other designs. The study designs of interventional and observational studies are given in Figures 4 and 5 respectively.
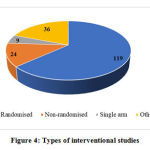 |
Figure 4: Types of interventional studies |
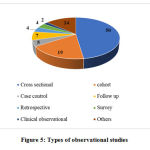 |
Figure 5: Types of observational studies |
Disease conditions studied
The analysis of disease conditions defined in the respective CTRI registrations shows that 132 studies among 188 interventional studies (70.21%) are conducted in COVID-19 with the specific COVID-19 disease code B972. One study each is conducted in acute respiratory distress syndrome with the code J80, acute respiratory failure with the code J960, malignant neoplasm of bronchus and lung with code not specified and respiratory failure unspecified with the code J969. The remaining 52 studies are conducted in healthy volunteers. The distribution of disease condition in interventional and observational studies is presented in Table 2 and Figure 6, respectively.
Table 2: Disease condition studied-Interventional studies
| Interventional studies (188) | |
| Disease condition studied | No. of studies |
| B972/ Corona virus as the cause of disease classified elsewhere | 132 |
| J80/Acute respiratory distress syndrome | 1 |
| J960/Acute respiratory failure | 1 |
| J969/ Respiratory failure, unspecified | 1 |
| Malignant neoplasm of bronchus and lung | 1 |
| Healthy volunteers | 52 |
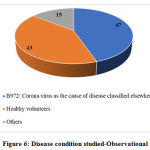 |
Figure 6: Disease condition studied-Observational |
Phase of clinical trials / studies
With regard to the phases of clinical studies registered, among the interventional studies, 3 studies are Phase I, 5 combined Phase I/II, 52 Phase II, 19 combined Phase II/III, 42 Phase III, 7 combined Phase III/IV and 7 Phase IV/post marketing surveillance. Among the three phase I studies, one study assesses safety of the homeopathic preparation CNVO1 in healthy volunteers, second study evaluates safety of cytokine cocktail therapy in healthy volunteers and the third study assesses the efficacy of low dose radiation therapy in COVID-19 pneumonia. The five combined Phase I/II studies include 4 studies that evaluate Ayurveda formulations and the fifth one evaluates the efficacy and safety of ozone therapy in mild to moderate COVID-19. The details of phase of study are not applicable (NA) for the remaining 53 interventional studies.
The observational studies are also classified into different phases in CTRI. One study, each, is Phase I and Phase II/III, 2 studies are post marketing surveillance studies and the other studies are categorized as not applicable (NA). The study categorized as Phase I is a cross sectional study sponsored by Dabur and it aims to generate data among healthcare workers who are engaged in COVID-19 care and on treatment with Ayurveda based immuno modulators consisting of three tablets, namely Mahasudarshan vati, Samshamani vati and Haridra. The Phase II/III observational study is sponsored by Dept. of AYUSH, Govt. of Telangana to generate data on incidence of COVID-19 among healthcare workers who are on Ayurveda raksha kit 1 which is a combination of three formulations, AYUSH 64, Samshamani vati and Chyavanprash. The two post marketing surveillance studies include one study of surveying the clinical acceptability of mouth dissolving turmeric lozenges in healthcare workers and the other study for the validation of rapid diagnostic kits in detection COVID-19 antibodies. The graphical representation of phases of interventional and observational studies is given in Figures 7 and 8.
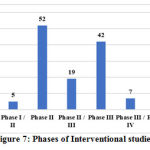 |
Figure 7: Phases of Interventional studies |
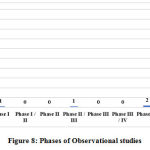 |
Figure 8: Phases of Observational studies |
Various types of interventions are evaluated in COVID-19 as detailed in the CTRI registration data. 41 studies are conducted with drugs (chemicals or small molecules), 15 with biologicals, 4 vaccines, 96 AYUSH medicines and 7 nutraceuticals. Besides, 6 studies are conducted with Yoga and Naturopathy.
The 41 studies (21.80% of 188 studies) registered for drugs include investigation of 28 molecules. Hydroxy chloroquine (HCQ) is the most investigated single agent in COVID-19 either being studied as monotherapy or in combination with other agents, in a total of 7 studies. The agents combined with HCQ are Nitazoxanide, Ciclesonide, Ivermectin and Azithromycin in these studies. Ivermectin is the second most studied drug, registered in 5 studies, with 4 studies investigating it as monotherapy and one study in combination with HCQ and Ciclesonide. Favipiravir is investigated in 3 studies, two studies sponsored by Glenmark, one investigating Favipiravir monotherapy and the other ‘Favipiravir+Umifenovir’ combination therapy and the third study, sponsored by Cipla, evaluating Favipiravir monotherapy. Chloroquine Phosphate (CQ) is evaluated in two studies, one for its efficacy when it is administered orally and the other for the evaluation of intranasal administration of Chloroquine, 0.03%. The other drugs investigated in COVID-19 include immuno-modulators, antivirals, anti helminthic agents and a few neurological and cardiovascular agents. The comprehensive list of medications evaluated in COVID-19 is provided in Table 3. The consolidated data on various study interventions is graphically presented in Figure 9.
Table 3: List of interventions evalauted in COVID-19 trials
| Drugs (small molecules) | Ayurvedha | Siddha |
| Chlorophyllin
2-deoxy-D-glucose oral powder Acalabrutinib C21 Chloroquine phosphate Chloroquine nasal drops Hydroxy chloroquine Nitaxozanide Niclosamide Eflornithine Imatinib Immunoglobulin Sepsivac Ivermectin Lithium Losartan N-acetyl cysteine Ciclesonide Nafamostat Sofusbuvir Lopinavir/Ritonavir Favipiravir Remdesivir Azithromycin Ulinastatin Resveratrol copper Mycobacterium W (Heat killed, autoclaved) Purified aqueous extract of Cocculus hirsutus (AQCH) Biologicals Convalescent plasma Interferon gamma Interferon alpha 2b Interferon beta 5. Itolizumab Tocilizumab |
AYUSH 64
ShatPlus Raj Nirwan Bati capsule Ayush Kwath Aayudh Advance Bhoumya Mulmina TM Mango Immunity Kit Shakti drops Tab. Bresol and Tab. Septilin Aragwadhadi kwath Turmeric mouth dissolving lozenge Guduchi ghan vati Ashwagandha (Withania somnifera) Samshamani vati Piper betal + swarnabhasma Yashtimadhu Shanshamani Azadvir drops Shirashadi Kasai Sudarshan Ghanvati and Vyaghradi Kwath Malla Chandrodaya + Ashthadashang Ghana vati Pranvir + Yashada Rasayana Arogya Kashayam-20 Ayurcov Dabur Chyawanpras MyVir tablets Zingivir-H Amrta Karuna Clevira tablet Ayush Raksha Kwath Astha Tinospora cordifolia Giloy Gaumutra Dashamula kwatha + Pathyadi kwatha
|
Kabasura kudineer
Amukkara churnam Nellikai legium Bramanandha bairavam Nilavembu kudineer Adathodai Manappagu
Unani
Joshanda decoction Khameera Marwareed Tiryaq-e-Arba
Homeopathy Tuberculinium 1M + Zincum Metallicum 6C + Chininum Arsenicosum 6C + Calc Phos 6x Arsenic album 30 C Bryonia alba 30C Bryonia Alba, Gelsemium, Antimonium Tartaricum, Crotalus Horridus Cadamba
Nutraceuticals ACT-12, ACT-13 Virulina tablets Neem capsules SSV formulation Thymoquinone Picovrid syrup |
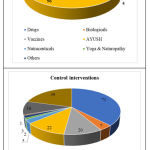 |
Figure 9: Types of Interventions |
The most commonly investigated biological therapy in COVID-19 is Convalescent Plasma. It is evaluated in 10 studies including the one-multi centre study proposed and conducted by ICMR, the PLACID trial. Council of Scientific and Industrial Research (CSIR) sponsors one trial, which is conducted in ID and BG Hospital, Kolkata. Wockhardt Ltd has proposed one trial and various Govt. and Private hospitals have proposed all the remaining trials. The Office of the Drugs Controller General of India (DCGI) has approved all the Convalescent Plasma trials registered. Among these trials, 5 are multi centre and 5 single centre trials. The trials proposed by ICMR and Max Super Specialty Hospital, Delhi are in moderate COVID-19 and the remaining 8 studies assess the efficacy of Plasma therapy in severe COVID-19. The five studies on the biological agents other than plasma therapy are conducted with Interferons including Interferon gamma and pegylated Interferon alfa 2b and Itolizumab and Tocilizumab.
BCG vaccine is evaluated in 4 vaccine trials registered in CTRI, till 14/07/2020. Three studies investigate the prophylactic role of BCG vaccine against COVID-19 while one study evaluates the efficacy of BCG as a therapeutic agent. ICMR initiated a BCG vaccine prophylactic study that aims at reducing the morbidity and mortality in elderly individuals in COVID-19 hot spots. JIPMER has initiated a prophylactic study with BCG vaccine among health care workers. The third prophylactic study is sponsored by Serum Institute of India Pvt. Ltd to evaluate the efficacy of BCG in reducing the incidence and disease severity among high-risk subjects. Medical Education and Drugs Department of Mumbai sponsors the only therapeutic study of BCG vaccine that is initiated in Sassoon General Hospital, Pune. It evaluates the effectiveness of BCG as a potential treatment in symptomatic COVID-19 positive patients.
Six studies are registered to evaluate the role of Yoga and Naturopathy in COVID-19. Among them, 2 studies are carried out in COVID-19 positive patients and the remaining 4 studies in healthy volunteers. Govt. Yoga and Naturopathy Medical College, Chennai, Tamilnadu sponsors a therapeutic study to investigate the efficacy of Yoga and Naturopathy as the main treatment in comparison with standard treatment in mild COVID-19 patients. It is conducted in 4 sites in Chennai, namely, Rajiv Gandhi Govt. General Hospital, Govt. Stanley Medical College Hospital, Govt. Kilpauk Medical College Hospital and Govt. Omandurar Medical College Hospital. The second therapeutic study is conducted in GS Ayurveda Medical College and Hospital, Hapur, Uttar Pradesh (UP) and sponsored by the same Institute. This study investigates the efficacy of Yoga and Naturopathy as an add-on therapy to two herbal formulations in asymptomatic and mild COVID-19 patients. Among the four studies conducted in healthy volunteers, one is sponsored by JIPMER, Pondicherry, one by AIIMS-Rishikesh and two studies by AIIMS-New Delhi. JIPMER investigates the effect of alternate nostril breathing and guided meditation practice in doctors and nursing staff to check whether it has significant impact on sleep quality and coping abilities amidst their work stress in COVID-19 pandemic. AIIMS – Rishikesh conducts a similar study of investigating the role of Yoga and Naturopathy in reducing the stress levels in healthcare workers. AIIMS-New Delhi conducts two studies, both are with the combination of an Ayurveda formulation and Yoga, while one study assesses the efficacy of the intervention in reducing the incidence of COVID-19 among healthcare workers, the other study, among individuals who are exposed to COVID-19 and quarantined.
Nutraceuticals are evaluated in 7 studies registered and among them 5 studies are conducted in COVID-19 positive patients and 2 studies in healthy volunteers. Among the 5 studies conducted in COVID-19 positive patients, 4 studies investigate the role of Nutraceuticals added to standard treatment in terms of clinical and viral outcomes. These 4 studies evaluate 4 different poly Nutraceutical formulations, namely Virulina, ACT 12 tablets and dry syrups, SSV tablets and PICOVRID syrup. The fifth study aims at assessing the efficacy of the Nutraceutical formulation, Thymoquinone as monotherapy in comparison with standard treatment in improving the clinical and viral outcomes. Between the two studies conducted in healthy volunteers, one study evaluates Neem capsule for its efficacy in reducing the incidence of infection in healthcare professionals and high-risk contacts of COVID-19 and the other study evaluates the same outcome with a different poly Nutraceutical formulation consisting of various ingredients including Vitamin D3.
Ninety six (96) studies are being conducted with AYUSH formulations in COVID-19. The number is almost more than twice the number of studies conducted for drugs and biologicals together. Various types of interventions from Ayurveda, Siddha, Homeopathy and Unani are experimented with the expectation that native medicines could offer the much-needed relief from COVID-19. Ministry of AYUSH has taken the initiative in this regard and sponsored 11 studies involving AYUSH formulations.
Arsenic Album or Arsenicum Album, a popular homeopathic medicine, is evaluated in 9 studies. It contains Arsenic Tri oxide as the main ingredient and is used as a general immunity booster. Out of nine studies, 5 studies evaluate its efficacy as therapeutic agent in COVID-19 and 4 studies as prophylactic agent in healthy volunteers. Kabasura kudineer, a Siddha concoction, is evaluated in 8 studies and among them 2 studies exclusively test Kabasura kudineer, whereas the other 6 studies test Kabasura kudineer given along with other herbal concoctions. The two studies that exclusively evaluate the therapeutic potential of Kabasura kudineer in asymptomatic COVID-19 are conducted in Tamilnadu, one study at Govt. Stanley Medical College and the other study in Govt. Theni Medical College. Among the other 6 studies that investigate combination of Kabasura kudineer and other concoctions, 2 studies evaluate the prophylactic potential among high-risk contacts and the remaining 4 studies assess the therapeutic potential in asymptomatic and mild to moderate COVID-19.
The Ayurveda formulations Ashwagandha and Guduchi are evaluated in 6 studies each. These formulations are immunity boosting agents usually recommended in the treatment and prophylaxis against respiratory infections. These are investigated for their therapeutic potential against COVID-19 in one study each and prophylactic potential in 5 studies each. Formulations such as AYUSH-64, AYUSH-Kwath are evaluated in 3 studies each and Dabur’s very famous Chyawanprash is evaluated in 4 studies. And there are several other AYUSH formulations being tested in more than 70 studies.
The analysis of control interventions indicate 71 trials (37.76%) have standard treatment as control. 20 trials (10.63%) do not have any control and 22 trials (11.70%) have placebo control. Though HCQ and Azithromycin are not yet fully accorded evidence-based approval for their use in COVID-19, they are used as control interventions in some of the trials, with HCQ alone in 3 trials, HCQ+Azithromycin in 2 trials and Azithromycin in 1 trial. The remaining trials have various other control interventions such as supportive therapy, Zinc and Vitamin C.
Study Outcomes
The interventional studies assess the treatment outcomes with varying measures of primary and secondary end points including clinical, viral, imaging, biomarker, mortality and safety. Out of 188 interventional studies, 37.23% of studies (70 studies) assess the clinical outcome as primary outcome measure. Among them 37 studies (19.68%) have clinical outcome as the only measure of primary outcome and the other studies have clinical outcome in combination with other primary outcomes like viral, imaging, biomarker and mortality. 64 out of 188 studies (20.7%) have primary outcome that does not include clinical measures and they have either only viral or combination of other non-clinical outcomes. 23 out of 188 studies (12.2%) have viral outcome as the only primary outcome measure. Mortality alone is measured as primary outcome in 6 studies and mortality in combination with other outcomes is measured in 8 studies. The distribution of primary and secondary outcomes of the interventional studies is given in Table 4.
Table 4: Study outcomes
| Interventional studies | Observational studies | ||
| Primary outcomes | Secondary outcomes | Primary outcomes | Secondary outcomes |
| Clinical – 37 | Clinical – 5 | Clinical – 1 | Biomarker – 1 |
| Biomarker – 2 | Biomarker – 3 | Clinical + others – 3 | Clinical + others – 1 |
| Clinical + others – 33 | Clinical + others – 43 | Viral – 1 | Mortality – 1 |
| Viral – 23 | Viral – 4 | Mortality – 3 | Others – 21 |
| Mortality – 6 | Mortality – 4 | Others – 18 | NA – 81 |
| Others (without clinical) – 64 | Others (without clinical) – 103 | NA – 79 | |
| NA – 23 | NA – 26 | ||
Other data elements
The other data elements compiled from the studies such as sponsor details and nature of funding, regulatory clearance, age of study participants, severity of COVID-19 included in the studies, sample size, method of randomization, blinding and concealment of treatment, trial duration and study status are categorized, analyzed and presented in Figure 10 to 18.
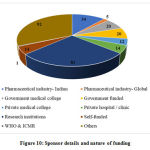 |
Figure 10: Sponsor details and nature of funding |
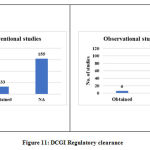 |
Figure 11: DCGI Regulatory clearance |
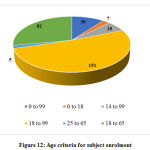 |
Figure 12: Age criteria for subject enrolment |
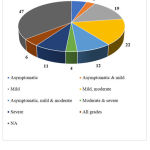 |
Figure 13: COVID severity distribution |
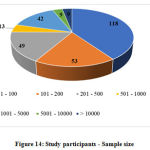 |
Figure 14: Study participants – Sample size |
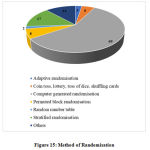 |
Figure 15: Method of Randomisation. |
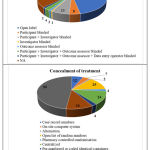 |
Figure 16: Blinding and concealment of treatment |
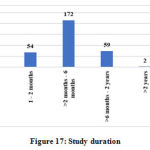 |
Figure 17: Study duration. |
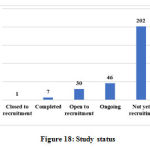 |
Figure 18: Study status |
Discussion
This study of collecting, compiling and analyzing the clinical trial data from the Clinical Trials Registry – India (CTRI), has given a lot of useful information on the way studies are planned and conducted in COVID-19. The geographical distribution of COVID-19 trials indicates that the states having more number of COVID-19 cases have more number of trials registered. The states and UTs such as Maharashtra, Tamilnadu, Delhi, Karnataka, Rajasthan and Uttar Pradesh have higher incidence of COVID-19 and they account for higher number of trials, with Delhi, a maximum of 76 trials, Rajasthan, 25 trials and the other states between 76 and 25. The states where the number of COVID-19 cases is low especially the North East states like Manipur, Mizoram, Nagaland, Sikkim, Tripura and a few UTs, there are no trials registered. It is expected that the areas with high disease burden will be desperate to find out solutions by way of doing more trials than the areas that have less disease burden and more over when the cases are low the patient recruitment will also be difficult justifying their less participation in research.
With regard to the interventions evaluated in COVID-19, AYUSH formulations outnumber the modern medications that include drugs, biologicals and vaccines. Out of 188 interventional studies, 51.06% of studies are for AYUSH formulations, 31.9% modern medications and 17.02% Nutraceuticals, Yoga and Naturopathy and others. This implies that the native Indian systems of medicines are in lead, currently, in the research on COVID-19. It also indicates that the stakeholders in AYUSH system of medicine including the Govt. agencies, pharmaceutical companies and AYUSH practitioners have prioritized the task of finding out solution to the COVID-19 pandemic.
Interventional studies constitute 64.16% (188 out of 293) of the total registered studies and among them, 63.29% (119 studies out of 188) are randomized trials and it can be anticipated that these studies may yield clinically useful information on COVID-19 treatment.
Though 293 studies have been registered for COVID-19 and 188 were interventional studies among these, there is no novel investigational new drug explored in COVID-19, except for one investigational new drug, namely C-21. This molecule is developed by Vicore Pharma AB, Sweden and has been primarily evaluated in Idiopathic Pulmonary Fibrosis and Pulmonary Fibrosis associated with systemic sclerosis and now the company is exploring its potential in COVID-19. The pharmacological information about this drug is not available in CTRI portal or public domain. As per the data available in CTRI, the drug is evaluated in a Phase II, multi national clinical trial for its efficacy on certain biomarker levels including CRP, IL6, IL10, TNF, CA 125 and Ferritin. In India, this study is initiated in 9 sites and the recruitment status shows “Not yet recruited”.
Most of the medicines evaluated in the CTRI registered interventional studies, either modern medications or traditional medicines, are all already in use for various other indications and only undergoing repurposing clinical experiments presently in COVID-19. The antiviral drugs evaluated are Sofosbuvir, Remdesivir, Lopinavir/Ritonavir and Favipiravir. There are many unrelated medications evaluated in COVID-19 that include drugs like anti helminthic drugs (Nitazoxanide, Eflornithine, Niclosamide, Ivermectin), antihypertensive Losartan, Lithium, N Acetyl cysteine, urinary trypsin inhibitor Ulinastatin and a serine protease inhibitor Nafamostat. There are other agents with possible theoretical explanations for their benefits in COVID-19, which are tested in some of the clinical studies, and they include inhalational corticosteroid Ciclesonide, antimalarials like Chloroquine and Hydroxyl chloroquine, tyrosine kinase inhibitors (Imatinib and Acalamrutinib) and biologicals (Tocilizumab and Itolizumab).
In our study, we could identify BCG vaccine as the only vaccine getting evaluated for prophylactic and therapeutic potential in COVID-19. But it is known from other sources that two vaccine trials (Phase I / II) other than the BCG vaccine studies are currently registered in CTRI and sponsored by Bharat Biotech and Cadila. These were registered on 01/07/2020 and 04/07/2020 respectively. But in our search we could not retrieve them with the key word “COVID-19”. The probable reason could be that the respective study titles do not have “COVID-19” and the sponsor companies have mentioned the disease condition as “healthy volunteers” at the time of CTRI registration.
The Indian Pharmaceutical companies have been sponsoring 34 clinical studies in COVID-19. Cadila Pharmaceuticals has been conducting 4 trials. Glenmark, Sun Pharma and Dabur India have been engaged in 2 trials. The other companies including Biocon biologics, Bharath Serum, Intas, Cipla, Dr Reddys Laboratories, Himalaya Drug Company and Wockhardt are associated with 1 trial each.
The analysis of current status of the studies indicates that 46 studies are ongoing, 7 completed, 30 open to recruitment and 202 studies not yet recruiting. It may seem that 68.94% of studies (202 out of 293) are not yet recruiting but there is a possibility that many studies must be ongoing or completed and the investigators might not have updated the information in CTRI.
As analyzed in this study, many scientists, researchers and physicians analyze the characteristics of clinical trials in this manner. But with regard to COVID-19, we are not able to access significant number of manuscripts dealing with clinical trial database analysis. Lu et al. (2020), has analyzed the characteristics of clinical trials registered in “ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (WHO ICTRP), Chinese Clinical Trials Registry (CHiCTR), Australian Clinical Trials Registry, Britain’s National Research Register (BNRR), Current Control Trials (CCT), and Glaxo Smith Kline Register” and published the data in the Journal of BMC Medicine which was made available online on 1 June 2020. They reported 220 clinical trials registered in these databases as on 27 Feb 2020. The authors analyzed study design, study type and patient characteristics among other information and observed that the Traditional Chinese medicines (TCM) were the most frequently investigated treatment either alone or in combination with other treatments and the other common medications experimented were the antiviral drugs and biologicals [7].
Jihan Huang et al. (2020), analyzed the characteristics such as study design, end points, number of samples, types of interventions of the clinical trials obtained from the Chinese Clinical Trials Registry (CHiCTR) and ClinicalTrials.gov. The authors reported that 262 interventional trials were registered for COVID-19 as on 10 March 2020 and 76.3% of studies were randomized parallel studies. 22.9% of trials considered symptom improvement as the primary outcome measure and 16.4% of trials considered viral clearance as the primary outcome. They also found that the most frequently evaluated intervention was the Traditional Chinese medicines (TCM), at 27.9%, which is similar to the observation of Lu et al. (2020) [8]. In our analysis of CTRI data also we found that the traditional systems of Indian Medicines (AYUSH) are evaluated in 51.06% of interventional studies which is significantly higher than the studies conducted for modern medications.
One of the limitations of the present analysis could be that we have used one single key word “COVID-19” to retrieve the data from CTRI and we did not include other relevant key words such as SARS CoV-2, Corona virus, Novel Corona virus, COVID, Corona virus disease and Novel corona virus disease. The other limitation could be that the data collection was done till 14 July 2020 and when the manuscript is published, the actual data and the trend of analysis may vary from what is presented in this article. Further, the authors purposefully do not elaborate the possible pharmacological mechanisms and hypothesis based on which the interventions are evaluated in these COVID-19 studies. Arunachalam Ruckmani et al., (2020) in their review article on clinical trials and potential therapeutic agents in COVID-19, have given a detailed account on the rationale for use of various drugs in COVID-19 and the evidences available in support of their use [9]. However, the present study will be the first of its kind in analyzing the COVID-19 clinical trial data from CTRI web portal, in this manner.
Conclusion
The present study shows that 293 clinical studies with the key word “COVID-19” have been registered in CTRI as on 14 July 2020 and among them 188 (64.16%) were interventional and 105 (35.83%) were observational studies. The interventions evaluated are modern drugs and biologicals, AYUSH formulations, Nutraceuticals, Yoga and Naturopathy. Most of the interventions are already existing and undergoing repurposing evaluations in the clinical studies and no new or novel drug is under clinical trials presently for COVID-19. Trials with AYUSH formulations constitute more than half of the clinical studies (51.06%) while modern medications are evaluated in 31.09% of the studies. 119 trials (63.3%) of the total interventional studies are randomized studies. The distribution of trials in various states indicates that where the incidence of COVID-19 is high, the number of trials is also high. 231 studies out of total 293 registered and 146 out of 188 interventional studies are expected to be completed within 6 months and hence the outcomes of these studies may provide valid information on the potential treatments in COVID-19.
Acknowledgement
The authors thankfully acknowledge Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Tamilnadu, India for the unconditional support they received form the Institute.
Conflict of Interest
The authors declare that they do not have any conflict of interest in the preparation and publication of this manuscript.
Funding Sourse
The authors did not receive any fund for the preparation and publication of this manuscript.
References
- World Health Organization (WHO), Coronavirus disease (COVID-19) Situation Report – 175, Data as received by WHO from national authorities by 10:00 CEST, 13 July 2020, Last accessed on 13 July 2020.
- Home page of the website of Ministry of Health, and Family Welfare Government of India. https://www.mohfw.gov.in/, Last accessed on 23 July 2020.
- Clinical Management Protocol: COVID-19, Directorate General of Health Services (EMR Division), Ministry of Health and Family Welfare, Government of India, Version 5, dated 03.07.20.
- Terms and Conditions @ www.clincialtrials.gov, https://clinicaltrials.gov/ct2/about-site/terms-conditions, Last accessed on 14 July 2020
- International Clinical Trials Registry Platform (ICTRP), World Health Organization (WHO), https://www.who.int/ictrp/en/, Last accessed on 14 July 2020.
- Home page of the website of Clinical Trials Registry- Indi, http://ctri.nic.in/Clinicaltrials/login.php, Last accessed on 14 July 2020.
- Lu L, Li F, Wen H, Ge S, Zeng J, Luo W, Wang L, Tang C, Xu N. An evidence mapping and analysis of registered COVID-19 clinical trials in China. BMC medicine. 2020 Dec;18(1):1-0.
CrossRef - Huang J, He Y, Su Q, Yang J. Characteristics of COVID-19 Clinical Trials in China Based on the Registration Data on ChiCTR and ClinicalTrials. gov. Drug Design, Development and Therapy. 2020 May 29;14:2159-64.
CrossRef - Arunachalam, Ruckmani and K R, Ilamathi and Radhakrishnan, Arunkumar and P M, Umesh Kumar, COVID-19: Clinical Trials and Potential Therapeutic Agents – A Narrative Review (July 10, 2020). Available at SSRN: https://ssrn.com/abstract=3653031 or http://dx.doi.org/10.2139/ssrn.3653031, Last accessed on 25 July 2020.
CrossRef








