Putu Dyah Widyaningsih1,2 , Putu Gita Pranata Putra1,2
, Putu Gita Pranata Putra1,2 , DG Wedha Asmara1,2
, DG Wedha Asmara1,2 , Erna Bagiari1,2
, Erna Bagiari1,2 , Agus Santosa3
, Agus Santosa3 , Harapan Harapan4,5,6,*
, Harapan Harapan4,5,6,* , Sri Masyeni1,2,
, Sri Masyeni1,2,
1Department of Internal Medicine, Faculty of Medicine and Health Sciences Universitas Warmadewa, Denpasar, Bali 80235, Indonesia
2Department of Internal Medicine, Sanjiwani Hospital, Denpasar, Bali 80235, Indonesia
3Department of Pharmacology, Faculty of Medicine and Health Sciences Universitas Warmadewa, Denpasar, Bali 80235, Indonesia
4Department of Microbiology, School of Medicine, Syiah Kuala University, Banda Aceh, Aceh 23111, Indonesia
5Medical Research Unit, School of Medicine, Syiah Kuala University, Banda Aceh, Aceh 23111, Indonesia
6Tropical Disease Centre, School of Medicine, Syiah Kuala University, Banda Aceh, Aceh 23111, Indonesia
Corresponding Authors E-mail:harapan@unsyiah.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2094
Abstract
The treatment of corona virus disease 2019 (COVID-19)remains in debate, and the use of chloroquine has not been validated by accurate clinical trials.The aim of this study was to provide the possible cardiotoxicity effect of chloroquine in patients with COVID-19. This study was a case-series of prolonged QT interval of COVID-19 patients treated with chloroquine in a hospital in Bali, Indonesia. There were two cases of COVID-19 with exhibited a prolonged QT interval after being administrated of chloroquine. The prolonged QT interval returned to normal after chloroquine was stopped.These cases alert us the cardiotoxicity effect of chloroquine and the need for serial electro-cardiography monitoring before and during therapy. In conclusion, although antiviral and anti-inflammation properties of chloroquine on COVID-19 are promising, its cardiotoxicity effects should be monitored closely for less harm to the patients.
Keywords
Chloroquine; Cardiotoxicity; COVID-19; long QT syndrome; LQTS
Download this article as:| Copy the following to cite this article: Widyaningsih P. D, Putra P. G. P, Asmara D. G. W, Bagiari E, Santosa A, Harapan H, Masyeni S. Chloroquine-induced Prolonged QT Interval in COVID-19 Patients in Indonesia: Case Series. Biomed Pharmacol J 2021;14(1) |
| Copy the following to cite this URL: Widyaningsih P. D, Putra P. G. P, Asmara D. G. W, Bagiari E, Santosa A, Harapan H, Masyeni S. Chloroquine-induced Prolonged QT Interval in COVID-19 Patients in Indonesia: Case Series. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/31b8rym |
Introduction
Corona virus disease 2019 (COVID-19) pandemic, cause by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a global health concern1, 2. Currently no specific treatment or vaccine are available against COVID-193-7, but chloroquine (CQ) or hydroxy chloroquine (HCQ) have been suggested as potential therapy of COVID-19based on its anti-inflammatory and antiviral effect8-10.The mechanism of action of CQ or HCQ through its capability to decrease the expression of phosphatidylinositol binding clathrin assembly protein (PICALM) could be valuable as aprophylactic candidate of COVID-19. Inhibition of PICALM expression, one of the three most abundant proteins in clathrin-coated pits constrains SARS-CoV-2endocytosis into host cells11. Other mechanisms by which CQ against SARS-CoV-2 are acidic environment inside lysosomes and late endosomes alteration, exosome release and phagolysosomal fusion, and host cytokine storm inhibition 12. The limitation of CQ has been widely published due to cardiotoxicity 13, hepatotoxicity 14,and hematotoxicity 15. The common cardiac toxicities due toCQ are not well demarcated. The most common side effect of CQ on the cardiac disturbance is prolonged QT interval (LQT), atrioventricular block (AV) block,and aprolonged QRS complex. LQT is the result of atypical repolarization of the ventricular myocardium resulting in lengthening of the QT interval on the electrocardiogram. In females, the normal corrected QT interval is 430-440 milliseconds (ms), with males slightly lower at 410-420 msand LQT when it is more than 500 ms16.
There is no report of cardiac ischemia as the side effect of CQ against COVID-19. Long term use of CQ has reported causing coronary arterial disease among SLE patients17 but not in short term use.We describe two cases of COVID-19 at Sanjiwani Hospital of Bali, presented unusual manifestation of CQ side effect on the cardiac rhythm, a case with ischemia at the anteroseptal lead of electro cardiography (ECG) while another case with usual CQ side LQT. This report warnsthe physician about the unusual manifestation of CQ adverse effect and the importance of ECG monitoring during CQ treatment.
Cases Report
Case 1
A 40-years-old woman presented with a chief complaint of cough and chest discomfort 12 days after contact with her husband, a positive COVID-19 patient. She experienced mild headaches and fever two days prior admission to the hospital. She had a history of bronchitis and hemorrhoid and worked as a seller at the local art market. She did not have any past medical history such as diabetes, hypertension, nor other comorbidities. Physical examination showed vital signs within normal limits, blood pressure of 120/70 mmHg, heart rate 92x/minute, respiratory rate 22x/minute, temperature axilla of 37.3oC, and oxygen saturation of 98% in room air. All other examination revealed to be normal.
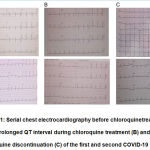
Laboratory examination showed white blood cell 7.21×103/μL, neutrophil 52.5% and lymphocyte 41.2%,hemoglobin 9.6 g/dL with hematocrit 30.2% (MCV 68.3 and MCH 21.7), thrombocyte 338×109/L, random blood glucose of 89 mg/dL, ureum 19.8 mg/dL, creatinine serum 0.55 mg/dL, sodium 141 mmol/L, potassium 3.3 mmol/L and chloride 107 mmol/L. Chest radiograph showed an increase in broncho-vascular marking in both lung fields. ECG showed normal sinus rhythm with a corrected QT interval (QTc) interval of 459 ms (Fig.1A). The patient was diagnosed with positive COVID-19 by real-time polymerase chain reaction (RT-PCR) with mild hypochromic microcytic anemia due to iron deficiency. The patient then was treated with 500 mg of azithromycin once daily, 500 mg of chloroquine sulfate twice daily, and 75 mg of oseltamivir twice daily along with a high dose of vitamin C.
On daily evaluation she appeared to be normal, her vital sign and physical examination within normal limit, cough disappear after 3 days of therapy. She kept complaining of headaches and sleeping difficulty during the night. A counseling session with psychiatric was scheduled and she was diagnosed with mild anxiety. A daily dose of 0.5 mg alprazolam was given with partial effect. On day 4thof therapy, she complained a frequent episode of nausea and vomiting followed by chest discomfort. An ECG was performed, and showed normal sinus rhythm with an increased QTc interval to 510 ms (Fig.1B). The therapy of azithromycin, oseltamivir, and chloroquine was then halted, and patient was put under close examination to an episode of cardiac abnormality. After four days of only supportive therapy, her QTc was returned to normal (Fig.1C). Her RT- PCR showed negative results two days later and she was then declared negative for COVID-19 after 10 days of hospital treatment and suggested to continue self-isolation at home.
Case 2
A 51-years-old male presented to the emergency department with a sore throat after one-weekof contact with a confirmed COVID-19 patient. He did not have other signs of COVID-19 such as fever, cough, runny nose nor shortness of breath. He denied any comorbidities such as diabetes, hypertension, nor other chronic illnesses. Physical examination revealed normal vital signs, normal heart, and lung sounds. Baseline ECG was normal sinus rhythm (Fig.1D), white blood cell 15.55×103/μL; absolute neutrophil count 7.16 x103/μL and lymphocyte 6.02×103/μL; hemoglobin 14.9 g/dL with hematocrit41.6% (MCV 85.8 and MCH 30.9). Thrombocyte 283×103/μL, random blood glucose of 112 mg/dL,ureum24.6 mg/dL, creatinine serum 0.77 mg/dL, sodium 142 mmol/L; potassium 3.4 mmol/L,chloride 104 mmol/L, aspartate transaminase 24 U/L, and alanine transaminase 34 U/L. Chest radiograph, the revealed bronchovascular patterns in both lungs. He was put on 500 mg of azithromycin once daily, 500 mg of chloroquine sulfate twice daily, and 75 mg of oseltamivir twice daily along with a high dose of vitamin C on admission. On the day 3rdof CQ treatment, there was an increase of QTc interval, become 530 ms (Fig.1E) and CQ was discontinued. On follow-up ECG, QTc interval returned to normal with normal sinus rhythm. He was discharged on the day 11thof his admission when the second RT-PCR was negative of SARS-CoV-2.
Discussion
Apart to treat malaria, CQis frequently used in the management of rheumatoid arthritis, systemic lupus erythematosus, and other connective tissue disorders 17-19.Recently, without strong evidence of efficacy, CQ has been proposed as an effective treatment option of COVID-19.Cardiac toxicities induced CQis LQT, QRS widening, Torsade de Pointes, cardiomyopathy, or ventricular arrhythmia. LQTis the most common cardiac adverse event of CQ treatment and this is the result of abnormal repolarization of the ventricular myocardium resulting 16. The mechanism by which HCQ or CQ causes LQT is not well understood. A study of sinoatrial node myocyte in guinea pig demonstrated inhibitory effects of HCQ on the hyperpolarization activated current ion channels along with delayed rectifier potassium currents (𝐼Kr), and L-type calcium ion currents (𝐼CaL)20.This may associate with a proposed mechanism by which intractable action potentials in cardiac myocytes induced prolongation of QT interval due to inhibition of depolarization and repolarization from abnormal ion currents.
In our presented cases, QT prolongation was more than 500 ms, denoting the high-risk group for malignant arrhythmia. There were no risk factors likely to serve as a risk factor to have cardiotoxicity due to CQ use in both patients such as liver disease and renal impairment 21. Discontinuation of CQ leaded to a dramatic delayed of LQT suggested the LQT due to CQ itself.With CQ/HCQ as one of the COVID-19 treatment candidates, the clinician needs to monitor the QT interval frequently 22. Further investigation into the mechanism of action of HCQ, and possible risk factors to have cardiac toxicities needs to be further elucidated.
Conclusion
During awaiting adequate randomized controlled clinical trials, many national guidelines recommended CQ/HCQ use as a therapeutic option of COVID-19. Although CQ/HCQ exhibit antiviral against SARS-CoV-2 and anti-inflammation properties on COVID-19 patients, its potential side effects especially cardiotoxicity should be considered to monitor during the therapy.
Acknowledgment
We would like to thank to the patients and HT Editorial Service for the assistance during manuscript preparation.
Conflict of Interest
Authors do not have any conflict of interests.
Funding Source
This study received no external funding.
References
- Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, Villamizar-Pena R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020; 34:101623.
CrossRef - Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): A literature review. J Infect Public Health. 2020; 13:667-73.
CrossRef - Sharun K, Tiwari R, Iqbal Yatoo M, Patel SK, Natesan S, Dhama J, et al. Antibody-based immunotherapeutics and use of convalescent plasma to counter COVID-19: advances and prospects. Expert Opin Biol Ther. 2020; 20:1033-46.
CrossRef - Frediansyah A, Tiwari R, Sharun K, Dhama K, Harapan H. Antivirals for COVID-19: A critical review. Clinical Epidemiology and Global Health. 2020; https://doi.org/10.1016/j.cegh.2020.07.006.
CrossRef - Rabaan AA, Al-Ahmed SH, Sah R, Al-Tawfiq JA, Al-Qaaneh AM, Al-Jamea LH, et al. Recent advances in vaccine and immunotherapy for COVID-19. Hum Vaccin Immunother. 2020:1-12.
CrossRef - Frediansyah A, Nainu F, Dhama K, Mudatsir M, Harapan H. Remdesivir and its antiviral activity against COVID-19: A systematic review. Clin Epidemiol Glob Health. 2020.
CrossRef - Keam S, Megawati D, Patel SK, Tiwari R, Dhama K, Harapan H. Immunopathology and immunotherapeutic strategies in severe acute respiratory syndrome coronavirus 2 infection. Rev Med Virol. 2020; 30:e2123.
CrossRef - Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020; 55:105938.
CrossRef - Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020; 382:2411-8.
CrossRef - Wilson KC, Chotirmall SH, Bai C, Rello J. COVID-19: interim guidance on management pending empirical evidence. Last updated April. 2020; 3.
- Hu TY, Frieman M, Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nature Nanotechnology. 2020; 15:247-9.
CrossRef - Tripathy S, Dassarma B, Roy S, Chabalala H, Matsabisa MG. A review on possible modes of actions of Chloroquine/Hydroxychloroquine: Repurposing against SAR-COV-2 (COVID 19) pandemic. International Journal of Antimicrobial Agents. 2020:106028.
CrossRef - Borba M, de Almeida Val F, Sampaio VS, Alexandre MA, Melo GC, Brito M, et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study). MedRxiv. 2020.
- Falcão MB, de Góes Cavalcanti LP, Filgueiras Filho NM, de Brito CAA. Case Report: Hepatotoxicity Associated with the Use of Hydroxychloroquine in a Patient with COVID-19. The American Journal of Tropical Medicine and Hygiene. 2020; 102:1214-6.
CrossRef - Maillart E, Leemans S, Van Noten H, Vandergraesen T, Mahadeb B, Salaouatchi MT, et al. A case report of serious haemolysis in a glucose-6-phosphate dehydrogenase-deficient COVID-19 patient receiving hydroxychloroquine. Infectious Diseases. 2020:1-3.
CrossRef - European Society of C. ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. ESC. 2020.
- Yang D-H, Leong P-Y, Sia S-K, Wang Y-H, Wei JC-C. Long-term hydroxychloroquine therapy and risk of coronary artery disease in patients with systemic lupus erythematosus. Journal of clinical medicine. 2019; 8:796.
CrossRef - Cusnir I, Dobing S, Jones N, Russell A. Antimalarial Drugs Alone May Still Have a Role in Rheumatoid Arthritis. JCR: Journal of Clinical Rheumatology. 2015; 21:193-5.
CrossRef - Lee YH. Chronic hydroxychloroquine/chloroquine exposure for connective tissue diseases and risk of Alzheimer’s disease. Annals of the rheumatic diseases. 2019; 78:e137.
CrossRef - Capel RA, Herring N, Kalla M, Yavari A, Mirams GR, Douglas G, et al. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current If: Novel electrophysiological insights and therapeutic potential. Heart rhythm. 2015; 12:2186-94.
CrossRef - Chorin E, Dai M, Shulman E, Wadhwani L, Cohen RB, Barbhaiya C, et al. The QT interval in patients with SARS-CoV-2 infection treated with hydroxychloroquine/azithromycin. MedRxiv. 2020.
CrossRef - Monzani A, Genoni G, Scopinaro A, Pistis G, Kozel D, Secco GG. QTc evaluation in COVID-19 patients treated with chloroquine/hydroxychloroquine. Eur J Clin Invest. 2020; 50:e13258.
CrossRef