Nasser Al-Nazwani1* , Najam Siddiqi2, Yahya Tamimi3 and Zoya Sheikh3
, Najam Siddiqi2, Yahya Tamimi3 and Zoya Sheikh3
1Department of Biochemistry, College of Medicine and Health Sciences, National University of Science and Technology, Sohar, Oman.
2Department of Anatomy and Neurobiology, College of Medicine and Health Sciences,National University of Science and Technology, Sohar, Oman.
3Department of Biochemistry, Sultan Qaboos University, Oman.
Corresponding Author E-mail: nassernazwani@nu.edu.om
DOI : https://dx.doi.org/10.13005/bpj/2123
Abstract
Low field electromagnetic radiation that is emitted from cell phones may induce morphological aberrations and change in gene expression of heat shock protein 70 (Hsp 70). Using Real Time PCR (RTPCR) and protein concentration methods, we studied changes in levels of Hsp70 mRNA and protein synthesis after exposure to the physical stimulus. We also evaluated the suitability of using chick embryo as a model to study alteration in heat shock proteins expression and translation using specific egg incubator set up. Zero-day fertilized chicken eggs were used and a popular mobile phone and service provider was selected with 1800 MHz frequency, power of 0.47 W/kg body and SAR 1.10 w/KG (head). The total daily exposure duration was 50 minutes in each 24 hours with variable maximum exposure time. Experimental samples were divided into controls with no exposure and experimental samples. The developing embryos were removed and different organs were isolated for estimation of Hsp70mRNA and protein. This physical stimulus caused a sequential increase of mRNA corresponding to the period of exposure. Liver tissue demonstrated higher levels of mRNA than heart tissue. Nonetheless, this increase in mRNA was not matched with an increase in the amount of protein of the corresponding mRNA in both tissues at different exposure times. Total protein in all tissues remained unchanged due to slow down of the clearance process and as an indication that the cell is struggling to preserve housekeeping proteins signaling attempt of survival.
Keywords
Cellular Stress; Chick Embryo; Electromagnetic Waves; Extracellular Mrna; Hsp70; Mrna Hsp70; Real Time PCR
Download this article as:| Copy the following to cite this article: Al-Nazwani N, Siddiqi N, Tamimi Y, Sheikh Z. Biochemical Analysis of the Differential Tissue Expression of Heat Shock Protein70 Following Exposure to Electromagnetic Waves. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Al-Nazwani N, Siddiqi N, Tamimi Y, Sheikh Z. Biochemical Analysis of the Differential Tissue Expression of Heat Shock Protein70 Following Exposure to Electromagnetic Waves. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3qWbeqL |
Introduction
Continuous exposure to low-field electromagnetic waves (EMW) remains to be a disputable public health hazard. People are continuously and silently exposed to low electromagnetic radiations whether at home or at work. Numerous studies have been conducted to provide conclusive evidence of the adverse health effects of electromagnetic waves exposure that were inconclusive. Nonetheless, several studies have provided enough scientific elucidations linking the continuous exposure of higher fields to conditions like childhood leukemia, dizziness, headache and sleep disturbances1, cancer2, and effects on the nervous system3. The accumulating evidence from animal and cellular models remains controversial with regard to the biological effects and the concomitant changes on the organisms and the recovery process. Moreover, there is still evident absence of clear consensus on the ideal experimental model and conditions that can elicit a proper measurable effect. This lack of proper model has created inconsistency on how to accurately measure the biological effects of EMW and therefore the potential health hazard. One way in which the biological effects of electromagnetic fields can be measured is by using animal models that can be exposed to the stimuli at different frequencies for various lengths of time after which the biological changes can be investigated and quantitated.
Cells respond to a variety of stimuli in which the precise biological effect is a reflection of the nature of the applied stress which depends on duration of the exposure. Among these effects are altered transcription of specific genes and translation of various proteins including heat shock protein 70 (Hsp70). Heat shock proteins play a major role in maintaining cellular homeostasis particularly in situations of stress. They are constitutively expressed but can be upregulated by cell stresses including heat (hence the name ‘heat-shock protein’), oxidative stress, infection, and so on4. HSPs play an important role in immunity and inflammation; recent work indicates that they modulate the function of regulatory T cells. These proteins have the potential to interact with a wide array of proteins and protein factors to facilitate proper folding patterns of specific proteins in addition to potentiating downstream effects. Hsp70 translocate into the plasma membrane following cellular stress and is released into the extracellular environment in a membrane-associated form that activates macrophages5. Extracellular heat shock proteins (eHSPs) in general help to modulate the immune system by activating the neutrophils6, chemotaxis7 and cytokine production by the immune cells8. It also increases enzymatic activities of ornithine decarboxylase and cytochrome oxidase both of which are measures to combat sudden stress trauma9.
The regulation of heat shock protein genes transcription is regulated by heat-shock factor (HSF) which interacts with heat shock elements (HSEs) in the heat shock protein promoter regions on the DNA10. On exposure to stimuli e.g. low -field electromagnetic waves stress, HSF is converted to the active form and bind to target HSE sequences on the DNA molecule ensuing fast transcription of the heat shock protein genes11. There are recent report linking HSP 70 with promoting cancer cell survival, tumorigenicity and anti-apoptotic activities such as blocking apoptotic signaling molecules12.
The evidence to support that the exposure to low-frequency EMW radiation might induce heat shock protein synthesis is still equivocal as there have been very few confirmatory studies on the validity of previous reports about the nature and extent of heat shock protein expression. However, reports of heat shock proteins that are induced in chick embryo as a consequence of low-frequency magnetic radiation have been validated13. The relative values of cellular Hsp mRNA transcripts in mammalian cells were found to be hundreds of folds higher after heat stress14. When comparing the amount of HSP resulting, there is only 2 to 4 fold increase suggesting that the translation efficiency of HSP mRNA under heat stress is modified15. Moreover, ribosomal recruitment by more Hsp70 mRNA is expected to slow down protein synthesis of housekeeping genes implying that in situations of stress, cytotoxic activity is more focused to fight and adapt to the stress conditions rather than to grow. It has been elucidated previously that levels of hsp70 is upregulated when cells endure stress as a result of ionizing radiation. Moreover, it has been confirmed that levels of hsp70 in increased in correlation with tumor progression and resistance to drugs and is associated with bad prognosis16. Hsp70 plays a major role in obstructing apoptosis by interfering with the execution pathways downstream. It is an inhibitor of pro-caspase-9 and can also bind to the apoptosome Apaf-117.Moreover; hsp70 can inhibit the translocation of the pro-apoptotic molecule BAX to the mitochondria in addition to its ability to bind to the cell membrane to abstract apoptosis through interfering with the pro-apoptotic stimuli.
Heat shock proteins can be used as biomarkers to detect cytotoxicity and environmental stress effecting normal cellular functions. In this study we used levels of Hsp70 mRNA and protein expression to quantitate severity of stress induced by irradiation of breed zero-day fertilized chicken eggs embryo with 1800 MHz frequency, power of 0.47 W/kg body and SAR 1.10 w/KG from a popular mobile phone and service provider.
Material and Methods
Breed zero-day fertilized chicken eggs (Gallus gallus domesticus) were collected and incubated in an egg incubator (Brower Houghton Iowa, USA model TH 120)18. Eggs were divided equally into two incubators for the treated and untreated samples. In the exposed group, a mobile phone was placed inside the 30-egg incubator in call receiving mode, while in the control group the mobile phone was not used. A popular mobile phone and service provider was selected with 1800 MHz frequency, power of 0.47 W/kg body and SAR 1.10 w/KG. Check embryo model has been used previously to study the effects of EMW on living biological systems19.
Experimental Design
The total daily exposure duration was 50 minutes in each 24 hours starting from day 1. The eggs (chick embryos) were sacrificed at day 10 (total exposure time 500 minutes) and day 15 (total exposure time 750 minutes). The developing embryos were removed at 10 and 15 days, liver and heart tissues were dissected, fixed in formalin, homogenized and processed for the estimation of Hsp70 mRNA and protein concentration.
After tissue homogenization, total RNA was extracted and Hsp70 mRNA was quantitated. Gradient based ultracentrifugation was employed to fractionate tissues into various cellular organelles after which cells were lysed and Hsp70 concentration was assessed.
RNA extraction
RNA was extracted from supernatant of the homogenized tissue using the Qiagen RNeasy Mini Kit (QIAGEN, CA, USA) and concentration was determined. cDNA synthesis was performed by using High-Capacity cDNA Reverse Transcription Kit (Applied BioSystems, Austin, TX). cDNA was then quantified using Nano-drop reader (Thermo-Fisher Scientific, USA)20.
Quantitative RT-PCR
TaqMan reagents were used to perform qRT-PCR. qRT-PCR was then carried out in the ABI 7500 Fast real time PCR machine (Applied Biosystems, Austin, TX). The relative expression level of hsp70 was calculated using the comparative delta Ct method by normalizing the cycle threshold values of hsp70 with those of GAPDH21.
Protein analysis
Differential centrifugation (Beckman J2-21 and ultracentrifuge, USA) was used to separate organs from the sacrificed chick embryos. Protein quantitation was then carried out using standard electrophoretic techniques. ELISA analysis was then performed using Hsp70 antibody obtained from SimpleStep (abcam, Cambridge, UK).
Results
Heat Shock Protein 70 mRNA Analysis.
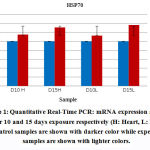
Liver and heart tissues expression levels of Hsp70 mRNA were quantitated using real time PCR (qRTPCR). Analysis was done after 10 and 15 days exposure to EMW. mRNA levels remained almost unchanged for the control samples while significant increase in the expression of HSP70 mRNA in the exposed groups was observed. The liver tissue expression of mRNA was more significant particularly after longer exposure to the physical stimulus (Fig. 1). Amount of increase of mRNA in the heart tissue decreased steadily with longer exposure to EMW radiation. Under normal circumstances levels of mRNA in various tissues were equal and inc reased only after adequate exposure to the stress stimulus. It is interesting to notice that mRNA levels in all tissues were identical demonstrating the universal role Hsp70 plays in cellular hemostasis across all tissue types.
Both heart and liver tissues are increasingly expressing higher levels of Hsp70 mRNA as exposure to EMW is prolonged further (Fig. 2) Higher levels of Hsp70 mRNA are produced in the liver tissue than in the heart tissue emphasizing the pivotal role liver plays in survival mechanisms. Such augmentation to express higher amounts of Hsp70 mRNA is an attempt to synthesize more amounts of the protein to help offset the stress stimulus.
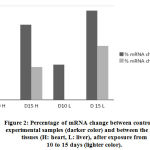 |
Figure 2: Percentage of mRNA change between control and experimental samples (darker color) and between the same tissues (H: heart, L: liver), after exposure from 10 to 15 days (lighter color). |
Cellular protein analysis
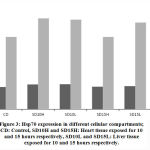
Cellular organells of hepatocytes and cardiomyocytes were extracted using differential centrifugation after carefull homogenisation at 4Cͦ. Diferential fractionation was caried out using refrigerated centrifuge Beckman J2-21, at 600xg for 10 min with sucrose gradient. (Fig.3). shows that cardiomyocytes cytoplasmic protein synthesis increased by 26% after 10 hours of exposure to the stimulus while the mitochondrial protein synthesis and after the same period increased by 11%. Exposure of the heart tissue to the same effect for 15 hours did not show same level of increase in protein synthesis as compared to the normal control. Similar pattern of protein expression was also observed in the liver tissue. After 10 hours exposure there was 24% and 13% increase in cytoplasmic and mitochondrial protein respectively (Fig.3). Similarly and compared to the normal control a noticeable decline in protein synthesis in both organelles of the liver was observed although the decline was less extreme than that of the heart tissue. Longer exposure durations provokes the cell to progressively adapt to survival tactics which will progressively become overwhelmed with apoptotic processes on prolonged exposure to the physical stimulus.
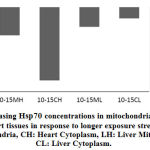
Longer exposure to EMW results in deceleration of Hsp70 protein expression particularly in the heart tissue. Although compared to the untreated control, increase in expression is still observed but the pace of expression has slowed down in the mitochondria indicating the tissue is striving to maintain steady level of Hsp70 expression. In comparing the same two tissues during the two periods the decrease becomes evident. In heart cytoplasm the decrease was from 26% to zero and in the mitochondria of the same tissue there was 10% decrease. Similarly, in the liver tissue and after longer exposure, there was 7% decrease in Hsp70 of mitochondria and 4% in the cytoplasm respectively. This finding might be suggestive of protein leakage in to the extracellular space as a result of membrane damage due to longer exposure to the stimuli. Similarly in the liver tissue, the initial surge in Hsp70 decreased with longer exposure although to a much lesser extent. Realizing the important role the liver plays in quenching stress effect provides a plausible explanation to the observation that the decrease in liver Hsp70 is less dramatic than in that has been shown with the heart (Fig. 4 and Fig. 5).
Extracellular and intracellular Hsp70
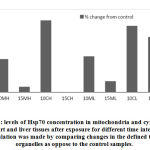
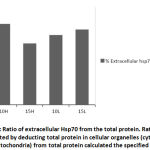
During stress effect, cell membrane becomes more porous and permeable allowing some molecules and proteins to leak into the extracellular space. With increasing amount of stress induced Hsp70, considerable amounts of the protein would cross the cell membrane to the outer space. It is postulated that this process is well controlled in order to stimulate several proinflammatory pathways22, 23, 24. From (Fig. 6) the ratio of extracellular Hsp70 is probably kept at a minimum at all occasions but increases in stress conditions due to extracellular membrane deformations as well as in order to play a role in the anticipated anti-stress response. An average of 26% of total cellular Hsp70 is translocated into the other side of the membrane (Fig. 7). The mechanism of how this process occur is not yet very well understood but there are accumulating evidence verifying the role this protein plays extracellularly to modulate inflammatory responses at least in times of stress.
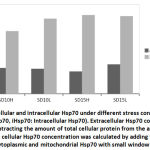 |
Figure 6: Extracellular and intracellular Hsp70 under different stress conditions (eHsp70: Extracellular Hsp70, iHsp70: Intracellular Hsp70). |
Extracellular Hsp70 concentration was calculated by subtracting the amount of total cellular protein from the amount of iHsp70 concentration. The cellular Hsp70 concentration was calculated by adding the concentrations of both cytoplasmic and mitochondrial Hsp70 with small window for error.
Discussion
Heat shock protein70 (Hsp70) is a multifaceted chaperon protein that is conserved across species and it is involved in many cellular metabolic processes. Its prime role as an anti-stress agent has been exploited heavily in an attempt to further elucidate its mode of action and identify its cellular distribution25, 26, 27. In this study Hsp70 was investigated for its cellular organelle distribution and pattern of deferential expression under stress conditions induced by EMW. Previous studies have demonstrated the overexpression of Hsp70 mRNA under various stress conditions but have not explained the magnitude and the subcellular compartments localization of the protein modification. Moreover, and in order to investigate the differential tissue and cellular mode of expression of Hsp70, an inexpensive and readily available chick embryo experimental model was used which proved to be reliable for this type of experiments.
Chick embryos grown in lab-based incubators were exposed to EMW for various lengths of time. The check embryos were sacrificed and organs separated by dissection in the anatomy lab of College of Medicine and Health Sciences at the National University of Science and Technology (NUST). Chick embryos were divided into two groups that were exposed either for 10 days or 15 days to the stress stimulus. Liver and heart tissues exposed to EMW have shown significant increase in levels of mRNA and Hsp70. EMW significantly altered the pattern in which Hsp70 is expressed and translated almost in all studied tissues. There was 18% increase in the expression of mRNA in the heart tissue after 10 days of exposure compared to the control group. This level of mRNA expression was more evident with prolonged exposure to EMW reaching up to 32% after 15 days compared to the control unexposed group. Similar results were observed with the liver samples where 15% and 38% increase was calculated after 10 days and 15 days of exposure respectively. Moreover, the magnitude of increase of mRNA after 15 days as opposed to 10 days was 14% in the heart tissue and 23% in the liver tissue. This corresponds to an increase rate of approximately two folds and two folds and a half in the heart and liver tissues respectively after longer exposure to the stimulus. Differences in mRNA levels after response to the effector stimulus may be attributed to the difference in copy number in each tissue giving an insight in the role this protein plays under specified conditions. This increase in mRNA was not paralleled by an equivalent increase in Hsp70 protein concentration in both tissues and under all given experimental conditions. Levels of mRNA and Hsp70 in the liver tissue was higher than in the heart tissue demonstrating the important role liver plays in combating cellular stress using Hsp70 as one of many mechanisms work to attenuate stress effect.
Total protein in both tissues remained almost unchanged for both exposed and control group possibly because cells under stress conditions and in order to survive need to quench the effects of stress by activating more of the housekeeping genes. Hsp70 is an anti-apoptotic28 agent that will promote cellular survival. Initially when cells are exposed to stress stimuli they respond in part by producing surplus amounts of Hsp70 mRNA through activating the heat shock transcription factors (HSFs). Moreover, the stimulation of Hsp70 expression is dependent on various genetic and environmental factors that orchestrate their effects in order to control the stress shock29. This stimulation can last as long as the stimulus is active and may generate excessive amounts of Hsp70 mRNA to the extent that it will start competing with the housekeeping genes mRNAs for a place on the cytoplasmic ribosome for translation. This competition and the limited number of cellular ribosomes results in lesser amounts of Hsp70 mRNA translation into a full functional protein. Moreover, Hsp70 gene transcription is active albeit processing into messenger RNA is slow due to the excessive number of housekeeping genes being transcribed at the same time. This suggests that the translation efficiency of Hsp70 mRNA under stress conditions is intensely modified for as yet unclear reasons30.
In recent studies31, 32, it was observed that the ratio between extracellular Hsp70 (eHsp70) and intracellular (iHsp70) may indicate the severity level of the stress applied33 Our study and in unpublished data, has demonstrated increasing concentrations of eHsp70 as the stress stimuli sustains for longer period particularly in the liver tissue. Cells undergo structural and morphological alterations at different levels including the cell membrane which becomes more leaky expelling Hsp70 to the extracellular side. In addition, membrane-bound Hsp70 might also become detached and therefore shed into the extracellular space24. It is possible to postulate from the above that the role of eHsp70 is to act as an anti-inflammatory agent and possibly helping in immune responses modulation and cytokines recruitment.
In order to evaluate the effect of the stress stimuli in inducing expression of Hsp70 in specific cellular compartments, we proposed to examine cytoplasm and mitochondria as an example in both heart and liver tissues. Our results have shown considerable variations within the cellular compartments of the same tissue or across different tissues that were examined under increasing exposure to EMW. In mitochondria of the heart, there was a decrease of 10% in the concentration of Hsp70 after 15 days of exposure compared to exposure for 10 days while in liver the decrease was only 7% between the two exposure intervals. Mitochondria are involved directly in the process of apoptosis initiation and this is opposite to the potential rule of Hsp70 as an anti-apoptotic protein even with longer exposure. Moreover, Hsp70 genes are not part of the mitochondrial genome therefore, it depends on the cytoplasm for its Hsp70 load. The cytoplasmic Hsp70 levels have shown a similar trend although more pronounced than in the mitochondrial. The calculated drop of Hsp70 concentration in the heart was 26% between 10 and 15 days of exposure and it was a mere 4% in the liver tissue. In conditions of stress, the liver activates several housekeeping genes to voice survival signals and in an attempt to maintain its role as a major protein synthesis organ, the housekeeping genes mRNA will occupy much of the locations on the ribosomes for translation resulting in less Hsp70 is synthesized. Continuous synthesis of mRNA will continue as long as the effector is active leading to an accumulation of Hsp70 mRNA surpassing the capacity of cellular ribosomes and instigating deformations of the cell membrane where Hsp70 will become translocated to the extracellular space. Translocation of Hsp70 has two dual purposes, serves to activate the immune system and at the same time reduces cellular molecular pressure that would alleviate apoptotic signals.
Conclusion,
This study demonstrates the reliability of using chick embryo models to study the effect of EMW on Hsp70 expression and tissue distribution. Our results demonstrate that the increasing synthesis of Hsp70 mRNA did not correspond with higher Hsp70 synthesis verifying the processes of transcription and translation at least in this case are not well orchestrated. Nonetheless, the observed differential expression of mRNA and Hsp70 in different tissues and cellular organelles of the same tissue indicate the multitude of roles Hsp70 plays not only across different tissues but also across different organelles of the same tissue. It is very interesting to attempt to measure the increasing concentrations of Hsp70 in the extracellular space which definitely would shed more light on the role of Hsp70 in modulating anti-apoptotic events not only inside the cell but also outside. The intracellular role of Hsp70 can be extrapolated from the current result as to promote the anti-apoptotic signals and provide enough of the protein to escape into the extracellular side. Hence the extracellular effects of Hsp70 are parallel to the intracellular effects in terms of enhancing survival but in a completely different mechanism.
More studies need to be conducted to further elucidate the mechanism of Hsp70 as an anti-apoptotic protein and to additionally verify the significance of the differential distribution across organelles and the role extracellular Hsp70 might play in attenuating immune system in response to EMW.
Acknowledgement
None
Conflict of Interests
There is no conflict of Interest.
References
- NRPB, ELF Electromagnetic Fields and the Risk of Cancer. Report of an Advisory Group on Non-ionizing Radiation. National Radiological Protection Board, Chilton. 12: 1–179 (2001).
CorssRef - Wakeford, R, The cancer epidemiology of radiation. Oncogene, 23: 6404-6428., (2004).
CorssRef - Regel SJ, Achermann P, Cognitive performance measures in bioelectromagnetic research–critical evaluation and recommendations. Environ. Health 10 (10): (2011).
CorssRef - Brenu EW, Staines DR, Tajouri L et al., Heat shock proteins and regulatory T cells. Autoimmune Dis, 813256 (2013).
CorssRef - Virginia L. Vega, Monica Rodríguez-Silva, Tiffany Frey, Mathias Gehrmann, Juan Carlos Diaz, Claudia Steinem, Gabriele Multhoff, Nelson Arispe and Antonio De Maio, Hsp70 Translocates into the Plasma Membrane after Stress and Is Released into the Extracellular Environment in a Membrane-Associated Form that Activates Macrophages. J Immunol: 180 (6): 4299-4307 (2008).
CorssRef - Ortega E, Giraldo E, Hinchado MD et al. “Role of Hsp72 and norepinephrine in the moderate exercise-induced stimulation of neutrophils’ microbicide capacity”. European Journal of Applied Physiology: 98: (3): 250–255 (2006).
CorssRef - Ortega E, Hinchado MD, Mart´ın-Cordero L, and Asea, A, The effect of stress-inducible extracellular Hsp72 on human neutrophil chemotaxis: a role during acute intense exercise. Stress: 12 (3): 240–249 (2009).
CorssRef - Horn P, Kalz A, Lim CL et al. Exercise-recruited NK cells display exercise-associated HSP-70. Exercise Immunology Review, 13: 100–111 (2007).
CorssRef - Byus CV, Kartun K, Pieper S and Adey WR, Increased ornithine decarboxylase activity in cultured cells exposed to low energy modulated microwave fields and phorbol ester low energy tumor promoters. Cancer Res. 48: 4222-4226 (1988).
CorssRef - Voellmy R, Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein gene expression in higher eukaryotes. Crit. Rev. Eukaryot. Gene Expr. 4: 357–401 (1994).
CorssRef - Morimoto RI, Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12: 3788–3796 (1998).
CorssRef - Murakami N, Kühnel A, Schmid TE, Ilicic K, Stangl S, Braun IS, Gehrmann M, Molls M, Itami J, Multhoff G. Radiat, Role of membrane Hsp70 in radiation sensitivity of tumor cells. Oncol. 10: 149 (2015).
CorssRef - Carmody S, Wu XL, Lin H, Blank M, Skopicki H and Goodman R, Cytoprotection by electromagnetic field-induced hsp70: A model for clinical application. J. Cell Biochem, 79: 453–459 (2000).
CorssRef - Finka A, Mattoo RUH, Goloubinoff P, Meta-analysis of heat- and chemically upregulated chaperone genes in plant and human cells. Cell Stress Chaperones, 16: 15–31 (2011).
CorssRef - Kiang JG, Gist ID, Tsokos GC, Regulation of heat shock protein 72 kDa and 90 kDa in human breast cancer MDA-MB-231 cells. Mol Cell Biochem, 204:169–178 (2000).
CorssRef - Reut Shalgi, Jessica A. Hurt, Irina Krykbaeva, Mikko Taipal, Susan Lindquist, Christopher B. Burge, Widespread Regulation of Translation by Elongation Pausing in Heat Shock, 49: (3): 439-452 (2013).
CorssRef - Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR, Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol, 2: (8): 469-75 (2000).
CorssRef - Ingole I., Ghosh SK, Exposure to radio frequency radiation emitted by cell phone and mortality in chick embryo (Gallus domesticus). Biomedical Research, 17: 205-210 (2006).
- Siddiqi N. A, Shalaby A, Al Kindi M , Al Ghafri F. Mobile Phone Electromagnetic Fields Affected the Hepatocytes in the White Leghorn Chicken Embryo: an Ultrastructural Study. Biomed Pharmacol J, 13: (1): 245-252 (2020).
CorssRef - Beltrame CO, Côrtes MF, Bandeira PT, and Figueiredo AMS, Optimization of the RNeasy Mini Kit to obtain high-quality total RNA from sessile cells of Staphylococcus aureus. Braz J Med Biol Res, 48: (12): 1071–1076 (2015).
CorssRef - Wu-Fienberg, Yuewei BS, Marquardt, et al. Engineering Primary Schwann Cells using Lentiviruses to Control GDNF Expression. Plastic and Reconstructive Surgery, 133: (3S): 105 (2014).
CorssRef - Whitham M and Fortes MB, Heat shock protein 72: release and biological significance during exercise. Frontiers in Bioscience, 13: (4): 1328–1339 (2008).
CorssRef - Borges TJ, Wieten L, van Herwijnen MJ et al. The anti-inflammatory mechanisms of Hsp70. Frontiers in Immunology, 3: (95) (2012).
CorssRef - Jones Q, Voegeli TS, Li G, Chen Y, and Currie RW, Heat shock proteins protect against ischemia and inflammation through multiple mechanisms. Inflammation and Allergy—Drug Targets, 10
4): 247–259 (2011).
CorssRef - Maxim Shevtsov, Gao Huile , Gabriele Multhoff, Philos, , Membrane heat shock protein 70: a theranostic target for cancer therapy. Trans R Soc Lond B Biol Sci, 373: 2016-3137 (2018).
CorssRef - Sujit Pujhari A, Marco Brustolin A, Vanessa M. Macias et al. Heat shock protein70 (Hsp70) mediates Zika virus entry, replication, and egress from host cells. Emerging Microbes & Infections, 8: 8-16 (2019).
CorssRef - Rodriguez-Iturbe B, Lanaspa MA, Johnson RJ, The role of autoimmune reactivity induced by heat shock protein 70 in the pathogenesis of essential hypertension. Br J Pharmacol, 176: (12): 1829-1838 (2019).
CorssRef - Anne-Laure Rerole, Gaetan Jego and Carmen Garrido, Hsp70: Anti-apoptotic and Tumorigenic Protein. Methods in molecular biology, 787: 205-30 (2011).
CorssRef - Shamovsky I, Nudler E, New insights into the mechanism of heat shock response activation. Cell Mol Life Sci, 65: 855–861 (2008).
CorssRef - Juliann G. Kianga George C. Tsokos, Heat Shock Protein 70 kDa: Molecular Biology, Biochemistry, and Physiology. Pharmacology & Therapeutics, 80: (2): 183-201 (1998).
CorssRef - Tytell M, Release of heat shock proteins (Hsps) and the effects of extracellular Hsps on neural cells and tissues. International Journal of Hyperthermia, 21: (5):445–455 (2005).
CorssRef - Whitham M and, Fortes MB, Heat shock protein72: release and biological significance during exercise. Frontiers in Bioscience, 13(4) 1328–1339 (2008).
CorssRef - Mauricio Krause,Thiago Gomes Heck,Aline Bittencourt et al. The Chaperone Balance Hypothesis: The Importance of the Extracellular to Intracellular HSP70 Ratio to Inflammation-Driven Type 2 Diabetes, the Effect of Exercise, and the Implications for Clinical Management. Mediators of Inflammation 2015: (2015).
CorssRef