Agung Nova Mahendra1 , I Nyoman Tri Pramartha2
, I Nyoman Tri Pramartha2  and Agung Ari Chandra Wibawa3
and Agung Ari Chandra Wibawa3
1Department of Pharmacology and Therapy, Faculty of Medicine, Universitas Udayana, Denpasar, Bali, Indonesia
2Medical Student, PSSKPD,Faculty of Medicine, Universitas Udayana, Denpasar, Bali, Indonesia.
3Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Universitas Mahasaraswati, Denpasar, Bali, Indonesia.
Corresponding Author E-mail: novamahendra@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2147
Abstract
Background: Bekulfruit from Northern region (Buleleng regency), Bali, Indonesia, is commonly consumed fresh in the island of Bali or processed as local delicacy and used as part of religious offerings. Up to date, there is no data regarding the taxonomy, phytochemical composition, and antioxidant properties of this Balinese fruit.This study was aimed to investigate total phenolic content, tannin content and antioxidant activity of the ethanol extract of bekul fruit obtained from Banjar district, Buleleng regency, Bali.
Methods: Total phenolic compound was quantified in terms of gallic acid equivalent (GAE) by using Folin-Ciocalteu method, mean while tannin content was determined in terms of tannic acid equivalent (TAE). IC50of the extract was determined using DPPH assay, and subsequently used in the calculation of antioxidant activity index (AAI) using the formula of Scherer and Godoy (2009).
Results: Bekul plant was revealed as Ziziphusjujuba Mill. Total phenolic and tannin content of the extract was 29.48 mg/100 g GAE and 91.06 mg/100 g TAE, respectively. Thevalue of IC50was 77.40 mg/ml, with antioxidant activity index (AAI) of 50.94.
Conclusion:Ethanol extract of bekul (Ziziphusjujuba Mill.) fruit contains phenolic and tannin compounds. This extract is found to scavenge free radicals and possess very strong antioxidant activityin vitro. Taken together, these findings lead to the notion that bekul fruit from Northern region of Bali, Indonesia, is a promising pharma food.
Keywords
Antioxidant Activity; Ethanol Extract; Total Phenolics; Tannins; Ziziphusjujuba mill Fruit
Download this article as:| Copy the following to cite this article: Mahendra A. N, Pramartha I. N. T, Wibawa A. A. C. Bekul Fruit, Potential Pharmafood from Northern Region of Bali Island, Indonesia: Selected Phytochemical Analyses and Antioxidant Activity of Its Ethanol Extract. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Mahendra A. N, Pramartha I. N. T, Wibawa A. A. C. Bekul Fruit, Potential Pharmafood from Northern Region of Bali Island, Indonesia: Selected Phytochemical Analyses and Antioxidant Activity of Its Ethanol Extract. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3wh7F16 |
Introduction
Endogenous antioxidant system is deployed by human body to mitigate the adverse effects of oxidative stress, thus maintaining the homeostasis. Unfortunately, in many pathological conditions, this system of mitigation is frequently overwhelmed and subsequently contribute to the genesis and progressivity of many diseases. Supplementation of exogenous antioxidantsin these situations will enhance the capacity of human body to attenuate oxidative stress and restore the homeostasis, as reviewed elsewhere.1 Natural products such as fresh fruits have long been known as good sources of exogenous antioxidants.2
A local fruit known in Bali as bekul fruit has not been investigated for its biomedical potentials, especially as a source of antioxidants. North Balinese bekul fruit, cultivated in Banjar District, Buleleng Regency, Bali, Indonesia is variable in size and tastes sweet with a hint of sourness. The skin is green and glossy, similar to that of Malang apple from East Java region, Java, Indonesia. The fruit is usually eaten immediately after harvest under fresh condition, or mixed with a special sauce (shrimp paste-based sauce) and made into local delicacy known as rujakbekul(a kind of Indonesian traditional spicy salad with bekul fruit as its main ingredient). The fruit is also used as part of offerings in Balinese Hindu (Sanatana Dharma) religious ceremonies. Thus, bekul fruit is mainly used for local consumption and for religious purpose.
To the best of our knowledge, there is lack of information regarding the taxonomy, phytochemical composition, and antioxidant activity of North Balinese bekul fruit. Therefore, it is important to determine the species and investigate selected phytochemical properties of bekul fruit cultivated in North Bali. Specifically, a study on taxonomy, selected phytochemical constituents and antioxidant activity of North Balinese bekul fruit ethanol extract was conducted as an initial effort to explore its potential biomedical benefits.
Materials and Methods
Species Determination
The whole live plant was obtained from a local bekul plantation in Banjar, Buleleng regency (North Bali region) and submitted to the office of LembagaIlmu Pengetahuan Indonesia/LIPI (Indonesian Institute of Sciences) at Eka Karya Bali Botanical Garden, Bedugul, Tabanan regency, Bali, for taxonomical analysis. The process of identification was done by three official botanists/taxonomists of LIPI. The plant was then kept in the botanical garden as trophy or voucher specimen.
Extraction of Bekul Fruit
Bekulfruits were air-dried and macerated in 80% ethyl alcohol for 48 hours. The macerate was then subjected to evaporation for removing water content, using a rotary vacuum evaporator. All procedures were done in Laboratory of Pharmacology and Therapy/Division of Drug Development and Laboratory Animal, Integrated Biomedical Laboratory Unit, Faculty of Medicine, Udayana University, Denpasar, Bali.
Total Phenolic and Tannin Content Analyses
Folin-Ciocalteu method was used in quantifying total phenolic content of bekul fruitethanolic extract, as described in our previous study. A part (100 mg) of the extract was dissolved in 85% methyl alcohol, and then subjected to vortex and filtration. The resulting filtrate and standard solution were mixed respectively with Folin-Ciocalteureagent and vortexed. The solutions were rested for 6 minutes and then mixed with 0.8 mL of 5% Na2CO3, vortexed and allowed to rest again for 30 minutes. These steps produced blue colour. The absorbances were measured at 760 nm. Linear regression curves were made to determine the formula y = ax + b, based on the absorbances and concentrations. The phenolic content of the sample was expressed as gallic acid equivalent (GAE) per gram of dry weight of the sample. UV-Vis spectrophotometry was used in the quantification of phenolics and tannins of bekul fruitethanolic extract. Tannin content was expressed as tannic acid equivalent (TAE). Quantitative phytochemical analyses were done in Laboratory of Food Analysis, Universitas Udayana, Denpasar, Bali.
Determination of Antioxidant Activity
The assay we used in determining antioxidant activity of the extract is a slightly-modified version of the assay done by Aldarraji et al.,3 as described previously in Dewi et al.4Five concentrations of the sample were made using methyl alcohol as solvent. Each 100 mL of sample was mixed with 700 mL DPPH solution (0.1 mM), shaken well, and then incubated for 30 minutes in dark room (RT).Gallic acid was used as positive control. The absorbance values were read at wavelength of 517 nm. Percentage of DPPH free radical inhibition by theextract was calculated using the formula:
The results were made into a graph to obtain the linear regression equation and IC50 value. The antioxidant activity of the extract is expressed as antioxidant activity index (AAI), calculated using formula proposed by Scherer and Godoy5:
Interpretation of AAI value was done based on the category proposed by Scherer and Godoy.5A test substance was considered to have poor antioxidant activity if AAI < 0.5, moderate antioxidant activity if between AAI is 0.5-1.0, strong antioxidant activity if AAI 1.0-2.0, and very strong whenAAI > 2.0.
Results
Plant Identification
The result of species identification is presented in Table 1. The certificate of the determination/identification was issued by Head of Exploration and Collection of Plants, EkaKarya Bali Botanical Garden – LIPI (certificate number: B-37/IPH.7/AP/I/2018).
Table 1: The Taxonomy of Bekul.
| Kingdom | Plantae |
| Division | Spermatophyte |
| Subdivision | Angiospermae |
| Class | Dicotyledonae |
| Order | Rosales |
| Family | Rhamnaceae |
| Genus | Ziziphus |
| Species | ZiziphusjujubaMill. |
Total Phenolic Content
The result of absorbance measurement for different concentrations of the sample is presented in Figure 1.
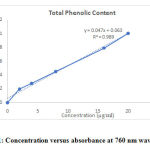 |
Figure 1: Concentration versus absorbance at 760 nm wavelength. |
Total Tannin Content
The measurement of total tannin content was based on the reagent manufacturer’s standard (Sigma Aldrich). The procedure was initiated by making extract preparations having different concentrations. The result of tannin analysis is shown in Figure 2. The regression (r) value was found to be 0.9742 (with y = 0.114x + 0.0957).
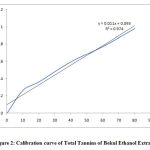 |
Figure 2: Calibration curve of Total Tannins of Bekul Ethanol Extract. |
Free Radical Scavenging Activity and Antioxidant Activity Index
Free radical scavenging capacity of bekulethanolic extract had been measured using 2,2 diphenyl-1-pycrilhidrazyl(DPPH) assay, and calculated using Formula 1. The IC50values were obtained from linear regression analysis of inhibition DPPH 0.1 mM. In this study, we obtained regression % inhibition y=0.6934x – 0.5106 with correlation coefficient value of 0.9485. The results of DPPH free radical scavenging activity and AAI quantification (based on calculation using formula 2) are shown in Table 2.
Table 2:IC50 and AAI ofBekul (ZiziphusjujubaMill.) Fruit EthanolExtract.
| Parameters of Antioxidant Properties | |
| IC50 | AAI |
| 77.40 µg/ml | 50,94 |
Discussion
In current study, we aimed to reveal the taxonomy of North Balinese bekul, and to investigate total phenolics, tannin contentand assessing in vitroantioxidant activity of bekulfruit ethanol extract. Taxonomically, Ziziphus jujube Mill. was revealed as the scientific name ofbekul.This species is known as Asian native plant.6Several relatives of bekul(Z. jujube Mill.) have been studied phytochemically, namely, Z. lotus, Z. mauritiana, Z. mucronata, Z. spina-christiandZ. xylopylorus.Bioactive phytocomponents and biomedicinal potentials of Z. jujube Mill. fruit had been discussed elsewhere.7In a recent review, Z. jujube Mill. fruit is known to be ethnopharmaceutically used in China to treat blood deficiency (as “Qi” tonifier), and had been discussed both as a prophylactic and therapeutic natural product to combat anemia.8
Quantitative phytochemical analysis revealed that bekul ethanol extract containedphenolicsas much as29.48 mg/100 g gallic acid equivalent (GAE). Total phenols content in our study was much lower than that of 7 cultivars of Chinese Z. jujube fruit ethanol extract.9 This difference may be caused partly by the concentration of the ethanol used during the extraction. The ethanol concentration in our study was lower that of Zhao et al., i.e., 80% versus 95%, respectively.9The ethanol extract of Z. spina-christi fruit from Oman was also shown to contain phenolic compounds and exhibit sound in vitro anti-inflammatory activity, compared to diclofenac sodium.10Fruit from other species, i.e., Z.mauritianaobtained from Nigeria had been also shown to contain phenolic content, with higher amount than the specimen in our study. Total phenolic compounds fromZ. mauritiana fruit aqueous extract was found to be 402.31±53.6mg GAE/100g.11Methanol extract of Z. xylopylorus fruit from Karnataka, India, was observed to contain 6262 mg GAE/100g.12The findings confirm that species and biogeographical factors are important determinants of secondary metabolite content, especially total phenolic compounds found in plants. The use of different solvents is also an important factor in determining the amount of extracted bioactive substances.
Tannin content in bekul fruit ethanol extract was revealed to be 91.06 mg/100 g TAE. Tannins in fruits of many plant species are a group protective compounds that act to protect plants against wide array of phytopathogens.13Condensed tannins (andflavanols) are assumed to take predominant role in determining the antioxidant activity ofripe Z. mauritianaLamk fruit.14Since ripe bekul(Ziziphus jujube Mill.) fruits were used in current study, tannins may be assumed to contribute significantlyto the antioxidant activity of its ethanol extract.There is currently lack of data regarding tannins content of Z. jujube Mill. fruit ethanol extract, using tannic acid as standard in the determination of tannin content. To tackle this issue we compare our result of tannin content determination to the findings of phytochemical (tannins) study on the relative of Z. jujubeMill., such as Z. mauritianaLam. Similar to our finding, tannins is also present in ripe Z. mauritianaLam. fruit obtained from Madya Pradesh, India. The ethanol extract of this Indian fruit was known to present in high amount (4+), but the exact amount awaits further investigation.15Fruit juice of Z. lotus was also revealed to contain both condensed and hydrolysable tannins.16 Moreover, proanthocyanidins (condensed tannins) is present in the ethanol extract of another part of genus Ziziphus, such as shown in of Z. mucronata Willd. subsp. mucronataWilld bark.17
Bekul fruitethanol extract was showed to exhibitI C50 value of 77.40 µg/mland very strong antioxidant activity (AAI > 2.0). Our specimen exhibited lower DPPH free radical scavenging activity than methanol extract ofZ. xylopylorus(Retz.) Willd.from Karnataka, India showed IC50 value 36.79µg/ml,12but higher activity than the extract of Nigerian Ziziphusmauritiana fruit (IC50 value = 338.45μg/ml).11Bekul fruit ethanol extract was revealed to possess very strong antioxidant activity, based onthe AAI value.Phenolics and tannins are secondary metabolites that ubiquitously exist in plants and their fruits.18,19In current study, phenolic compounds and tannins may contribute synergistically to the significant in vitro antioxidant activity of bekul (Ziziphus jujube Mill.) ethanol extract.
It is possible that other parts of bekul plant may also contain significant amount of phenolic compounds and other bioactive substances. Choi et al. found that South Korean Z. jujube seeds fractionated using different solvents exhibited substantial amount of total phenolics (ranging from 7,90 + 0,47 mg GAE/g to 102,05 + 2,42 mg GAE/g fraction).20 Both of our findings and that of Choi et al.20 promote the importance of conducting comparative phytochemical studieson various parts of North Balinese bekul plant, applying various solvents in the extraction process.Other factor that can be attributed to the variation of phytochemical composition, is the ripening stage of fruit. A study revealed that phenolic and tannin profiles of Z. mauritianaLamk fruits can be influenced by ripening or maturation stage.14 Therefore, it is also essential to consider this factor in studying the phytochemical composition of various parts of North Balinesebekul plant.
In order to be incorporated safely in clinical practice, a natural product mustnot be proved as genotoxic agent. Genotoxicity is a disruptive feature of an agent that may cause damage to DNA and/or cyto components that regulate the behaviour and functions of chromosomes.21 Recently, Indian Z. jujuba fruit ethanol extract had been known to exhibit non-genotoxicity, both in vitro and in vivo. Moreover, the extract had been also revealed to attenuate DNA alkylation in vivo,22 thus can be viewed as a phyto-antigenotoxin. The findings can be considered as preliminary signs of promising application in clinical settings, especially in the context of therapeutic safety. It is mandatory to extend this type of study in clinical settings to elucidate the safety profile of Z. jujubeMill. fruit extract before assessing its health-promoting efficacy in humans.
Attenuated amount of endogenous antioxidants may predispose human bodyto oxidative stress and its associated damages. Oxidative stressis known to play significant roles inpathological entities such as cardiovascular disease (CVD), acute and chronic kidney disease (CKD), neurogenerative diseases (NDs), diabetes and cancer.23 Oxidative stress is also known tobe associated with inflammaging ina diverse array of pathological states, as reviewed elsewhere.24Since oxidative stress is linked to many crippling health problems, it is important to adhere to healthy life style to promote health and halt the progression of diseases. One of the most convenient health-promoting ways is to consume natural products, such as fruits rich in antioxidants or high in antioxidant activity. Bekul (Z. jujube Mill.) fruit from northern region of Bali, Indonesia,is a promising example of such natural products.
Conclusion
North Balinese bekulwas found to be Ziziphus jujube Mill. Theethanol extract of its fruit had been revealed to possess phenolic compounds and tannins. The extract also exhibited free radical scavenging activity andvery strong antioxidant property, based on DPPH assay and AAI value, respectively. Taken together, these findings indicate that bekulfruit from Northernregion of Bali, Indonesia, is a pharma food with promising antioxidant activity.
Acknowledgement
The authors would like to acknowledge I GedeWiranatha, S.Si., M.Si., (laboratory technician, Department of Pharmacology and Therapy, Faculty of Medicine, Udayana)for the technical help during the extraction process, and GustuWidiKencana Putra(laboratory technician, Laboratory of Food Analysis, UniversitasUdayana, Denpasar, Bali) for the technical assistances in phytochemical analyses and IC50 determination.
Conflict of Interest
The authors declare that they do not have any conflict of interest.
Funding Source
The authors receive no grants from any funding agency or institution.
References
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutrition Journal. 2016. https://doi.org/10.1186/s12937-016-0186-5
CrossRef - Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, et al. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. International Journal of Molecular Sciences. 2017. https://doi.org/10.3390/ijms18010096
CrossRef - Aldarraji Q, Halimoon N, Majid N. Antioxidant activity and total phenolic content of earthworm paste of Lumbricus rubellus (red worm) and Eudrilus eugenia (African night crawler). J Entomol Nematol [Internet]. 2013;5(3):33–7. Available from: https://www. researchgate.net/publication/264159663_Antioxidant_activity_and_total_phenolic_content_of_earthworm_paste_of_Lumbricus_rubellus_red_worm_and_Eudrilus_eugenia_African_night_crawler doi: 10.5897/JEN2013.0075
CrossRef - Dewi NWS, Mahendra AN, Putra GWK, Jawi IM, Sukrama DM, Kartini NL. Ethanolic extract of the powder of red earthworm (Lumbricus rubellus) obtained from several organic farmlands in Bali, Indonesia: Analysis of total phenolic content and antioxidant capacity. Bali Med J. 2017; https://doi.org/10.15562/bmj.v6i3.730
CrossRef - Scherer R, Godoy HT. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem [Internet]. 2009;112(3):654–658. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0308814608007218 doi: https://doi.org/10.1016/j.foodchem.2008.06.026
CrossRef - Jin X. Jujuba— Ziziphus jujuba. In: Exotic Fruits. 2018. https://doi.org/10.1016/b978-0-12-803138-4.00034-4
CrossRef - Gao Q-H, Wu C-S, Wang M. The Jujube (Ziziphus Jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. J Agric Food Chem [Internet]. 2013;61(14):3351−3363. Available from: https://pubmed. ncbi.nlm.nih.gov /23480594/ doi: 10.1021/jf4007032
CrossRef - Chen J, Tsim KWK. A Review of Edible Jujube, the Ziziphus jujuba Fruit: A Heath Food Supplement for Anemia Prevalence. Front Pharmacol [Internet]. 2020;11:1–12. Available from: https://pubmed.ncbi.nlm.nih.gov/33324222/ doi: 10.3389/ fphar.2020 . 593655
CrossRef - Zhao H, Zhang H, Yang S. Phenolic compounds and its antioxidant activities in ethanolicextracts from seven cultivars of Chinese jujube. Food Sci Hum Wellness [Internet]. 2014;3(3–4):183–90. Available from: https://www.sciencedirect.com /science/ article/ pii/S2213453015000038 doi: https://doi.org/10.1016/j.fshw.2014.12.005
CrossRef - Alhakmani F, Khan SA, Ahmad A. Determination of total phenol, in-vitro antioxidant and anti-inflammatory activity of seeds and fruits of Zizyphus spina-christi grown in Oman. Asian Pac J Trop Biomed [Internet]. 2014;4(Suppl 2):S656–60. Available from: https://www.sciencedirect.com/science/article/pii/S2221169115300666 doi: 10.12980/APJTB.4.2014APJTB-2014-0273
CrossRef - Okala A, Ladan M, Wasagu R, Shehu K. Phytochemical Studies and In Vitro Antioxidant Properties of Ziziphus mauritiana Fruit Extract. Int J Pharmacogn Phytochem Res. 2014;6(4):885–8.
- Raghavendra H, Prashith K, Akarsh S, Ashwini H. Phytochemical Analysis, Antifungal and Antioxidant Activity of Leaf and Fruit of Zizyphus xylopyrus (Retz.) Willd. (Rhamnaceae). Sci Technol Arts Res J [Internet]. 2015;4(4):83–8. Available from: https://www. ajol.info/index.php/star/article/view/145824 doi: http://dx.doi.org/10.4314/star.v4i4.12
CrossRef - Yahia Y, Benabderrahim, Mohamed Ali Tlili N, Bagues M, Nagaz K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PLoS One [Internet]. 2020;15(5):1–16. Available from: https:// journals.plos.org/plosone/article?id=10.1371/journal.pone.0232599 doi: 10.1371/journal.pone.0232599
CrossRef - Suzie Z, Adrien S, Guillaume C, Didier M-A-M, Sylvie R, Dominique P, et al. Changes in antioxidant activity during the ripening of Jujube (Ziziphus mauritiana Lamk). Food Chem [Internet]. 2013;150:448–56. Available from: https://pubmed.ncbi.nlm.nih.gov /24360474/ doi: : 10.1016/j.foodchem.2013.11.022
CrossRef - Rathore SK, Bhatt S, Dhyani DS, Jain A. Preliminary Phytochemical Screening of Medicinal Plant Ziziphus mauritiana Lam. Fruits. Int J Curr Pharm Res [Internet]. 2012;4(3):160–2. Available from: https://www.researchgate.net /publication/281377329 _Preliminary_phytochemical_screening_of_medicinal_plant_Ziziphus_mauritiana_Lam_fruits
- Benidir M, El Massoudi S, El Ghadraoui L, Lazraq A, Benjelloun M, Errachidi F. Study of Nutritional and Organoleptic Quality of Formulated Juices from Jujube (Ziziphus lotus L.) and Dates (Phoenix dactylifera L.) Fruits. Sci World J [Internet]. 2020;2020:1–9. Available from: https://pubmed.ncbi.nlm.nih.gov/32292296/ doi: 10.1155/2020/9872185
CrossRef - Olajuyigbe OO, Afolayan AJ. Phenolic content and antioxidant property of the bark extracts of Ziziphus mucronata Willd. subsp. mucronata Willd. BMC Complement Altern Med [Internet]. 2011;11(130):1–8. Available from: https://pubmed.ncbi.nlm.nih.gov/ 22176659/ doi: 10.1186/1472-6882-11-130
CrossRef - Brglez Mojzer E, Knez Hrnčič M, Škerget M, Knez Ž, Bren U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules (Basel, Switzerland). 2016. https://doi.org/10.3390/molecules21070901
CrossRef - Montes-Ávila J, López-Angulo G, Delgado-Vargas F. Tannins in fruits and vegetables: Chemistry and biological functions. In: Fruit and Vegetable Phytochemicals: Chemistry and Human Health: Second Edition. 2017. https://doi.org/10.1002/9781119158042.ch13
CrossRef - Choi J, An X, Lee BH, Lee JS, Heo HJ, Kim T, et al. Protective Effects of Bioactive Phenolics from Jujube (Ziziphus jujuba) Seeds against H2O2–induced Oxidative Stress in Neuronal PC-12 Cells. Food Sci Biotechnol [Internet]. 2015;24(6):2219–27. Available from: https://link.springer.com/article/10.1007/s10068-015-0296-4 doi: https://doi.org/10.1007/s10068-015-0296-4
CrossRef - López-Romero D, Izquierdo-Vega JA, Morales-González JA, Madrigal-Bujaidar E, Chamorro-Cevallos G, Sánchez-Gutiérrez M, et al. Evidence of Some Natural Products with Antigenotoxic Effects. Part 2: Plants, Vegetables, and Natural Resin. Nutrients [Internet]. 2018;10(12):1–46. Available from: https://pubmed.ncbi.nlm.nih.gov/30544726/ doi: 10.3390/nu10121954
CrossRef - Goswami P, Banerjee R, Mukherjee A. Potential antigenotoxicity assessment of Ziziphus jujuba fruit. Heliyon [Internet]. 2019;5:e01768. Available from: https://www.sciencedirect.com/science/article/pii/S2405844019302348 doi: https://doi.org/ 10.1016/j.heliyon.2019.e01768
CrossRef - Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72. https://doi.org/10.2147/CIA.S158513
CrossRef - Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, et al. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int J Mol Sci [Internet]. 2019;20(18):1–39. Available from: https:// pubmed. ncbi.nlm.nih.gov/31510091/ doi: 10.3390/ijms20184472
CrossRef









