MWH Abdul Aziz1, SZ Raduan1, AH Roslida2, ZA Zakaria2, A Zuraini2 and MN Hakim2,3*.
1Faculty of Medicine and Health Sciences, University Malaysia Sarawak, Lot 77, Seksyen 22,KTLD Jalan Tun Ahmad Zaidi Adruce, 93150 Kuching, Sarawak, Malaysia.
2Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang Selangor, Malaysia.
3Institute of Bioscience, Universiti Putra Malaysia, 43400 UPM Serdang Selangor, Malaysia.
Corresponding Author E-mail: nazrulh@upm.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2099
Abstract
Hibiscus rosa-sinensis has been traditionally used by local communities to treat fever. However, there are only limited data have been published to support the antipyretic effects. The objective of this study is to investigate the antipyretic properties and possible mechanism of the ethanol extracts of Hibiscus rosa-sinensisL. (red colored flower) and Hibiscus rosa-sinensisvar. Alba (white colored flower). Phytochemical analysis, heavy metals screening and acute toxicity test were done to evaluate the safety of extracts. The first model ran induced fever in rats by injecting Brewer's Yeast subcutaneously and then treated with 4 extracts at dosage 5 & 50 mg/kg. The dosages used for the study were obtained by the acute toxicity test. Ibuprofen was used as a reference drug, with dose 100 mg/kg. Temperatures of rats were measured using a digital thermometer. The results were expressed as mean ± S.E.M. and analyzed using the SAS system. The results of the study showed that white flower extract 5mg/kg and 50 mg/kg significantly (p <0.05) reduced the total temperature when compared to positive control group. Therefore, this research suggests the probability for its therapeutic effectiveness as plant-based antipyretic agent as claimed by traditional medicine practitioners.
Keywords
Antipyretic; Hibiscus Rosa-Sinensis; Toxicity
Download this article as:| Copy the following to cite this article: Aziz M. H. A, Raduan S. Z, Roslida A. H, Zakaria Z. A, Zuraini A, Hakim M. N. Anti-Pyretic Activity of two Varieties of Hibiscus Rosa Sinensis L. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Aziz M. H. A, Raduan S. Z, Roslida A. H, Zakaria Z. A, Zuraini A, Hakim M. N. Anti-Pyretic Activity of two Varieties of Hibiscus Rosa Sinensis L. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3s58cQZ |
Introduction
Fever is a frequent medical sign of increased internal body temperature of human to level above normal, which is 36.8 ±0.7 °C (98.2±1.3 °F). Pyrexia or fever is an important brain-mediated response occurring as part of the acute phase reaction triggered by pyrogens during infections (Fabricioet al., 2005). It is the nearly universal and most important non-specific immune mechanism designed to combat the harmful effects of invading pathogens to neutralize or restore health of the afflicted host. Fever is the response of our body, involving the release of endogenous pyrogens (IL-1, TNF-α, etc.) by immune cells, the transfer of these immune signals to the brain, coordinated response of several brain regions to increase the thermoregulatory set point and consequently body temperature (Fairbanks et al., 2000).
Fever is usually accompanied by sickness behavior as the body’s attempts to counteract the newly-perceived hypothermia and reach the new thermoregulatory set-point.A feverish individual may complaints of feeling hot, followed by chills and trembling despite an increased body temperature, increases in heart rate, muscle tone and shivering. Symptoms of lethargy, somnolence, anorexia, depression, hyperalgesia and mental confusion which have the general feeling of discomfort putting additional strain on patients which interpret fever as a syndrome indicative of a dangerous and potentially deadly illness. Hyperpyrexia is when body temperature higher than 41.7 ºC that needs medical emergency because it approaches the upper limit compatible with human life. As body temperature escalates, body organs begin to fail may lead to unconsciousness and coma will result. Therefore, potential benefits must be weighed against risks of fever, in any circumstances, be brought under control when fever escalates to hyperpyrexia which threaten life where tissue damage is imminent (Blumenthal, 1998).
The use of anti pyretics is mostly due to their moderating effects of the discomfort level and consequent alleviation of the anxiety of afflicted patients. Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the oldest and most successfully used drugs for the alleviation of pain, fever, and inflammation by inhibiting prostaglandins (PGs) synthesis. However, NSAIDs are associated with a number of side effects, which have dose-dependent manner. The most common side effects are nausea, vomiting, diarrhea, constipation, decreased appetite, rash, headache, and drowsiness. NSAIDs may also cause gastrointestinal ulcers, serious cardiovascular events, hypertension, acute renal failure, and worsening of preexisting heart failure (Vonkeman and Laar, 2008).
Hibiscus rosa-sinensis L. belongs in family Malvaceae, locally known as ‘Bunga Raya’, is a well-known shrub among local herbal practitioners for its use as natural refrigerant. It is an evergreen flowering shrub native to East Asia that cultivated as ornamental plant producing large and varying bright colors blooms. Cultivars are available with singleor double flowers in shades of white, pink, red, yellow, peach,or orange, or combinations of these. The leaves are medium-textured, glossy dark green in color (Gilman, 1999).
The plant extracts are claimed to possess pharmacological activities such as anti-pyretic and anti-inflammatory effects(Singh et al., 1978; Masaki et al., 1995). In medicine, however, the red flowered variety is preferred (Adhirajanet al., 2003). Hence, the present study is to focus on the specific investigation on the potential of anti-pyretic effect of leaf and flower ethanol extract of two variants of Hibiscus rosa-sinensisL.(red) and Hibiscus rosa-sinensis L. var. Alba (white) using Sparague-Dawley rats.
Materials and Methods
Plant Material
The matured flowers and leaves of H.rosa-sinensis L. were collected from local areas of Perpustakaan Sultan Abdul Samad, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia. Whereas flowers and leaves of H. rosa-sinensis var. Alba were taken from the areas of Normal Medical Centre, Kuching, Sarawak, Malaysia. Voucher specimens of H. rosa-sinensis L. flowers and leaves and H.rosa-sinensis var. Alba flowers and leaves have been deposited in the Herbarium Institute of Bioscience, UPM, Serdang, Selangor, Malaysia (ACP 0087 and ACP 0143 respectively). These were dried as in oven with 42°C of temperature and stored in airtight glass jar until it is used.
Preparation of Extracts
Preparations of ethanol extracts were done by slightly modifying the method described by Sachdewa and Khemani (2003) and Zakaria et al. (2005). The dried flowers and leaves were ground into powder form. 40g of the powder was extracted with 400 ml of 95% ethanol for 48 hrs. The extract was filtered using What man Filter Paper No.1 and concentrated in a rotary evaporator, at 40ºC to yield. Finally, the concentrated extract was stored at 4ºC until further use. The extracts were labeled as RF (red flower), RL (red leaf), WF (white flower) and WL (white leaf).
Animal
Sprague-Dawley male rats of approximately same age group, having body weight 150-200g housed in standard cages at constant temperature of 26 ± 4ºC under a 12/12-hr light-dark cycle, acclimatized for at least one week before the experiment. The rats were feed with standard rat pellet diet and water ad libitum. Five rats were housed per cage, in order to provide them with sufficient space and to avoid unnecessary morbidity and mortality (Sachdewa and Khemani, 2003). All experiments were conducted from 9 a.m. until the end of the experimental time period. The use of animal and animal facility in this study had been approved (UPM/FPSK/PADS/BR-UUH/00285) by Animal Care and Use Committee (ACUC), Faculty of Medicine and Health Sciences, Universiti Putra Malaysia. The experimental procedures were carried out in strict compliance with the institutional Animals Ethics Committee regulations and the ethical guidelines for investigations of experimental pain in conscious animals (Zimmermann, 1983).
Chemicals and Drugs
Brewer’s Yeast (BY) (Saccharomyces cerevisiae and Ibuprofen were purchased from SIGMA-Aldrich. For pharmacological studies, Ibuprofen and Hibiscus extracts were given intra peritoneally by suspension in distilled water.The doses employed are expressed as mg of the crude extract per kg body weight. BY was dissolved in normal saline (0.9% sodium chloride) and dilutions were made fresh on the day of experiment.
Phytochemical Screening
The presence of some important phytochemical constituents that usually exhibit biological activities such as alkaloids, saponins, flavonoids, tannins, triterpenes and steroids were tested by simple qualitative and quantitative methods of Trease and Evans (1989) and Sofowara (1993). Phytochemical screening to detect alkaloids, saponins, flavonoids, tannins, polyohenolic compounds and triterpenes/steroids were carried out according to Farnsworth (1966) as previously described (Zakaria et al. 2006).
Heavy Metal Screening of Raw Samples
The procedure applies for the determination of trace metal such as Arsenic (As), Mercury (Hg) and Lead (Pb) in the sample. (Rao and Talluri, 2007).The concentration was calculated using the equation below (Jarvis et al., 1996):
Concentration of analyte in dry sample (ppm) = 
where:
C = Concentration of analyte in the digested sample calculated from standard calibration graph (mg/L)
D = Dilution factor (10X)
V= Volume of digested sample (L)
W= Weight of sample (kg)
Acute Toxicity Test
The acute toxicity study was carried out by the fixed-dose procedure (FDP) of 5, 50, and 500 mg/kg body weight. At each dose level tested, results will be available from 5 animals. Each animal was categorized into one of three groups (dead; showing signs of toxicity; unaffected) provided that there is reasonable agreement about what constitutes a toxic sign. Initially the single IP dose of 5 mg/kg was administered individually in 5 rats for each extract. Any mortality and signs of toxicity were observed within 14 days after the administration of test extracts. The next dose of 50 mg/kg was administered in 5 rats for each extract and was also observed for the next 14 days. Finally the dose was increased to 500mg/kg and the rats were observed for mortality and toxicity.Peripheral blood was collected from the effected groups for liver enzyme function test (ALT, ALP and AST) via liver function test kit and organs (kidney and liver) histological examination was done under the microscopy magnification of 100x and 400x (Hashemi et al 2008).
Effects of extracts on Body Temperature of Normal Rats.
50 Sprague-Dawley rats were divided into 10 groups, comprising five in each group as listed in the Table 1 below. The temperature of each rat was measured rectally using digital thermometer at one hour time intervals, before administration of each extract which was given intraperitoneally, and for each hour after the extracts administered until the 5th hour. The testing doses of extracts were obtained after the determination of acute toxicity dose that was done earlier which are 5 and 50 mg/kg of each extracts.
Table 1: Summary of Normal Body Temperature Test Groups.
| Treatment Groups (n=5)
(non-fever) |
Treatment | |
| Negative Control | 0.9 ml Normal Saline | |
| Reference Drug | 100 mg/kg Ibuprofen | |
| Red | Flower | 5 mg/kg Red Flower |
| 50 mg/kg Red Flower | ||
| Leaf | 5 mg/kg Red Leaf | |
| 50 mg/kg Red Leaf | ||
| White | Flower | 5 mg/kg White Flower |
| 50 mg/kg White Flower | ||
| Leaf | 5 mg/kg White Leaf | |
| 50 mg/kg White Leaf | ||
Effect of extracts in Brewer’s Yeast-induced Pyrexia.
Hyperthermia was induced in rats as previously described (Panthonget al., 2007; Zakaria et al. 2008). Anti-pyretic activities of extracts were measured by slightly modifying the method described by Bruguerolleet al. (1994) and Teotinoet al. (1963). 55 Sprague-Dawley rats were divided into 11 groups of 5 rats each as shown in Table 3.3. The rats were clustered into 3 large groups; control, reference and test groups. All of the rats (except negative control group) were induced to pyrexia with 10ml/kg of 20% w/v BY injected subcutaneously in the dorsum area 19 hrs prior to injection of treatments peritoneally. The control groups were consisted of positive control group and negative (not induced) control group. Both groups were given only normal saline for treatment. On the other hand, the reference group will be treated with reference drug Ibuprofen 100 mg/kg dose.
For test groups, the rats were divided into 8 groups and the rats were given extracts as treatment as shown in Table 2. The temperature of each rat was measured by recording the rectal temperature using digital thermometer, which was inserted in the rectum at 0hr and 19hr, then for every hour until 24hr and baby oil was used as lubricant.
Table 2: Summary of Brewer’s Yeast Anti-Pyretic Test Groups.
|
Treatment Groups (n=5) (Pyrexia Induced)
|
Treatment | |
| Positive Control | 0.9% Normal Saline + BY | |
| Negative Control | 0.9% Normal Saline | |
| Refrence Drug | 100mg/kg Ibuprofen + BY | |
| Red | Flower | 5 mg/kg Red Flower + BY |
| 50 mg/kg Red Flower + BY | ||
| Leaf | 5 mg/kg Red Leaf + BY | |
| 50 mg/kg Red Leaf + BY | ||
| White | Flower | 5 mg/kg White Flower + BY |
| 50 mg/kg White Flower + BY | ||
| Leaf | 5 mg/kg White Leaf + BY | |
| 50 mg/kg White Leaf + BY | ||
Statistical Analysis.
In this study, the results were analyzed using SAS System version 9 and presented as mean ± standard error of mean (SEM). The statistical significance among the groups was assessed using two-way analysis of variance (ANOVA) while the statistical differences between each treatment group and control group are evaluated using Duncan test. The significance of differences between the time for pre-treatment and post-treatment studies were evaluated using repeated measures. P-values less than 0.05 were considered an indication of significance.
Result
Phytochemical Screening
The phytochemical screenings of each plant extracts were done for alkaloids, saponins, flavonoids, tannins and polyphenolic compound, triterpenes and steroid. Alkaloids, triterpenes, tannins and polyphenolic compound were not detected in all of the extracts. Table 3 below shows the result of phytochemical constituents’ detection (flavonoids, triterpenes, tannins, alkaloids, saponins and steroids) in the extracts. Flavonoids were detected in all of the extracts but were found higher in both H. rosa-sinensis (red)and H. rosa-sinensis var. Alba (white)flower extracts. However, triterpenes, tannins and alkaloids were not detected in all four extracts. Saponins were detected higher in H. rosa-sinensis (red) flower, leaf and H. rosa-sinensis var. Alba (white) leaf than in H. rosa-sinensis var. Alba (white) flower extract. Steroids were also detected in all four extracts with the highest concentration in H. rosa-sinensis (red) leaf followed by H. rosa-sinensis (red) flower, H. rosa-sinensis var. Alba (white) leaf and H. rosa-sinensis var. Alba (white) flower extracts.
Table 3: Phytochemical constituents of H.rosa-sinensis L. (Red) andH.rosa- inensisvar. Alba (white).
| Constituents | Flower | Leaf | ||
| H.rosa-sinensis (red) | H.rosa-sinensis var. Alba (white) | H.rosa-sinensis (red) | H.rosa-sinensis var. Alba (white) | |
| Flavonoids | ++ | ++ | + | + |
| Triterpenes | – | – | – | – |
| Tannins | – | – | – | – |
| Alkaloids | – | – | – | – |
| Saponins | ++ | + | ++ | ++ |
|
Steroids |
++ | + | +++ |
++ |
Note: For saponins: +, 1-2 cm froth; ++, 2-3 cm froth; +++, >3 cm froth.
For flavonoids, tannins, titerpenes and steroids: +, weak color; ++, mild color; +++, strong color.
For alkaloids: +, negligible amount of precipitate; ++, weak precipitate; +++, strong precipitate.
Heavy Metal Screening.
Heavy metal screenings were done and the results are expressed as mean of element in sample (ppm) (mean ± SEM) for all four extracts (RF, WF, RL and WL), were displayed in Table 4(a)-(d) below. RF extract shows the lowest amount of Mercury (Hg) (0.04 ± 0.01 ppm) than the rest of the extracts. However, WF extract shows low amount of Hg and lowest amount of As and Pb. Both RL and WL extracts show high amount of Pb which are 2.07 ± 0.08 ppm and 3.24 ± 0.05 ppm respectively. As RL showed highest amount of As, WL extract showed highest amount of Hg and Pb than the rest of the extracts.
Table 4 (a): Element Concentration (ppm) in H. rosa-sinensis (red) flower (RF).
| Element | H.rosa-sinensis (red) flower (ppm) | Maximum limit (ppm)
(NPC, MOH) |
| As | 0.58 ± 0.01 | 5 |
| Hg | 0.04 ± 0.01 | 0.5 |
|
Pb |
0.66 ± 0.00 |
10 |
Note:Values were presented as Mean Element Concentration (ppm) ± S.E.M.
NPC: National Pharmaceutical Control Bureau
MOH: Ministry of Health Malaysia
Table 4 (b): Element Concentration (ppm) in H. rosa-sinensis var. Alba (white) flower (WF).
| Element | H.rosa-sinensis var. Alba (white) flower (ppm) | Maximum limit (ppm)
(NPC, MOH) |
| As | 0.18 ± 0.01 | 5 |
| Hg | 0.05 ± 0.00 | 0.5 |
| Pb | 0.53 ± 0.09 | 10 |
Note: Values were presented as Mean Element Concentration (ppm) ± S.E.M.
NPC: National Pharmaceutical Control Bureau.
MOH: Ministry of Health Malaysia.
Table 4 (c): Element Concentration (ppm) in H. rosa-sinensis (red) leaf (RL)
| Element | H. rosa-sinensis (red) leaf (ppm) |
Maximum limit (ppm) (NPC, MOH) |
| As | 0.75 ± 0.02 | 5 |
| Hg | 0.05 ± 0.00 | 0.5 |
| Pb | 2.07 ± 0.08 | 10 |
Note: Values were presented as Mean Element Concentration (ppm) ± S.E.M.
NPC: National Pharmaceutical Control Bureau.
MOH: Ministry of Health Malaysia.
Table 4 (d): Element Concentration (ppm) in H. rosa-sinensis var. Alba (white) leaf (WL).
| Element | H. rosa-sinensis var. Alba (white) leaf (ppm) |
Maximum limit (ppm) (NPC. MOH) |
| As | 0.40 ± 0.01 | 5 |
| Hg | 0.08 ± 0.01 | 0.5 |
| Pb | 3.24 ± 0.05 | 10 |
Note: Values were presented as Mean Element Concentration (ppm) ± S.E.M
NPC: National Pharmaceutical Control Bureau.
MOH: Ministry of Health Malaysia.
Acute Toxicity Testing.
Histological Examinations (Liver & Kidney).
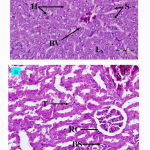
The rats were treated with extracts (5, 50 and 500 mg/kg) and histological examinations of liver and kidney were observed, and the results showed no obvious damage of organ tissue samples treated with 500 mg/kg (H: Hepatocytes, L: Liver Lobules, S: Sinusoids; T: Renal Tubules, BS: Bowman’s Space, RC: Renal Corpuscles).
Extracts Treated Rat Blood Analysis (Liver Function Test).
Liver function test was done consisting the detection of Alanine transaminase (ALT), Alkaline phosphatase (ALP) and Aspartate aminotransferase (AST) activities in blood serum of rats. The values are shown in Table 5(a)-(c) below.
Table 5 (a): Effect of various concentration of leaves and flowers ethanol extracts of H. rosa-sinensis(red) and H. rosa-sinensis var. Alba (white) on plasma level of Alanine transaminase (ALT) in rat.
| Concentration | H. rosa-sinensis
(red) |
H. rosa-sinensis var. Alba (white) | ||
| Flower | Leaf | Flower | Leaf | |
| 0 | 0.88 ± 0.08ax | – | – | – |
| 5 | 0.89 ± 0.06ax | 0.73 ± 0.07by | 0.89 ± 0.06ax | 0.83 ± 0.04ay |
| 50 | 0.90 ± 0.13ab | 0.93 ± 0.03ab | 0.88 ± 0.06a | 0.82 ± 0.06a |
| 500 | 0.74 ± 0.07bx | 0.95 ± 0.11abx | 1.08 ± 0.13ax | 0.86 ± 0.05ax |
Note: Data were presented as mean ± standard error mean (SEM) in IU/L; n = 5
ab: Comparison of means between columns significant at p<0.05
xy: Comparison of means between rows significant at p<0.05.
Table 5 (b): Effect of various concentration of leaves and flowers ethanol extracts of H. rosa-sinensis (red) and H. rosa-sinensis var. Alba (white) on plasma level of Alkaline phosphatase (ALP) in rat.
| Concentration | H. rosa-sinensis
(red) |
H. rosa-sinensisvar. Alba (white) | ||
| Flower | Leaf | Flower | Leaf | |
| 0 | 294.35 ± 6.56a | – | – | – |
| 5 | 277.28 ± 5.69ax | 277.54 ± 27.36ax | 300.96 ± 20.87ax | 290.82 ± 11.28ax |
| 50 | 286.02 ± 7.61ax | 307.00 ± 4.46ay | 284.00± 6.68ax | 290.34 ± 24.55axy |
| 500 | 289.21 ± 16.06ax | 287.66 ± 6.50ax | 292.95 ± 10.48ax | 259.09 ± 10.40by |
Note: Data were presented as mean ± standard error mean (SEM) in IU/L; n = 5
ab: Comparison of means between columns significant at p<0.05.
xy: Comparison of means between rows significant at p<0.05.
Table 5 (c): Effect of various concentration of leaves and flowers ethanol extracts of H. rosa-sinensis (red) and H. rosa-sinensis var. Alba (white) on plasma level of Aspartate aminotransferase (AST) in rat.
| Concentration | H. rosa-sinensis
(red) |
H. rosa-sinensisvar. Alba (white) | ||
| Flower | Leaf | Flower | Leaf | |
| 0 | 1.94 ± 0.11a | – | – | – |
| 5 | 1.91 ± 0.17ax | 1.81 ± 0.04ax | 1.76 ± 0.03ax | 2.01 ± 0.10ay |
| 50 | 1.98 ± 0.07ax | 1.72 ± 0.04bx | 1.73 ± 0.05ax | 1.75 ± 0.06bx |
| 500 | 1.75 ± 0.03bx | 1.73 ± 0.08bx | 1.92 ± 0.11bx | 1.97 ± 0.04ax |
Note: Data were presented as mean ± standard error mean (SEM) in pg/ml; n = 5
ab: Comparison of means between columns significant at p<0.05.
xy: Comparison of means between rows significant at p<0.05.
Effect of Extracts on Body Temperature of Normal Rats.
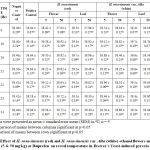
The effects of the ethanol extracts of H.rosa-sinensis(red) and H. rosa-sinensis var. Alba (white) on the normal body temperature in rats are shown in Table 6. The results are expressed as mean rectal temperatures (mean ± SEM) for Control group (RF dose 0 mg/kg), Reference Drug group (Ibuprofen 100 mg/kg), and 8 groups of different treatment extracts (RF5, WF5, RL5, WL5, RF50, WF50, RL50 and WL50), at a specific time intervals that is from 0 hr (normal state) until 24 hr. It was found that Ibuprofen at doses of 100 mg/kg body weight caused significant reduction of body temperature at the 1st hour following administration and no significant decrease in temperature at other times.
All extracts at doses 5 mg/kg and 50 mg/kg body weight administered to normal rats showed no significant lowering of body temperature with no significant difference between variant of H. rosa-sinensis.
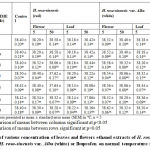 |
Table 6: Effect of various concentration of leaves and flowers ethanol extracts of H. rosa-sinensis(red) and H. rosa-sinensis var. Alba (white) or Ibuprofen on normal temperature rat. |
Brewer’s Yeast-Induced Pyrexia Test
The subcutaneous injection of yeast suspension markedly elevated the rectal temperature at the 19thhr after administration. Results of the antipyretic effect of the extracts were displayed in Table 7 at a specific time interval that is from 0 hr (normal state) until 24thhr whereas 19thhr is the peak temperature for pyrexia. Treatment with extracts at doses 5 mg/kg and 50 mg/kg body weight decreases the rectal temperature of the rats in a dose dependent manner. The anti-pyretic effect started as early as 1st hr.
In treatment time, after the subcutaneous injection of 20% BY w/v suspension (10ml/kg) in control group (RF dose 0 mg/kg) induced a progressive increase in rectal temperature by 4.05%, from 0 hr (38.04 ± 0.07°C) to 19thhr (39.58 ± 0.14°C). The control group showed significant increase (p<0.05) in rectal temperature at 19th to 24th. Treatment with Ibuprofen shows significant reducing in rectal temperature when compared to control (RF dose 0 mg/kg) from the 1sthr of administration until the 5th hr.
Treatment by dose 5 mg/kg RF showed no significant antipyretic effect on lowering the rectal temperature when compared to control (RF dose 0 mg/kg). However, treatments with 50 mg/kg dose RF only showed significant lowering (p<0.05) on rectal temperature starting on the 1st until the 2ndhr after administration when compared to control group but none at other times. Ibuprofen treated group significantly reducing the rectal temperature from the 1sthr of administration and remained until the 5thhr after (time 20 hr until 24 hr).
Treatment with WF surprisingly revealed a significant effect on reducing the rectal temperature (p<0.05) for both 5 and 50 mg/kg doses. The antipyretic effect started from the first hour after its administration of both doses WF on BY-induced pyrexia rats. Both shown significant effect at all time except for 22 hr of 5 mg/kg WF treated, and the last hour (24 hr) of 50 mg/kg WF treated group when compared to control (RF dose 0 mg/kg) group. Moreover, when looking at all the times of treatments of these 3 treatment groups, all of the temperatures from time 20 hr to the last 24 hr showed no significant difference (p>0.05) between all of the times. In conclusion, the potency of dose 50 mg/kg of WF can be compared to as ibuprofen in the antipyretic effect on reducing the rectal temperature of pyrexia rats.
On the other hand, the data analysis of RL treatments (dose 5 and 50 mg/kg) showed no significant effect in reducing the rectal temperature except for the first hour after administration of 50 mg/kg RL only which is 38.96 ± 0.14ºC when compared to control (RF 0 mg/kg) 39.64 ± 0.07ºC at the same time.
Treatment with WL showed no significant (p>0.05) antipyretic effect at all times after 5 mg/kg WL administered to the pyrexia rats. However, at higher dose, 50 mg/kg WL showed antipyretic effect at the 1st (20 hr) and 2ndhr (21 hr) after it has been administered when compared to control (RF dose 0 mg/kg) group.
Discussion
The results of phytochemical screening of both H.rosa-sinensis L. and H.rosa-sinensisvar. Alba flower and leaf ethanol extracts showed presence of flavanoids and saponins. As we know, flavonoids and saponins are two compounds that can contribute in anti-pyretic activity. Both saponins and flavonoids have found wideapplications in the fields of medicine, pharmacy and food industries as pharmacologically active principles (Schopke and Hiller, 1990).
Heavy metal screening for the leaf of both H.rosa-sinensis L. and H.rosa-sinensis var. Albacontained high concentration of lead (2.07 ± 0.08 ppm and 3.24 ± 0.05 ppm respectively). However, the values were considered safe as compared to maximum limit of lead (10.0 ppm) in traditional medicine products set by Revision Drug Registration Guidance Document (2012). These might be due to long term exposure of leaf to the environment rather than freshly blooming flower. Location of leaf of H.rosa-sinensis var. Albaalong roadside might contribute to high concentration of lead than leaf of H.rosa-sinensis L. Thus, the vehicles usage of leaded petrol can be the main reason of accumulation of lead in the leaf of H.rosa-sinensis var. Alba along roadside.
The use of lead as a petrol additive has been a disaster for public health.Leaded petrol contributed as the main cause of lead exposure than other sources worldwide.By contaminating air, dust, soil, drinking water and food crops, it has caused harmfully high human blood lead level (BLL), especially in children (Organisation for Economic Cooperation & Development/United Nations Environment Program, 1999). Recent National Health and Nutrition Survey data on lead exposure in the United States indicates that the average of BLL in the population is 1.6 μg/dL and 1.9 µg/dL in children 1-5 years of age (CDC, 2004 (A)).
Natural remedies are believed to be free of toxic effects and health risks. Nevertheless, ethnic remedies had been proven to contain lead and other heavy metals also toxic substances. Certain branches of ayurvedic medicines contain heavy metals because the metals are thought tohave therapeutic benefits for particular ailments. As been reported to Centre of Disease Control and Prevention (CDC), traditional remedies contained lead (Roche et al., 2005; Muziet al., 2005; CDC, 2004 (B); 1981; 1982; 1983 (A) 1983 (B); 1984; 1989; 1993; 1999;2002; 2005). In 2004, three cases of lead poisoning by ayurvedic medicines were reported to CDC (CDC, 2004(B)). Thus, lead detection in leaf of both H.rosa-sinensis L. and H.rosa-sinensis var. Alba might be due to its naturally contained of lead. However, these high values detection were still under the maximum limit which can lead to lead poisoning.
Lead is toxic to multiple organ systems even at low levels considered as safe. Small amounts of heavy metals are common in our environment and diet even necessary for good health (International Occupational Safety and Health Information Centre, 1999; Roberts, 1999) but great amounts of them will contribute to acute or chronic toxicity (poisoning). Toxicity of heavy metals will occur as they are unable to be metabolized by the body thus accumulated in the soft tissues. Heavy metals may enter the human body through food, water, air, or absorption through the skin in agriculture, manufacturing, pharmaceutical, industrial, or residential settings (Roberts, 1999).
In fetus and infant, lead can cross the placenta and penetrate the blood-brain barrier. Thus bring loss of intelligence and disruption of behavior and effectsare permanent and untreatable. According to Lanphea ret al. (2000), lead can bring harm to the infant brain even at blood levels as low as 5 μ/dl. Acutely, leadaccumulates in the proximal renal tubules, causing aminoaciduria and renal glycosuria due to diminished reabsorptionas well as hyperuricaemia secondary to diminish rate secretion and inhibition of guanine aminohydrolase by lead (Pollock and Ibels, 1988). Chronic lead exposure caused progressive tubularatrophy and interstitial fibrosis. The consequences include chronic renal insufficiency with benignurinary sediment, contracted kidneys and hypertension (Marsden, 2003).
Acute toxicity testing using the fixed-dose procedure was proposed by the British Toxicology Society in 1984 to reduce the number of animals used in acute toxicity testing and to avoid lethality as an endpoint where possible and later was adopted by the OECD in 1992. Rather than using lethality, the FDP relies on clear toxic signs observed at a number of fixed dose levels that are applicable to the cut-off points in the European Economic Community (EEC) acute toxicity classification system (Lipnicketal., 1995).
The result of 500mg/kg of test extracts administered to the rats showed only evident toxicity with no mortality. As mentioned by Whitehead and Curnow (1992), evident toxicity is a general term describing clear signs of compound-related toxicity, but not such as to cause very severe pain, distress or mortality. Therefore dose of test extracts were stopped at 500mg/kg as the dose was categorized as unclassified and therefore used to determine testing dose (via ratio calculation).
The organs (liver and kidney) of the rats histologically showed no sign of tissue damage 14 days after the extracts were administered. It was also confirmed by liver function test (AST, ALP and ALT) showed no significant value (p>0.05) compared to normal of each enzyme and between doses. Based on the results, it is safe to assume that the ethanolic extracts of flower and leaf of H.rosa-sinensis L. and H.rosa-sinensis var. Albaare not harmful.
The ethanolic extracts were administered to treat fever in rats. Fever or pyrexia is a complex and non-specific hostdefense response to invasion by infectious pathogens; as such it is not per se injurious to theafflicted host.It develops in combination with activation of various physiologic, autonomic, immunologic, and behavioral mechanisms. Fever is not uniquely a signof infection,as it may result from non-infectious diseases, as in stress, autoimmune,neoplastic and granulomatous disorders.
Since there are many factors to be considered, it is not easy to identify the role of a single mediator in the development of pyrexia. The febrile response in classical model of pathogenesis is mediated by the release of pyrogenic cytokines like IL-1β, IL-6 and TNF-α into the bloodstream and acts on central nervous system (CNS) which increases the synthesis of PGE2 near pre-optic hypothalamus area thereby triggering the hypothalamus to elevate the body temperature (Spacer and Breder, 1994) in response to exogenous pyrogens. The degree of febrile response is different regarding the etiology of fever and also varies with the species of animals.
The current study consisted of model of BY-inducedpyresis for better characterization of the anti-pyretic activity of the extracts. Anti-pyretic such as Ibuprofen is known to reduce fever by depressing inflammatory messages at both peripheral sites of tissue inflammation and within the CNS thermoregulatory sites. These agents suppressed peripheral production of pyrogenic cytokines such as TNF-α and IL-1β, while lowering the thermoregulatory set point by blocking central COX production of PGE2 (Aronoff, 2001). In some cases, antipyretics can slightly lower the normal temperature as seen in normal temperature rats test (Table 6).
BY is an exogenous pyrogen, where systemic injection into rats induced the formation of endogenous pyrogens such as IL-1β and TNF-α. This endogenous pyrogen will induced the synthesis of prostaglandins to alter the thermoregulatory set point, causing body to initiate heat-promoting mechanism.
BY model induced both TNF-α and PLA2 (PGs synthesis) (Kluger, 1991). Ibuprofen at dosage 100 mg/kg significantly reduced the pyresis induced by BYby blocking TNF-α and PGs synthesis. 50 mg/kg H. rosa-sinensis (red) flower,5 mg/kg and 50 mg/kg H. rosa-sinensis var. Alba (white)significantly (p<0.05) inhibited the BY pyresis model. Analysis showed significant difference (p<0.05) in variants, parts and doses in treatments. As mentioned by Souza et al. (1992), flavonoids are known to possess anti-pyretic and anti-inflammatory properties and flavonoid compounds have been detected from the extracts and it might therefore be responsible for the anti-pyretic effect of the extracts.
Main goal in treating pyrexia is to prevent the body’s temperature from going too high. NSAIDs are commonly prescribed to moderate or eliminate the discomfort level of afflicted patients in the treatment of pain, fever and inflammation. NSAIDs as non-selective inhibitors of COX enzyme, act on both COX-1 and COX-2 isoenzymes. There are beneficial and detrimental effects of NSAIDs with their ability to inhibit PGs synthesis through a directblockage of COX, where COX-1 is important for normal physiologic functions and the COX-2 is induced by various inflammatory stimuli (Sheeba and Asha, 2009).
Since it was clear that most antipyretics drug work by inhibiting the COX enzyme and reducing the levels of PGs (Aronoff and Neilson, 2001), the BY-induced pyrexia rat model was employed to investigate the antipyretic properties of H. rosa-sinensis ethanol extract.
Ibuprofen is a non-selective COX inhibitor possessing anti-inflammatory, antipyretic and analgesic properties. Ibuprofen has been use as over-the-counter (OTC) drugs with the best tolerated drug of its class for pain relief without any obvious major health issues. Adverse reactions to ibuprofen are dose and duration dependent; it is as effective as aspirin and more effective than paracetamol. Ibuprofen have the least risk of gastrointestinal complications compared with other NSAIDs and is considered relatively benign in overdose (Moore, 2007).
The present studies showed that the intraperitoneal administration of ethanol extracts, especially H. rosa-sinensis var. Alba (white) did contributed in the antipyretic properties to a certain degree of extent. 50 mg/kg of H. rosa-sinensis var. Alba (white) flower extract decreased the rectal temperature in BY-induced pyrexia test until the time of 24th hour. Although the extracts may be beneficial in management of fever, a much in-depth studies are still needed to further determine and confirm the pharmacodynamic of the active compounds.
Further studies involving the purification of the chemical constituents of the plant and investigations of the mechanism of action may result in the development of a potent antipyretic agent with high efficacy and better therapeutic index. Nevertheless, the results from this initial study provide the basis for further investigation and isolation of the biologically active component.
Conclusion
The results of this study have indicated the antipyretic potential H. rosa-sinensisL. and H. rosa-sinensis var. Alba extracts.Therefore the results suggest the probability for its therapeutic effectiveness as plant-based antipyretic agent as claimed by traditional medicine practitioners of our local community. This study also suggests that flower of H. rosa-sinensis can be taken as prophylactic to prevent pyrexia. This provides an alternative way to overcome the side effects produced by chemically synthesize pharmaceutical drugs. For example, prolonged use of NSAIDs has detrimental gastrointestinal complications.
References
- Adhirajan, N., Kumar, T.R., Shanmugasundaram, N. and Babu, M. (2003). In vivo and in vitro evaluation of hair growth potential of Hibiscus rosa-sinensisJournal of Ethnopharmacology.88:235-9.
CrossRef - Aronoff, D.M. and Neilson, E.G. (2001). Antipyretics: mechanisms of action and clinical use in fever suppression. The American Journal of Medicine. 111(4): 304–315.
CrossRef - Blumenthal, I. (1998). What parents think of fever.Family Practice. 15: 513-518.
CrossRef - Bruguerolle, B. and Roucoules, X. (1994). Time dependent changes in body temperature rhythm induced in rats by Brewer’s yeast injection. Chronobiology International. 11:180-186.
CrossRef - (1981). Lead poisoning from lead tetroxide used as a folk remedy-Colorado.Morbidity and Mortality Weekly Report 30: 546-547.
CrossRef - (1982). Use of lead tetroxide as a folk remedy for gastrointestinal illness.Morbidity and Mortality Weekly Report 30: 647-648.
CrossRef - (1983)(A). Folk remedy-associated lead poisoning in Hmong children-Minnesota.Morbidity and Mortality Weekly Report 32: 555-556.
- (1983)(B). Lead poisoning from Mexican folk remedies-California. Morbidity and Mortality Weekly Report 32: 554-555.
- (1984). Lead poisoning associated death from Asian Indian folk remedies Florida.Morbidity and Mortality Weekly Report 33: 643-645.
- (1989). Epidemiologic notes and reports cadmium and lead exposure associated with pharmaceuticals imported from Asia-Texas. MMWR Morbidity and Mortality Weekly Report 38: 612-614.
- (1993). Lead poisoning associated with use of traditional ethnic remedies- California, 1991-1992.Morbidity and Mortality Weekly Report 42: 521-524.
- (1999). Adult lead poisoning from an Asian remedy for menstrual cramps Connecticut, 1997.Morbidity and Mortality Weekly Report 53: 27-29.
- (2002). Childhood lead poisoning associated with tamarind candy and folk remedies-California, 1999-2000.Morbidity and Mortality Weekly Report 51: 684-686.
- CDC, (2004)(A). Blood lead levels-United States, 1988-1991. Morbidity and Mortality Weekly Report 54:545-548.
- (2004)(B). Lead poisoning associated with ayurvedic medications-five states, 2000- 2003.Morbidity and Mortality Weekly Report 53:582-584.
- (2005). Lead poisoning associated with use of litargirio-Rhode Island, 2003.Morbidity and Mortality Weekly Report 54: 227-229.
- Fabricio, A.S.C., Rae, G.A., D’Orléans-Juste, P. and Souza, G.E.P. (2005). Endothelin-1 as a central mediator of LPS-induced fever in rats. Brain Research. 1066:92–100.
CrossRef - Fairbanks, C.A., Schreiber, K.L., Brewer, K.L., Yu, C.G., Stone, L.S., Kitto. F.F., Nguyen, H.O., Grocholski, B.M., Shoeman, D.W., Kehl, L.J., Regunathan, S., Reis, D.J., Yezeirski, R.P., Wilcox, G.L. (2000). Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proceedings of the national Academy of Sciences. 97(79): 10584-10589.
CrossRef - Farnsworth, N.R. (1996). Biology and phytochemical screening of plants. Journal of Pharmaceutical Sciences. 55: 225-276.
CrossRef - Gilman, E.F. (1999). Hibiscus Rosa-Sinensis. Fact Sheet FPS-254. Cooperation Extension Service. University of Florida, pp: 1-3.
- Hashemi, S.R., Zulkifli, I., Bejo, M.H., Farida, A., Somchit, M.N (2008). Acute toxicity study and phytochemical screening of selected herbal aqueous extract in broiler chickens. International Journal of Pharmacology.4(5): 352-360.
CrossRef - International Occupational Safety and Health Information Centre. (1999). Metals.In Basics of Chemical Safety, Chapter 7.Geneva: International Labour Organization.
- Jarvis, K.E., Gray, A.L., Houk, R.S. (1996). Handbook of inductively coupled plasma mass spectrometry. pp:172-224. London and New York. Blackie Academic and Professional Chapman & Hall.
- Kluger, M. J. (1991) Fever: role of pyrogens and cryogens. Physiology Review. 71:93–127.
CrossRef - Lanphear, B.P., Dietrich, K., Auinger, P. and Cox, C. (2000). Cognitive deficits associated with blood lead concentrations <10 mg/dl in US children and adolescents. Public Health Report. 115: 521-529.
CrossRef - Lipnick, R.L., Cotruvo, J.A., Hill, R.N., Bruce, R.D., Stitzel, K.A., Walker, A.P., Chu, I., Goddard, M., Segal, L., Springer, J.A. and Myers, R.C. (1995). Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food and Chemical Toxicology, Volume 33, 3: 223-231.
CrossRef - Marsden, P.A. (2003). Increased body lead burden – cause or consequence of chronic renal insufficiency? The New England Journal Medicine. 348: 345-347.
CrossRef - Masaki, H. S., Sakaki, S., Atsumi, T. and Sakurai, H. (1995). Active oxygen scavenging activity of plant extracts. Biological and Pharmaceutical Bulletin. 18:162–166.
CrossRef - Muzi, G., Dell’Omo, M., Murgia, N., Curina, A., Ciabatta, S. and Abbritti, G. (2005). Lead poisoning caused by Indian ethnic remedies in Italy. La 96: 126-133.
- Moore, N. (2007). Ibuprofen: A journey from prescription to over-the-counter use. Journal of the Royal Society of Medicine. 48:2–6.
- Organisation for Economic Cooperation & Development/ United Nations Environment Programme. (1999). Phasing lead out of gasoline: an examination of policy approaches in different countries. Paris: OECD.
- Panthong A., Norkaew P., Kanjanapothi D., Taesotikul T., Anantachoke N., Reutrakul V. (2007). Anti-inflammatory, analgesic and antipyretic activities of the extract og gamboge from Garniciahanburyi Hook f..Journal of Ethnopharmacology 111: 335-340.
CrossRef - Pollock, C.A. and Ibels, L.S. (1988). Lead nephropathy – a preventable cause of renal failure. International Journal Artificial Organs 11: 75-78.
CrossRef - Rao, R.N. and Talluri, M.V.N.K. (2007). An overview of recent applications of inductively coupled plasma-mass spectrometry (ICP-MS) in determination of inorganic impurities in drugs and pharmaceuticals. Journal of Pharmaceutical and Biomedical Analyisis 43: 1-13.
CrossRef - Revision Drug Registration Guidance Document, (2012). National Pharmaceutical Control Bureau Ministry of Health Malaysia: Petaling Jaya, Malaysia.
- Roberts, J.R. (1999). Metal toxicity in children. In Training Manual on Pediatric Environmental Health: Putting It into Practice 1999 Jun. Emeryville, CA: Children’s Environmental Health Network http:// www. cehn. org/cehn/trainingmanual/pdf/manual-full.pdf
- Roche, A., Florkowski, C., Walmsley, T. (2005). Lead poisoning due to ingestion of Indian herbal remedies. New Zealand Medical Journal. 118: U1578.
- Sachdewa, A. and Khemani, L.D. (2003). Effect of Hibiscus rosasinensis Linn. ethanol flower extract on blood glucose and lipid profile in streptozotocin induced diabetes in rats. Journal of Ethnopharmacology. 89:61–66.
CrossRef - Schopke, T.H. and Hiller, K. (1990). Triterpenoidsaponins, Part 6. Die Pharamazie. 45: 313-342.
- Sheeba, M.S. and Asha, V.V. (2009). Cardiospermumhalicacabum ethanol extract inhibits LPS induced COX-2, TNF-α and iNOS expression, which is mediated by NF-κB regulation, in RAW264.7 cells. Journal of Ethnopharmacology. 124:39–44.
CrossRef - Singh, N., R. Nath, A. K. Agarwal & R. P. Kohli. (1978). A pharmacological investigation of some indigenous drugs of plant origin for evaluation of their antipyretic, analgesic and anti- inflammatory activities. Journal of Research and Education in Indian Medicine. Yoga Homeopathy. 13: 58–62.
- Sofowara, A. (1993). Medicinal Plants and Traditional medicine in Africa. Ibadan: Spectrum Books Ltd.
- Souza, M.F., Rao, V.S., Silveira, E.R. (1992). Anti-anaphylactic and anti-inflammatory effects of ternatin, a flavonoid isolated from Egletesviscosa Brazilian Journal of Medicaland Biological Research. 25(10): 1029-32.
- Spacer, C.B. and Breder, C.D. (1994). The neurologic basis of fever. New England Journal of Medicine. 330: 1880-1886.
CrossRef - Teotino U.M., Friz L.P., Gandini A., Dellabella D. (1963). Thio Derivatives of 2,3-Dihydro-4h-1,3-Benzoxazin-4-One. Synthesis and Pharmacological Properties. Journal of Medicinal Chemistry 6: 248-250.
CrossRef - Trease G.E., Evans W.C. (1989). A Text Book of Pharmacognosy. London: Bailliere Tindal Ltd.
- Vonkeman, H.E. and Laar, M.A.F.J. (2008). Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention. Seminars in arthritis and rheumatism. 39(4):294–312.
CrossRef - Whitehead, A. and Curnow, R.N. (1992). Statistical Evaluation of the Fixed-Dose Procedure. Food and Chemical Toxicology. 30(4): 313-324.
CrossRef - Zakaria, Z.A. ,Safarul, M., Valsala, R., Sulaiman, M.R., Fatimah, C.A., Somchit, M.N., Mat Jais, A.M (2005). The influences of temperature and naloxone on the antinociceptive activity of Corchorus olitorius L. in mice. Naunyn-Schmiedeberg’s Archives of Pharmacology. 372: 55-62
CrossRef - Zakaria, Z.A., RadenMohd. Nor, R.N.S., Hanan Kumar, G., Abdul Ghani, Z.D.F., Sulaiman, M.R., Rathna Devi, G., Mat Jais, A.M., Somchit, M.N., Fatimah, C.A. (2006). Antinociceptive, anti-inflammatory and antipyretic properties of Melastomamalabathricum leaves aqueous extract in experimental animals. Canadian Journal of Physiology and Pharmacology. 84(12): 1291-1299
CrossRef - Zakaria, Z.A., Abdul Ghani, Z.D.F., RadenMohd. Nor, R.N.S., Gopalan, H.K, Sulaiman, M.R., Mat Jais, A.M., Somchit, M.N., Kader, A.A., Ripin, J. (2008). Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopterislinearis leaves in experimental animal models. Journal of Natural Medicines. 62(2):179-187.
CrossRef - Zimmermann, M. (1983). Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Journal of Pain. 16:109-110.
CrossRef