Narendar Koyagura1* , V. Hemanth Kumar2
, V. Hemanth Kumar2 and Chandrakumar Shanmugam3
and Chandrakumar Shanmugam3
1Department of Pharmacology, RVM Institute of Medical Sciences and Research Center (KNR University of Health Sciences), Laxmakkapally, Mulugu, Siddipet Dist. Telangana, India-502279
2Department of Pharmacology, MVPS Dr Vasantrao Pawar Medical College, Nashik, Maharashtra, India- 422003
3Department of Pathology, RVM Institute of Medical Sciences and Research Center (KNR University of Health Sciences), Laxmakkapally, Mulugu, Siddipet Dist., Telangana, India 502279
Corresponding Author Email: narendar.kumar23@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2107
Abstract
This study explores the anti-diabetic, insulin sensitizing and hypolipidemic activity of Coccinia indica (C.indica) leaf extract (ethanolic) in glucocorticoid induced insulin resistance (IR). A 12 day study with 5 groups of 30 male Wistar albino rats, with 6 rats in each was conducted. The rats in all the groups except group 1 received dexamethasone (8mg/kg/i.p.) from 7th to 12th day to induce IR. The groups 1 and 2 received 2% gum acacia orally for 12 days whereas the groups 3 & 4 received oral ethanolic extract of C.indica leaf in the dose of 1 and 2 gm/kg, respectively. The standard control (group 5) received metformin (1gm/kg) orally for 12 days. Fasting serum glucose, insulin and lipid levels were estimated at the beginning and end of the study. The insulin sensitivity indices (homeostatic model assessment of insulin resistance and sensitivity, fasting glucose to insulin ratio, hepatic & atherogenic indices) were calculated. The body weight was monitored on alternate days. The liver weight, volume and histopathology were also done. Compared to group 2 rats, the group’s 3 & 4 demonstrated significant(p<0.05) dose dependent lowering of serum glucose, insulin and lipids as well as lowered IR, improved insulin sensitivity and reduced hepatic steatosis. Additionally, group 2 rats had low body weight and hepatomegaly. This extract demonstrated significant anti-diabetic, hypolipidemic and insulin sensitizing activity. Hence it can be used as an effective alternative for treating type2 diabetes mellitus.
Keywords
Coccinia Indica; Diabetes Mellitus; Dexamethasone; Hyperlipidemia; Hepatic Steatosis; Insulin Sensitivity
Download this article as:| Copy the following to cite this article: Koyagura N, Kumar V. H, Shanmugam C. Anti-Diabetic and Hypolipidemic Effect of Coccinia Indica in Glucocorticoid Induced Insulin Resistance. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Koyagura N, Kumar V. H, Shanmugam C. Anti-Diabetic and Hypolipidemic Effect of Coccinia Indica in Glucocorticoid Induced Insulin Resistance. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/2YhEJq7 |
Introduction
Diabetes mellitus (DM) is characterized by hyperglycemia either due to absolute or relative deficiency of insulin secretion or action.1 Insulin resistance (IR), found in type 2 DM, is a state of impaired glucose utilization by the peripheral target tissues like liver, skeletal muscle and adipose tissue due to reduced insulin response. Initially, IR leads to compensatory hyperinsulinemia via beta cell hypersecretion which progresses to persistent hyperglycemia, dyslipidemia, and endothelial alterations.2 Glucocorticoids (GC) contribute to IR due to its counter regulatory effect on insulin to produce hyperglycemia and hyperlipidemia. Furthermore, the GC enhances the release of excess fatty acids from adipose tissue and facilitates triglycerides (TG) synthesis in liver, contributing to fatty liver.3, 4
The indices of IR and insulin sensitivity (IS) are widely used in research and clinical trials as well as clinical practice. These indices quantify IR and IS, and may serve as specific markers of early organ damage.5 The homeostatic model assessment (HOMA) of IR and IS are simple indices employed to calculate IR, insulin secretion and IS.6 In addition, another specific index that measures IS is fasting glucose to insulin ratio (FGIR) .7 The hepatic index (HI), an indicator of hepatic steatosis analyzes the progression of non-alcoholic fatty liver disease whereas the atherogenic index (AI) indicates the risk/occurrence of atherosclerosis and coronary artery diseases.8, 9
Many local plants were used in the traditional medicine for treating DM. Coccinia indica (C.indica), a Cucurbitaceae family member, is commonly known as ivy gourd is a climbing tropical perennial vine.10 It is commonly grown in India and its fruit is consumed as vegetable. Traditionally the leaves and the shoot were used in Ayurvedic and Unani Medicine. The aqueous and ethanolic leaf extracts exhibited hypoglycemic activity11 and depressed glucose-6-phosphatase enzyme activity.12 To the best of our knowledge no other study has evaluated the hypoglycemic, insulin sensitizing and hypolipidemic effect of ethanolic leaf extract of C.indica in GC-induced IR in Wistar Albino rats hence, the current study. Additionally the hypolipidemic effects were confirmed by histopathologic examination of liver tissues.
Materials and Methods
Plant Material
Fresh leaves of C.indica plant were collected from the farms of Nalgonda town, Telangana, India. The leaves were authenticated and stored until extraction and analysis in the Department of Pharmacognosy, SDM Centre for Research in Ayurveda and Allied Sciences, Udupi, Karnataka, India.
Extract Preparation
The leaves were dried under shade and then finely powdered using a blender. The powdered leaves (3000 gm) was mixed with 16L of absolute ethanol in a flask and allowed to stand for 24hrs. Then it was filtered and concentrated by rotary evaporator. Finally 132 gm of dried yield was obtained and stored in a stopper glass bottle until the study.13
Phytochemical Analyses
Preliminary phytochemical analysis of ethanolic extract of C.indica was assessed to find the presence of active chemical compounds by using standard methods.14
Experimental Design
Animals
Thirty adult male Wistar albino rats weighing 250-300gms were obtained and housed in polypropylene cages in the animal house facility. In accordance with the Committee for the Purpose of Control and Supervision on Experimental Animals (CPCSEA), they were maintained under optimum temperature (22ºC±2ºC) and 12h day:12h night cycle. They had free access to pellet food (Hindustan Lever Limited, Mumbai, India) and water ad libitum. This study was approved by the Institutional Animal Ethics Committee (KSHEMA/IAEC/02/2013).
Drugs and Reagents
The drugs and reagents including GC dexamethasone (DEX) (Zydus pharmaceuticals, India), Metformin (Mahalakshmi Chemicals Private Limited, Hyderabad), Ketamine (Neo laboratories, Mumbai), Biochemical Reagents (Agappe Diagnostic Private Limited, Bangalore), Enzyme-Linked Immunosorbent Assay (ELISA) kit (Gen X Bio Health Sciences Private Limited, New Delhi), Hematoxylin & Eosin (H&E) Reagents (Bio Lab Diagnostics Private Limited, Mumbai) were procured and were used in the study.
Methodology
Thirty male Wistar albino rats were categorized into five groups with 6 rats in each and were studied for 12 days. The details of five categorical groups are given below. All the rats were weighed using electrical balance on alternate days beginning from day 1 and ending on day 12 before sacrifice. The blood glucose, insulin and lipid levels of all the rats were recorded before starting the experiment as well as at the end of the study (12th day). For which fasting blood samples were collected from overnight fasted rats by retro-orbital sinus puncture under ketamine anesthesia intraperitoneally (50mg/kg). The safe dosage of ethanolic extract of C.indica in Wistar rats was determined using acute toxicity study prior to the experiment as was done earlier.15 The experiment was conducted as follows –
Group 1: Normal control group – were given only 2 % gum acacia orally (10mg/kg/day) from 1- 12 days.
Group 2: Diabetic control group received 2% gum acacia orally/day from 1-12 days along with intraperitoneal DEX injection (8mg/kg/day) from 7-12 days.
Group 3 and 4: Rats in these test groups were given ethanolic extract of C.indica orally [group III (1 gm/kg/day) & group IV (2gm/kg/day) respectively] from 1-12 days along with intraperitoneal DEX injection (8mg/kg/day) from 7-12 days.
Group 5: The standard control group rats were given oral metformin (1gm/kg/day) from 1-12 days along with intraperitoneal DEX injection (8mg/kg/day) from 7-12 days.
At the end of the study the rats were sacrificed by cervical dislocation. The liver was dissected, its weight and volume was recorded. The liver volume was measured by calculating volume of saline displaced by liver in 100ml of saline. Later it was fixed in 10% neutral buffered formalin solution for further histopathology examination.
Fasting Serum Glucose Levels
The blood samples collected in plain and anticoagulated (calcium oxalate & sodium fluoride) vacutainers were centrifuged at 2000 RPM for 15-20 minutes to obtain the serum. Then, serum glucose levels were measured by glucose oxidase – peroxidase method (GOD-POD) using automated analyzer. The values were expressed as mg/dl.
Insulin Assay
Serum insulin levels were estimated by ELISA method and the results were expressed as ng/ml.
Serum Lipid Profile
Serum total cholesterol (TC), HDL-Cholesterol (HDL-C) and LDL-Cholesterol (LDL-C) levels were assessed by Cholesterol oxidase – phenol aminophenazine (CHOD-PAP) method. Serum TG levels were estimated by glycerol 3-phosphate oxidase – phenol aminophenazine (GPO-PAP) method using automated analyzer. The results were expressed as mg/dl.
Calculation of Insulin Sensitivity Indices
The insulin sensitivity indices were calculated using formulas. HOMA-IR = [Fasting insulin × fasting glucose]/405, HOMA-IS = 10000/ [fasting insulin × fasting glucose], FGIR = Fasting glucose concentration / Fasting insulin concentration, HI = liver weight/ body weight x 100 and AI = Total cholesterol – HDL cholesterol / HDL cholesterol.
Histopathological Examination
The formalin fixed liver was processed in an automated tissue processor and then embedded in paraffin wax block. Sections of 5µm thickness were cut using microtome. These sections were mounted onto the slides and were stained with standard H & E reagents. Subsequently, the slides were examined under light microscope by the pathologist (CS) for the assessment of the extent of steatosis (40X).18
Statistical Analysis
All the values were expressed as mean ± standard deviation (SD). The statistical analyses were done by One way Analysis of Variance (ANOVA) followed by Tukeys multiple comparisons post hoc test using SPSS version 20.0 statistics software. The p value of < 0.05 was taken as statistical significance.
Results
Phytochemical Findings
The qualitative phytochemical screening of ethanolic extract of C.indica leaf revealed the presence of triterpenoids, alkaloids, steroids, carbohydrates, tannins, flavonoids, coumarins, phenols, resins and quinones.
Serum Insulin Levels and Anti-Diabetic Effect
The diabetic control group (group 2) exhibited significantly (p<0.05) higher fasting serum insulin (19.04 vs. 2.76 ng/ml)(Confidence Interval(CI):15.23-17.33) and glucose (272.27 vs. 103.37 mg/dl) (CI:159.90-177.88) levels when compared to the normal control group (group 1) (Table 1 & 3). The test (groups 3 & 4) and metformin (group 5) groups demonstrated significantly lower levels of serum insulin (7.41, 6.02 & 6.37 ng/ml) (CI:10.59-12.68, 11.97-14.07 & 11.62-13.71) and glucose (147.10, 122.51 & 130.91 mg/dl) (CI:116.17-134.14, 140.76-158.73 & 132.36-150.33) when compared to the diabetic control group (group 2) in a dose-dependent manner (Table 1 and 3).
Effect on Lipid Profile
The diabetic control group (group 2) had significantly (p<0.05) higher serum lipid levels (TC & LDL-C), but lower HDL-C when compared to the normal control group (group 1) (Table 1 and 3). The test groups 3 & 4 and the metformin group 5 had lower TC, LDL-C and higher HDL-C levels. Specifically, the group 4 rats had much lower TC, LDL-C and higher HDL-C levels than the group 5 (Table 1 and 3).
Effect on Insulin Sensitivity Indices
The insulin resistance (IR) was determined using insulin sensitivity indices. The group 2 rats demonstrated an increased HOMA-IR levels and decreased HOMA-IS, FGIR levels indicating development of IR. In addition group 2 rats exhibited increased HI and AI indices indicating hepatic steatosis and hyperlipidemia. Whereas, the test groups 3 & 4 and the metformin group 5 had normal indices (Table 2 and 3).
Table 1: Serum glucose, insulin and lipid profile among Categorical groups
| Categorical Group | Glucose
(mg/dl) |
Insulin
(ng/ml) |
TC
(mg/dl) |
TG
(mg/dl) |
HDL-C
(mg/dl) |
LDL-C
(mg/dl) |
| Group 1 | 103.37±0.27 | 2.76±0.11 | 82.98±2.84 | 57.49±2.49 | 27.25±0.90 | 44.23±2.98 |
| Group 2* | 272.27±3.36 | 19.04±0.27 | 224.51±2.72 | 176.73±3.20 | 12.16±0.81 | 177.01±3.19 |
|
Group 3 |
147.10±2.23 | 7.41±0.24 | 106.97±2.66 | 98.46±2.58 | 20.18±0.74 | 67.10±1.95 |
| Group 4** | 122.51±1.21 | 6.02±0.23 | 103.40±2.54 | 86.83±1.45 | 22.88±0.61 | 63.15±2.73 |
| Group 5 | 130.91±0.70 | 6.37±0.24 | 105.35±3.24 | 93.92±0.95 | 20.94±0.36 | 65.63±2.91 |
Each group has 6 rats (n=6), The group 2* compared to the other groups had higher levels of all the parameters. **Group 4 compared to group 3 had dose dependent lowered levels of all parameters
Table 2: Insulin Sensitivity indices among Categorical groups
| Categorical Group | HOMA-IR | ISI | FGIR | Hepatic Index | Atherogenic Index |
| Group 1 | 16.92±0.69 | 1.47±0.06 | 1.57±0.06 | 1.37±0.03 | 2.06±0.12 |
| Group 2* | 307.17±4.45 | 0.08±0.01 | 0.60±0.01 | 7.16±0.23 | 17.94±1.42 |
| Group 3 | 64.67±2.84 | 0.39±0.02 | 0.83±0.02 | 2.64±0.05 | 4.33±0.19 |
| Group 4 | 43.76±1.90 | 0.57±0.02 | 0.85±0.03 | 1.77±0.04 | 3.53±0.16 |
| Group 5 | 49.46±1.93 | 0.50±0.02 | 0.86±0.03 | 2.46±0.04 | 4.03±0.13 |
Each group has 6 rats (n=6), The group 2* had higher insulin resistance, hepatic index, atherogenic index and low insulin sensitivity compared to the other groups.
Table 3: Confidence Intervals (CIs) of Serum glucose, insulin, lipid profile and insulin sensitivity indices between group 2 and other rat groups
| Parameter | Group 2 Vs. 1 | Group 2 Vs. 3 | Group 2 Vs. 4 | Group 2 Vs. 5 |
| Glucose | 159.90-177.88 | 116.17-134.14 | 140.76-158.73 | 132.36-150.33 |
| Insulin | 15.23-17.33 | 10.59-12.68 | 11.97-14.07 | 11.62-13.71 |
| TC | 128.32-154.74 | 104.32-130.75 | 107.90-134.32 | 105.95-132.37 |
| TG | 108.50-129.97 | 67.53-89.00 | 79.16-100.63 | 72.06-93.53 |
| HDL-C | -18.42 – -11.75 | -11.35 – -4.69 | -14.06 – -7.39 | -12.11 – -5.44 |
| LDL-C | 119.69-145.86 | 96.82-122.99 | 100.78-126.95 | 98.29-124.46 |
| HOMA-IR | 277.70-302.79 | 229.96-255.04 | 250.86-275.95 | 245.16-270.25 |
| ISI | -1.53 – -1.24 | -0.44 – -0.16 | -0.63 – -0.34 | -0.56 – -0.27 |
| FGIR | -1.14 – -0.80 | -0.40 – -0.06 | -0.42 – -0.08 | -0.43 – -0.09 |
| HI | 5.27-6.29 | 4.01-5.02 | 4.87-5.89 | 4.19-5.20 |
| AI | 12.83-18.92 | 10.56-16.65 | 11.35-17.44 | 10.85-16.94 |
All these CIs indicate significance with a p value of <0.05.
Effect on Body weight
Significantly (p<0.05) decreased body weights (179.17 gm) were observed in the group 2 rats whereas groups 3, 4 (247.83, 256.5 gm) and group 5 (250.00 gm) had increased body weight(Figure 1A).
Effect on Liver Weight and Volume
Of all the groups the group 2 when compared to group 1, 3, 4 & 5 rats had enlarged liver by weight (12.83 vs. 3.55, 6.55, 4.55 & 6.15 gm) and volume (14.52 vs. 2.88, 5.50, 3.60 & 4.50 ml) (Figure 1B).
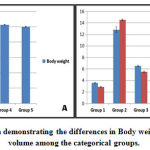 |
Figure 1: Bar diagram demonstrating the differences in Body weight, Liver weight and volume among the categorical groups. |
(A) The diabetic control group 2 rats exhibited significantly reduced body weight when compared to the other group rats (p<0.0001). (B) In comparison to the other group rats the diabetic control group 2 rats had marked hepatomegaly as manifested by increase in both weight and volume of the liver (p<0.0001).
Histopathological Examination
The rats in groups 1 & 5 (Figure 2A, E) did not show any steatosis whereas, the group 2 (Figure 2B) exhibited extensive hepatic macro-vesicular steatosis throughout the lobule. The test groups 3 & 4 showed almost normal liver histology except for focal mild microvesicular steatosis (Figure 2C, D).
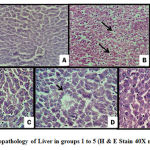 |
Figure 2: Histopathology of Liver in groups 1 to 5 (H & E Stain 40X magnification). |
(A) Normal control (group1) – Normal architecture without steatosis (B) Diabetic control (group2) – partial loss of architecture with extensive macrovesicular steatosis (arrows) (C) Test group (3) – Normal architecture with microvesicular steatosis (arrows) (D) Test group (4) – Normal architecture with focal microvesicular steatosis (arrow) (E) Metformin group (group5) – Normal architecture without steatosis.
Extract Dosage Significance.
Though not statistically significant the groups 3 and 4 exhibited dosage dependent variation in all of the above parameters (data not shown).
Discussion
Globally, the rapid increase in the incidence of type 2 DM poses a demand for the quest of novel therapeutic drugs and necessitates addition of alternative medicine. As a result number of studies has been conducted to assess the utility of herbal medicine in type 2 DM.19 Our study evaluated the antidiabetic, hypolipidemic and possible insulin sensitizing effect of ethanolic extract of C.indica on GC induced IR in Wistar albino rats. Wherein the rats belonging to the diabetic control group due to GC induced IR had impaired glucose and lipid homeostasis. Elevated free fatty acid levels in these rats may suppress cell membrane expression of GLUT-4 transporter thereby, decreases glucose uptake leading to impaired glucose metabolism.20 GC by enhancing hepatic gluconeogenesis and lipogenesis eventually lead to IR state in these rats which was further confirmed by the insulin sensitivity indices.21
The C.indica plant as an antidiabetic drug has been used since ancient times of Ayurveda.22 Earlier studies on C.indica leaf extract reported hypoglycemic activity possibly due to the insulin stimulatory effect.23 But, the mechanism of action is not well understood. Interestingly, the hypoglycemic effect of C.indica leaf in obese hyperglycemic db/db mice, was due to insulin sensitizing action by the triterpenes compound (dehydrotrametenolic acid) and was also due to the activation of peroxisome proliferator activated receptor (PPAR)-ϒ.24 Similarly in our study the ethanolic extract of C.indica leaf demonstrated insulin sensitizing effect that helped in overcoming the IR induced by GC in Wistar albino rats.
Prophylactic oral administration of ethanolic extract of C.indica (groups 3 & 4) prevented the GC induced hyperinsulinemia and hyperglycemia which was comparable to the effect of metformin in these rats (group 5). This distinct insulin sensitizing activity of C.indica extract might be attributed to its effect on glucose transporters whose function was impaired by GC treatment. It is postulated that C.indica extract up-regulates glucose utilization by the peripheral tissues by enhancing the GLUT1 and GLUT4 expression leading to lowered IR.25 Furthermore, it also corrects the glucose-6-phosphatase and lactate dehydrogenase enzymes in glycolytic pathway as well as lipoprotein lipase enzyme in lipolytic pathway. Additionally, it also increases glucose oxidation by stimulating glucose-6-phosphatase.26, 27 All these mechanisms underlie the dose dependent improvement in glucose and insulin levels in IR rats (groups 3 & 4) exerted by C.indica extract.
The rats with GC induced diabetes (group 2) have elevated lipid levels which can be attributed to decreased insulin sensitivity of peripheral tissues especially liver and also due to decreased TG hydrolysis because of diminished lipoprotein lipase activity.28 Prophylactic treatment with C.indica extract for 12 days (groups 3 & 4) prevented the elevation in lipid levels in a dose dependant manner which can be due to the normalization of lipoprotein lipase activity leading to decrease in cholesterol levels.29
Pathologically, the livers of the diabetic control (group 2) rats demonstrated extensive macrovesicular steatosis and degenerative changes in hepatocytes manifesting as hepatomegaly. The suppressed β-oxidation of fatty acids and elevated fatty acid synthesis may underlie the development of hepatic steatosis and increased cholesterol levels in these rats [Peckett AJ Repeated]. The test groups (groups 3 & 4) showed lower lipid levels and only focal microvesicular steatosis indicating protective effect of C.indica extract against hepatic steatosis and hyperlipidemia.30
GC administration was associated with significant (30%) reduction in the body weight in diabetic control (group 2) rats. This is probably due to the catabolic effect of GC on the muscle and bone mass. However, prior treatment with C.indica extract in group 3 & 4 rats maintained the body weight owing to its insulin sensitizing activity which negated the catabolic effects of GC.31
The anti-diabetic activity of ethanolic extract of C.indica is due to the presence of phytochemical compounds which has been explored previously.32, 33 We also demonstrated the rich presence of active therapeutic ingredients like triterpenoids, flavonoids and saponins in the C.indica extract by qualitative phytochemical analysis. These ingredients probably helped in the correction of metabolic abnormalities induced by GC, though the value of individual compound of the extract is not known. Most likely, the triterpene compound of C.indica extract by increasing the PPAR-ϒ expression, promotes insulin sensitization and peripheral glucose utilization.34
To conclude, ethanolic extract of C.indica leaf exhibited significant anti-diabetic, insulin sensitizing and hypolipidemic activity on GC induced IR in Wistar albino rats. Hence, the C.indica leaf extract may play an important role in the management of type2 DM by enhancing the insulin signaling in peripheral tissues through various mechanisms. Further studies are required to explore the underlying mechanisms as well as for the quantitative isolation of specific active compounds of C.indica extract that might potently reduce IR, enhance glucose utilization and correct the IR in type 2 DM and other disorders like metabolic syndrome.
Acknowledgement
We thank Mr. Goparaju Anumolu (Consultant Statistician cum Tutor, Department of community medicine, RVM Institute of Medical Sciences & Research center, Siddipet, Telangana, India) for his statistical help.
Conflict of Interest
There are no conflicts of interest to be disclosed.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Kerner W, Bruckel J. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocr. Diab., 2014; 122: 384-386.
CrossRef - Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, et al. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diab., 2003; 52: 2490-2496.
CrossRef - Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metab., 2011; 60: 1500-1510.
CrossRef - Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. J. Biochem. Mol. Biol., 2015; 154: 94-103. Available from doi: 10.1016/j.jsbmb.2015.07.020.
CrossRef
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing Insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. J. Physiol. Endocrinol. Metab., 2008; 294: 15-26.
CrossRef - Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia., 1985; 28: 412-419.
CrossRef - Vuguin P, Saenger P, Dimartino-Nardi J. Fasting glucose insulin ratio: a useful measure of Insulin resistance in girls with premature adrenarche. Clin. Endocrinol. Metab., 2001; 86: 4618-4621.
CrossRef - Tang W, Zeng L, Yin J, Yao Y, Feng L, Yao X, et al. Hugan Qingzhi exerts anti-inflammatory effects in a rat model of Nonalcoholic Fatty Liver Disease. Based. Complementary. Altern. Med., 2015; 2015: 01-13. Available from https://doi.org/10.1155/2015/810369
CrossRef - Hassan S, El-Twab SA, Hetta M, Mahmoud B. Improvement of lipid profile and antioxidant of hypercholesterolemic albino rats by polysaccharides extracted from the green alga Ulva lactuca Linnaeus. Saudi. J. Sci., 2011; 18: 333-340.
CrossRef - Deokate UA, Khadabadi SS. Pharmacology and phytochemistry of Coccinia indica. J. Pharmacognosy. Phytother., 2011; 3: 155-159.
- Mukherjee K, Ghosh NC, Datta T. Coccinia indica as potential hypoglycaemic agent. Indian. J. Exp.Biol., 1972; 10: 347-349.
- Hossain MZ, Shibib BA, Rahman R. Hypoglycemic effects of Coccinia indica: inhibition of key gluconeogenic enzyme, glucose-6-phosphatase. Indian. J. Biol., 1992; 30: 418-420.
- Shibib BA, Khan LA, Rahman R. Hypoglycaemic activity of Coccinia indica and Momordica charantia in diabetic rats: depression of the hepatic gluconeogenic enzymes glucose-6-phosphatase and fructose-1, 6-bisphosphatase and elevation of both liver and red-cell shunt enzyme glucose-6-phosphate dehydrogenase. Biochem. J., 1993; 292: 267-270.
CrossRef - Yadav RN, Agarwala M. Phytochemical analysis of some medicinal plants. J. Phytol., 2011; 3: 10-14.
CrossRef - Njue L.G, Ombui J.N, Kanja L.W, Gathumbi J.K, Nduhiu.J.G. Evaluation of oral toxicity level of ethyl acetate extract, from garlic (allium sativum) in onorrh dawleys rats as per OECD guidelines 423. J. Food. Sci. Tech., 2015; 2: 056-064.
- Baset MA, Osama MA, Lobna AA, Margit S. Maternal rat diabetes mellitus deleteriously affects insulin sensitivity and beta-cell function in the offspring. Diabetes. Res., 2013. Available from: http://www.dx.doi.org/10.1155/2013/429154.
CrossRef - Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome 1. J. Clin. Endocrinol. Metab., 1998; 83: 2694-2698.
CrossRef - Eissa F, Zidan N. Haematological, biochemical and histopathological alterations induced by Abamectin and Bacillus thuringiensis in male albino rats. ABiol. Hung., 2010; 61: 33-44.
CrossRef - Pang GM, Li FX, Yan Y, Zhang Y, Kong LL, Zhu P, et al. Herbal medicine in the treatment of patients with type 2 diabetes mellitus. Chin. Med. J., 2019; 132: 78-85.
CrossRef - Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes., 2000; 49: 677-683.
CrossRef - Shalam MD, Harish MS, Farhana SA. Prevention of dexamethasone-and fructose-induced insulin resistance in rats by SH-01D, a herbal preparation. Indian. J. Pharmacol., 2006; 38: 419-422.
CrossRef - Yadav G, Mishra A, Tiwari A. Medical properties of ivy gourd (Cephalandra indica): A review. Int. J. Pharmaceu. Res. Dev., 2010; 2: 124-126.
- Dhanabal S, Koate C, Ramanathan M, Elango K, Suresh B. The hypoglycemic activity of Coccinia indica Wight & Arn. and its influence on certain biochemical parameters. Indian. J. Pharmacol., 2004;36: 249-250.
- Sato M, Tai T, Nunoura Y, Yajima Y, Kawahima S. Dehydrotrametenolic acid induces preadipocyte differentiation and sensitizes animal models of non insulin dependent diabetes mellitus to insulin. Biol. Pharm. Bull., 2002; 25: 81–86.
CrossRef - Purintrapiban J, Keawpradub N, Jansakul C. Role of the water extract from Coccinia indica stem on the stimulation of glucose transport in L8 myotubes. Songklanakarin. J. Sci. Technol., 2006; 28: 1199-1208.
- Kohli S, Kumar PN. Combined effect of Coccinia indica leaf extract with acarbose in type II diabetes induced neuropathy in rats. J. Innov. Pharm. Biol. Sci., 2014; 1: 77-87.
- Kamble SM, Kamlakar PL, Vaidya S, Bambole VD. Influence of Coccinia indica on certain enzymes in glycolytic and lipolytic pathway in human diabetes. Indian. J. Med. Sci., 1998; 52: 143-146.
- Appel BU, Fried SK. Effects of insulin and dexamethasone on lipoprotein lipase in human adipose tissue. Am. Physiol. Endocrinol. Metab., 1992; 262:695-699.
CrossRef - Pari L, Venkateswaran S. Protective effect of Coccinia indica on changes in the fatty acid composition in streptozotocin induced diabetic rats. Die Pharmazie- J. Pharm. Sci. Res., 2003; 58: 409-412.
- Manjula S, Ragavan B. Hypoglycemic and Hypolipidemic effect of Coccinia indica Wight & Arn in alloxan induced diabetic rats. Sci. Life., 2007; 27: 34-37.
- Venkateswaran S, Pari L. Effect of Coccinia indica on blood glucose, insulin and key hepatic enzymes in experimental diabetes. Biol., 2002; 40: 165-170.
CrossRef - Teoh SL, Das S. Phytochemicals and their effective role in the treatment of diabetes-mellitus: a short review. Phytochem. Rev., 2018; 17: 1111-1128.
CrossRef - B Gaikwad S, Krishna Mohan G, Sandhya Rani M. Phytochemicals for diabetes management. Crop., 2014; 5: 11-28.
CrossRef - Kuriyan R, Rajendran R, Bantwal G, Kurpad AV. Effect of supplementation of Coccinia cordifolia extract on newly detected diabetic patients. Diabetes. care., 2008; 31: 216-220.
CrossRef







