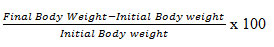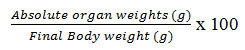Nwogueze Bartholomew Chukwuebuka1*, Ojieh Anthony Emeka2, Ovuakporaye Simon Irikefe2, Wilson Josiah Iju3, Ogbutor Udoji Godsday4 Olowe Gideon Temitope2, Eke Chidinma Nwanneamaka2, Aloamaka Chukwuemeka Peter5
1Department of Human Physiology, College of Health Sciences, Evangel University, Akaeze, Ebonyi State Nigeria.
2Department of Human Physiology, Faculty of Basic Medical Sciences, Delta State University Abraka, Delta State Nigeria.
3Department of Anatomy, Faculty of Basic Medical Sciences, Delta State University, Abraka. Delta State Nigeria.
4Department of Physiotherapy, Federal Medical Centre Asaba, Delta State Nigeria.
5Department of Human Physiology, Nnamdi Azikwe University, Awka, Anambra State Nigeria.
Corresponding Author E-mail: bukasono123@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2038
Abstract
Stress as it relatesto infertility has become a global issue attracting public health concern. The present study examined the morphological changes in ovarian histology in response to oxidative stress-induced in female rats following exposure to different stressors. 92 rats of 12-14weeks old weighing between 120-160g were used for the study. Three (3) different stress models were utilized for stress induction at the rate of 1, 3, and 5hours per day for 1, 2, and 3weeks respectively. At the end of stress induction durations, bodyweights were obtained and the rats were euthanized via cervical dislocation while the ovary weights were carefully isolated and their weights recorded. The harvested ovary was sectioned, mounted on slides, stained, and observed under the microscope for histopathological investigations. Findings from this study established that exposure to restraint mirror or intruder stressor significantly (p<0.05) altered the body and/or ovary weights of the rats irrespective of the rate of exposure when compared to the control group. Cellular degeneration, infiltration, and atretic follicular changes were observed in the ovarian histology of rats in response to stress-inducedchanges caused by exposure to restraint or intruder stressors, whereas, equivalent exposure of the rats to mirror stressor did not result in any observed degenerative changes in the histology of the ovary studied. Our study revealed that exposure to restraint or intruder stressor points towards the existence of stress contributes towardsorgan/body weight changes and cellular damage inthe ovarian tissuespossiblycausing pathogenesis in reproductive capacity of females.
Keywords
Ovarianhistology: Reactive Oxygen Specie; Stress; Stressors; Weights; Wistar Rat
Download this article as:| Copy the following to cite this article: Chukwuebuka N. B, Emeka O. A, Irukefe O. S, Iju W. J, Godsday O. U, Temitope O. G, Nneamaka E. C, Peter A. C. Stress-Induced Morphological Changes of Ovarian Histology in Female Wistar Rats. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Chukwuebuka N. B, Emeka O. A, Irukefe O. S, Iju W. J, Godsday O. U, Temitope O. G, Nneamaka E. C, Peter A. C. Stress-Induced Morphological Changes of Ovarian Histology in Female Wistar Rats. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/38He4rq |
Introduction
The term stress is often used to describe the threatening situation and the anxious response [1]. In the light of the above, Akter,[2] considered stress as unpredictable example of psychological appraisals, physiological responses, and social propensities that happen in reaction to an apparent unevenness between situational requests and the resources expected to adapt to them. Truly, stress has been conceptualized as an event or stimulus, which may be considered a stressor that is imposed upon an individual and may include internal states such as hunger and environmental factors like in the case of natural disaster. Maintaining an effective response to different kinds of stressor is physiologically useful and beneficial for survival [3]. According to stress research exerts, pathology and disease conditions in stressful conditions occur within reproductive ovaries when excessive ROS are generated[4]
Every day, women face multiple stress challenges of various types, intensities, and durations that can act synergistically. This makes ‘‘real life’’ stress (i.e., the combination of stressors an individual is exposed to daily) extremely unable to replicate in clinical or laboratory settings [5]. The biological collaboration among infertility and stress has led to the response of the brain to stimulate the secretion of stress hormones, especially, the hypothalamus-pituitary axis and the female reproductive organs. Stress has been connected with numerous degenerative diseases, maladies, and syndrome disorders [6], thus, interfering with the normal functions of the ovaries [7]. Stress can alter ovarian functions and cause deleterious effects during gestation period on the developing fetus, which often contributes to early onset of stress-induced impairment in the follicular development [8,9] Wu et al., 2011, Ward et al., 2013);
The ovary is a dual female reproductive gonad that periodically produces and releases an ovum (oocytes) needed for fertilization in the mid-point of each menstrual cycle. It is also an endocrine gland that is crucial in enhancing fertility outcomes.[10, 11,12] Stress leads to series of altered physiology such as; steroidogenesis, angiogenesis, maturation, ovulation, blastocysts development, fertilization of ovum, implantation of ovum, and maintenance of pregnancy [13,14,15].Furthermore, different studies have supported the ability of free radical species following increased production of free radicals resulting in oxidative damageand the altered reproductive function ofthe ovary [16,17,18], uterine tubes and embryos [19],ovarian functions, embryonic resorption [15],in addition to stress-induced impairment in the follicular development [20],recurrent pregnancy loss [21], reduction in steroidogenesis [22], irregular estrus cyclicity [23],interference in the potentials of oocytes development [24,25],blockage of ovulation[26].
Pregnancy begins with fertilization of the ovum by the combination of sperm following series of ovarian functions, development of blastocyte, increased secretion of reproductive hormones. Specifically, stress has been proven to contribute to reproductive failures in females causing deleterious effects during the gestation period especially on the developing fetus thereby contributing to early onset of many pathological conditions [27], and to a large extent fetal death [28]. All thepathologiesthat affectsthe female reproductive processes have been traced to stress-induced morphological damage within the ovary, thus capable of been a factor for infertility[23,29].Considering, that findings from the study of Nwogueze et al, [30] which has established that stress induced by exposure to restraint, mirror and intruder stressors alters antioxidant stress markers at the rate of 1, 3, and 5hours per day for 1, 2 and 3weeks, it becomes necessary for thispresent study to examine the impact of stress-induced morphological changes in the ovarian histological in female rats using similar stressors.
Materials and Methods
Study Design
This study is an experimental design and was invasive in nature. The focus of the design is to ascertain Morphological Changes of Ovarian Histology in Female Wistar Rats via comparisons between a control group against experimental groups (rats exposed to three different stress models in time and duration dependent fashion).
Experimental animal and sample size
Ninety two (92) healthy female Wistar rats of 12-14weeks old weighing between 120-160g were procured and used for the study. Animals used for the experiment were obtained from Emma Maria Research & Diagnostic Laboratory, Abraka, Delta State and they were transported in plastic cages to the animal unit of Delta State University, Abraka. The rats were kept in wooden cages and housed in a well-ventilated, 12-12hours light and dark cycles animal house while maintaining a temperature of 22±230C and humidity of 30-40%. The rats were allowed unrestricted access to standard rat chow and normal tap water.
Inclusion and Exclusion Criteria
Not applicable
Randomization
The rats were divided into twenty eight (28) experimental groups with each consisting of four (4) rats each as described below:
Group 1: Served as the normal control rats that received basic feed.
Group 2 – 4: Rats were exposed to restraint stress or at a rate of 1, 3 and 5hours per day for 1week.
Group 5 – 7: Rats were exposed to restraint stress or at a rate of 1, 3 and 5hours per day for 2weeks
Group 8 – 10: Rats were exposed to restraint stress or at a rate of 1, 3 and 5hours per day for 3weeks.
Group 11 -1 3: Rats were exposed to mirrored chamber stress or at a rate of 1, 3 and 5hours per day for 1week
Group 14 – 16: Rats were exposed to mirrored chamber stress or at a rate of 1, 3 and 5hours per day for 2weeks.
Group 17 – 19: Rats were exposed to mirrored chamber stress or at a rate of 1, 3 and 5hours per day for 3weeks.
Group 20 – 22: Rats were exposed to intruder stress or at a rate of 1, 3 and 5hours per day for 1week.
Group 23 – 25: Rats were exposed to intruder stress or at a rate of 1, 3 and 5hours per day for 2weeks.
Group 26 – 28: Rats were exposed to intruder stress or at a rate of 1, 3 and 5hours per day for 3weeks.
Ethical Statement
Formal approval was obtained from the Bioethics Committee on Animal Studies of the Faculty of Basic Medical Sciences, Delta State University, Abraka for experimental protocol, and research guidelines for animal welfare and protection (Permit Number: REC/FBMS/DELSU/19/58).
Experimental Procedures
Weight measurement:
To obtain the percentage (%) change in body weights of the female Wistar rats, the weights of the animals were obtained before the respective experimental procedures and after the end of stress procedure using the electronic weighing balance (G&G-301, England). The mean values were calculated by the formula below:
The ovaries of some of the Wistar rats were carefully isolated after the adhering adipose tissue on the isolated organs was carefully removed. The organs were washed properly using normal saline and sterile paper was used to remove excess fluid and the relative organ weights of the ovaries were recorded and calculated by the formula below:
Induction of stress
The experimental female Wistar rats were induced with three (3) different natures of stress models; restraint stress or, mirror chamber stress or, and paradigm intruder stress or as shown in Figure 1 using the methods described by Nwogueze et al. [31] for a stress time interval of 1, 3, and 5 hours per day for 1, 2, and 3 weeks durations respectively. The restraint stress or was designed to induce physical stress to a greater extent, the cylindrical chamber was constructed to fit the rats allowing only backward and forward movements but limiting lateral. The mirror chamber stress or was designed to induce anxiety stress in the Wistar rats to a larger extent and the wooden chamber was constructed to have three different boxes with each having three mirror walls and an opaque black coloured wall on each compartment. The paradigm intruder stress or was designed to induce psychosocial stress in the animals to a greater extent using aggressive male cat in the same partitioned cage made up wire gauze.
 |
Figure 1: Different Types of Stress Models Used in the Study(A) Restraint stressor (B) Mirror chamber stressor (C) Paradigm intruder stressor |
Histopathological studies
At the end of the experiments in which rats were exposed to different stressors, the rats were euthanized via cervical dislocation and the ovaries from the rats were carefully isolated and washed properly using distilled water. Thereafter, the organs were cut into pieces of about 0.5 cm2 sizes and fixed in 10% formalin for at least 2 days adopting the methods of Bancroft and Gamble,[32].The samples were dehydrated using an automated tissue processor (Leica ASP300, Germany) which contained 12 beakers (10 glass beakers and 2 thermostatically control electric metal beakers) containing paraffin wax (Leica EG1160, Germany) mounted on wooden blocks. Using a microtome (Leica RM2155), the blocks containing the samples were sectioned to a size of 4.5×4.5×4.0μm and then mounted on glass slides using a hot plate (Leica HI1220, Germany).The slides were treated in the sequence of 70%, 90% and 100% ethanol respectively for 2 minutes each and then rinsed in tap water. Using Harris’s haematoxylin and Eosin stain, the sections were stained and then viewed under a light microscope.
Statistical Methods
Values obtained were expressed as Mean Standard Error of Mean (SPSS software version 22 – Chicago, USA) while One-way ANOVA statistic was used to compare means across groups and Fisher’s Least Square Difference (LSD) was used for the Post Hoc Test. For significance level, P-value <0.05 was considered.
Results
Figure 2 below comparatively illustrates the percent change in body weights of rats following exposure to the different stressors for 1 week. The result obtained revealed that there was a decline in body weights of rats exposed at the rate of 1, 3, and 5 hours per day for 1 week when compared to the control levels, irrespective of the stressors applied. Mean comparison revealed that the observed decrease in the body weights was significant (P<0.05) for rats exposed to mirror or intruder stress or at the rate of 1 hour per day.Also, exposing the rats to restraint stress or at the rate of 3 hours and 5 hours per day or the intruder stress or at the rate of 5 hours per day caused a significant (P<0.05) reduction in body weights when compared with control levels. Meanwhile, the percentage reduction in the body weights of rats was severe when exposed to restraint stress or at the rate of 3 hours or 5 hours per day or when exposed to intruder stress or at the rate of 5 hours per day for 1 week.
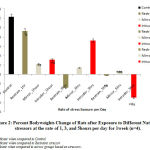 |
Figure 2: Percent Body weights Change of Rats after Exposure to Different Nature of stressors at the rate of 1, 3, and 5 hours per day for 1 week (n=4). |
Figure 3 below comparatively shows the percent change in body weights of rats following exposure to the different stressors for 2 weeks. The result obtained revealed a reduction in the body weights of rats exposed at the rate of 1, 3, and 5 hours per day for 2 weeks when compared to the control levels, irrespective of the stressors applied. Mean comparison revealed that the observed decrease in the body weights was significant (P<0.05) when rats were exposed to mirror or intruder stress or at the rate of 3 hours and 5 hours per day when compared tot he body weights of rats in the control group. The percent loss in body weights was more severe in rats exposed to restraint stress or at the rate of 1 hour and 5 hours per day and intruder stress or at the rate of 5 hours per day for 2 weeks.
 |
Figure 3: Percent Body weights Change of Rats after Exposure to Different Nature of stressors at the rate of 1, 3, and 5 hours per day for 2 weeks (n=4). |
Figure 4 below comparatively represents the percent change in bodyweights of rats following exposure to the different stressors for 3weeks. The results obtained revealed that there was a gradual return of bodyweights beyond the control level in rats exposed to intruder stressor at the rate of 1hour and 3hours per day and also, in rats exposed to mirror stressor at the rate of 5hours per day when compared with the control bodyweights. Mean comparison revealed that the observed increase in the bodyweights was significant (P<0.05) in rats exposed to mirror stressor at the rate of 5hours per day while there was a significant (p<0.05) decrease in rats exposed to intruder stressor at the rate of 5hours per day when compared to control levels.
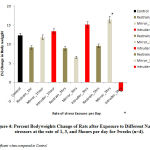 |
Figure 4: Percent Body weights Change of Rats after Exposure to Different Nature of stressors at the rate of 1, 3, and 5 hours per day for 3 weeks (n=4). |
Figure 5 below comparatively represents the absolute change in ovary weights of rats following exposure to the different nature stressors for 1 week. The results obtained revealed that the weights of the ovary were significantly (p<0.05) decreased in rats exposed to intruder stress or at the rate of 1 hour and 5 hours per day for 1 week when compared to the control level. Meanwhile, there was weight gain in rats exposed to the mirror or restraint stress or irrespective of the rate of exposure. Although, the increased observed was significant (p<0.05) when compared to control.
 |
Figure 5: Change in AbsoluteOvary Weight of Rats after Exposure to Different Nature of stressors at the rate of 1, 3, and 5hours per day for 1week (n=4). |
Figure 6 below comparatively illustrates the absolute change in ovary weights of rats following exposure to the different nature stressors for 2 weeks. The results obtained shows that the weight of the ovary was decreased in rats exposed to intruder stress or at the rate of 5 hours per day for 2 weeks when compared to the control level. Meanwhile, there was weight gain in the other experimental groups exposed to the different nature of stressors irrespective of the rate of exposure, although, the observed increase was not statistically significant.
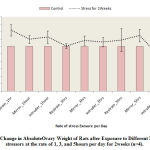 |
Figure 6: Change in Absolute Ovary Weight of Rats after Exposure to Different Nature of stressors at the rate of 1, 3, and 5 hours per day for 2 weeks (n=4). |
Figure 7 below comparatively illustrates the absolute change in ovary weights of rats following exposure to the different nature stressors for 3 weeks. The results obtained revealed that there was reduction in weights of the ovary of rats exposed to restraint and intruder stressors at the rate of 1 hour day and also the ovary was significantly (p<0.05) decreased in rats exposed to restraint stress or at the rate of 3 hours per day when compared to the control level. Meanwhile, there was a significant (p<0.05) gain in body weights of rats exposed to mirror stress or at the rate of 1 hour and 3 hours per day. Also, there was a significant (p<0.05) increase in the ovary of rats exposed to intruder stress or at the rate of 3 hours per day.
 |
Figure 7: Change in AbsoluteOvary Weight of Rats after Exposure to Different Nature of stressors at the rate of 1, 3, and 5hours per day for 3weeks (n=4).
|
Figure 8 below illustrates the photo micrograph of control rat and the experimental rats exposed to restraint stressor at the rate of 1, 3, and 5 hours per day for 1 week. It was observed that the ovary photo micrograph of control rat as well as rats exposed to restraint stressors at the rate of 1 hour and 3 hours respectively revealed normal histological architecture of the ovary with Follicular antrium (FA), Primary Oocyte (O), Theca follicli (blue arrow), corona radiata (green arrow) and Zona granulosa (ZG) well defined while the ovary of rats exposed to restraint stressor at the rate of 5hours per day showed cellular degeneration of follicular antrium, primary oocyte, theca follicli, corona radiata and zona granulosa.
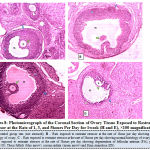 |
Figure 8: Photo micrograph of the Coronal Section of Ovary Tissue Exposed to Restraint Stress or at the Rate of 1, 3, and 5 hours Per Day for 1 week (H and E), ×100 magnification. |
Figure 9 illustrates the photo micrograph of control rat and the experimental rats exposed to mirror stress or at the rate of 1, 3, and 5 hours per day for 1 week. The results obtained revealed that the ovary of control rat as well as rats exposed mirror stressors at the rate of 1 hour, 3 hours, and 5 hours per day revealed normal ovary histology with features of primary and secondary follicles showing various stages of maturation, follicular antrium, primary oocyte, theca follicli, corona radiata and zona granulosa.
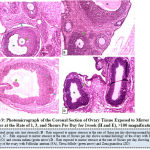 |
Figure 9: Photo micrograph of the Coronal Section of Ovary Tissue Exposed to Mirror Stress or at the Rate of 1, 3, and 5 hours Per Day for 1week (H and E), ×100 magnification. |
Figure 10 below represents the photomicrograph of control rat and the experimental rats exposed to intruder stressor at the rate of 1, 3, and 5hours per day for 1week. The results revealed that the control rat shows normal histological architecture of the ovary when compared to ovary of rats exposed to intruder stressor at the rate of 1hour per day with the zona granulosa showing areas of cellular degeneration. Meanwhile, the ovary of rats exposed the same stressor at the rate of 3hours per day shows degeneration of follicular antrium, primary oocyte, theca follicli, corona radiata and zona granulosa while rats exposed at the rate of 5hours per day presents absence of primary oocyte and noticeable features of atretic follicles, hemorrhagic and infiltration of cells.
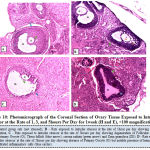 |
Figure 10: Photomicrograph of the Coronal Section of Ovary Tissue Exposed to Intruder Stressor at the Rate of 1, 3, and 5hours Per Day for 1week (H and E), ×100 magnification. |
Figure 11 below showed the photo micrograph of control group and the experimental rats exposed to restraint stressor at the rate of 1, 3, and 5 hours per day for 2 weeks. The results obtained revealed that the ovary histology of control rat as well as rats exposed to restraint stress or at a rate of 1 hour, presents both the primary and secondary follicles at various stages of maturation, with normal histological architecture of the ovary. Rats exposed to similar stressor at the rate of 3hours per day showed absence of primary oocytes and degeneration of zona granulosa while rats exposed at the rate of 5hours per day showed congestions of blood vessels, absence of the primary oocytes and features of atretic follicles.
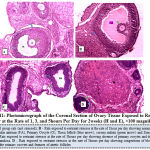 |
Figure 11: Photo micrograph of the Coronal Section of Ovary Tissue Exposed to Restraint Stress or at the Rate of 1, 3, and 5 hours Per Day for 2 weeks (H and E), ×100 magnification. |
Figure 12 below represents the photo micrograph of control rats and the experimental rats exposed to mirror stress or at the rate of 1, 3, and 5 hours per day for 2 weeks. The results obtained showed that the ovary of control rat as well as rats exposed to mirror stressors at the rate of 1 hour, 3 hours, and 5 hours per day presents normal histological architecture of the ovary with follicular antrium, primary oocyte, theca follicli, corona radiata and zona granulosa.
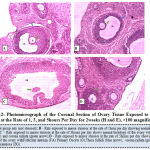 |
Figure 12: Photo micrograph of the Coronal Section of Ovary Tissue Exposed to Mirror Stress or at the Rate of 1, 3, and 5 hours Per Day for 2 weeks (H and E), ×100 magnification. |
Figure 13 below represents the photo micrograph of control rats and the experimental rats exposed to intruder stress or at the rate of 1, 3, and 5 hours per day for 2 weeks. The results revealed that the control rat and rats exposed to intruder stress or at the rate of 1hour per day shows normal histological architecture of the ovary when compared to ovary of rats exposed to 3 hours and 5hours per day showed infiltrated follicular antrium, primary oocyte, theca follicli, corona radiata and zona granulosa.
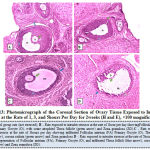 |
Figure 13: Photo micrograph of the Coronal Section of Ovary Tissue Exposed to Intruder Stress or at the Rate of 1, 3, and 5 hours Per Day for 2 weeks (H and E), ×100 magnification. |
Figure 14 below represents the photo micrograph of control rat and the experimental rats exposed to restraint stress or at the rate of 1, 3, and 5hours per day for 3weeks. The results revealed that the control rats had normal histological architecture of the ovary when compared to ovary of rats exposed to restraint stress or at the rate of 1 hour per day that showed some features of hemorrhagic, degenerated, atretic and infiltrated follicles. Rats exposed to restraint stressor at the rate of 3hours per day showed presence of nuclear in the primary oocyte and cellular degeneration of the zona granulosa while rats exposed to similar stress or at the rate of 5 hours per day revealed absence of primary oocyte, features of hemorrhagic and infiltrated inflammatory cells.
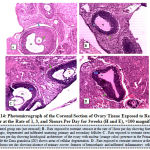 |
Figure 14: Photo micrograph of the Coronal Section of Ovary Tissue Exposed to Restraint Stress or at the Rate of 1, 3, and 5 hours Per Day for 3 weeks (H and E), ×100 magnification. |
Figure 15 below represents the photo micrograph of control rat and the experimental rats exposed to mirror stressor at the rate of 1, 3, and 5 hours per day for 3 weeks. The result obtained revealed that the ovary of control rats as well as experimental rats exposed to mirror stressors at the rate of 1 hour, 3 hours, and 5 hours per had normal histological architecture of the ovary showing follicular antrium, primary oocyte, theca follicli, corona radiata and zona granulosa with presence of both the primary and secondary follicles.
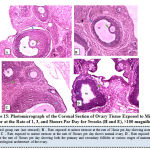 |
Figure 15: Photomicrograph of the Coronal Section of Ovary Tissue Exposed to Mirror Stressor at the Rate of 1, 3, and 5hours Per Day for 3weeks (H and E), ×100 magnification. |
Figure 16 below represents the photomicrograph of control rats and the experimental rats exposed to intruder stressor at the rate of 1, 3, and 5hours per day for 3weeks. The results revealed that the control rats and experimental rats exposed to intruder stressor at the rate of 1hour per day shows normal histological architecture of the ovary when compared to ovary of rats exposed to similar stressor at the rate of 3hours per day which showed features of remains of degenerated primary oocyte, corona radiata and Zona granulosa (ZG) and irregular zona pellucida. Rats exposed to intruder stressor at the rate of 5hours per day showed absence of primary oocyte and features of degenerated, hemorrhagic and infiltrated cells.
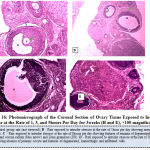 |
Figure 16: Photomicrograph of the Coronal Section of Ovary Tissue Exposed to Intruder Stress or at the Rate of 1, 3, and 5 hours Per Day for 3 weeks (H and E), ×100 magnification. |
Discussion
Stress is an unavoidable part of our daily life that can change the immunological, neurochemical, and endocrine elements of the body. Oxidative stress has been identified as a causative factor in problems relating to maternal pregnancy[33] and stress-induced morphological changes in the ovary of rat[30]. Excessive ROS productions are thought to be critical in the etiology of infertility, thus, Tremellen,[34] identified the prevalence to be responsible for 30% to 80% of infertility issues. Stress-induced changes possibly arise from several endogenous and exogenous factors or interactions between each. The imbalance between free radical species and antioxidant system scavenging potentials is critical in understanding the physiologic and pathologic process involved altered reproductive capacity of females.
Findings from this present study have revealed that there was a significant (p<0.05) decrease in percentage change in the bodyweights of rats following exposure to different stress models at the rate of 1hour, 3hours, and 5hours per day for 1 and 2weeks respectively. This finding agrees with the reports by Anil et al. [35] who revealed that exposure of mice to mirror chamber stressor caused stress-induced bodyweights loss, and inhibited locomotion activity. In the present study, the bodyweights of Wistar rats exposed to restraint stressor, mirror chamber stressor, and paradigm intruder stressor were marked affected and it was observed that the responses of the rats on weekly basis varied depending on the stressor applied, suggesting that responses of rats to exposure to stressors depends, at least to some degree on the extent of stress-induced in the respective rats. According to Rai et al.[36]exposure to stress can initiate various pathways capable of releasing free radicals which cause cellular damages and loss in the bodyweights in rats.
Findings from our study observed that there was a gradual return of the bodyweights to almost the control levels in rats exposed to restraint and mirror stressors for up to 3weeks irrespective of the rate of exposure. However, the rats exposed to the intruder stressor showed a progressive decrease in the bodyweights and this was significant (p<0.05) when exposed to a rate of 5hours per day. In support of the observation made in our experiments on bodyweights changes, similar reports were made by Berton et al.[37] and Razzoli et al.[38] that exposure of rats to intruder stressor decreased the bodyweights of the experimental animal and interfered with food intake levels, while increased anxiety and hyperlocomotion in Lewis rats. Moreover, therecovery in the bodyweights to almost the basal levels agrees as observed in this study can be connected to the explanations of Nagaraja and Jeganathan, [39] who suggested that such changes could be due to adaptation or habituation in the animal following continued exposure to the stressor.
Findings from this study showed a significant (p<0.05) decrease in relative ovary weights in rats exposed to the different nature of stressor at the rate of 1hour, 3hours, and 5hours per day for 1, 2 and 3weeks respectively. Nayanatara et al.[40] and Liu et al.[41] separately also reported variable effect of exposure of rats to stressors on organ weights. While the former reported that the weights of the uterus and ovary were decreased, the later reported increased kidney, liver, and brain weights. This was supported by the findings of Nancy et al.[42] who asserted that the hallmark of stress response include decreased total body weights or altered organ weights since stress perceived by the brain results in adaptive responses in other organ systems via the neuroendocrine pathways. The authors maintain that the magnitude of the organ response is commensurate with the magnitude of the stress. Based on available data from this study, it is not possible to provide precise mechanisms for the variable responses of internal organs following exposure of rats to a particular stressor.
Histopathological changes of the ovary revealed that rats exposed to restraint and paradigm intruder stressor at the rate of exposure 1, 3, and 5hours per day for 1week showed cellular degeneration of zona granulose, presence of hemorrhagic and infiltrated inflammatory cells, infiltrated follicular antruim, reduction in the number of ovarian follicles while no histopathological alterations were noticed in the ovaries of rats exposed to mirror stressor. Such alterations in ovarian tissues agree with the assertion of Bo et al.[43] who suggested that oxidative stress activates the HPA-axis to impair oocyte via an indirect mechanism leading to apoptosis of follicular cells, however,Agarwal et al.[44] reported that in the event of oxidative stress, corona radiata and zona pellucida are disrupted while granulosa cells have greatly degenerated in the process of cellular autophagy leading to cell death.
Subsequently, the findings of this study showed that the ovary of rats exposed to restraint stressor at the rate of 1hour per day for 2weeks revealed the presence of hemorrhagic, degenerated and infiltrated maturing follicles, while for rats exposed at the rate of 3hours per day to the same stressor, there was noticeable cellular degeneration of zona granulose and for rats exposed to a similar stressor at the rate of 5hours day showed absence of primary oocyte, alongside the presence of hemorrhagic and infiltrated cells. This finding validates the reports of Iwa et al.[45] Our result also revealed similar degenerative changes in the ovaries from rats exposed to intruder stressors, but no histopathological changes were observed in the ovary of rats exposed to mirror stressor. This is consistent with the report of Devine et al.[46] that stress causes infiltration and degeneration of granulosa cells.
The histology of ovary of rats exposed to restraint and intruder stressors for 3weeks irrespective of the rate of exposure showed evidence of degenerated primary oocytes, corona radiate, and zona granulose especially with exposure of rats at the rate of 3hours per day showing features of atretic follicles. Furthermore, rats exposed to similar stressor at the rate of 5hours per day showed absence of primary oocytes, hemorrhagic and infiltrated inflammatory cells.Ovarian tissues of rats exposed to mirror stressor showed normal morphology. This finding was similar to the observation of Khalaf et al. [47] who noted presence ofthin and irregular zona pellucida, degenerated cells, infiltrated macrophages, and atretic follicles in the ovarian tissues caused possibly by oxidative stress-induced by haloperidol and clozapine drug. Although, our study differs from theirs as the oxidative stress was induced by exposure to restraint, mirrored or intruder stressor.
Our study has some obvious limitations. First, it was a novel stress research which deferred by the comparative assessment of three different stress models utilized for stress induction at varying rate (time) of exposure and duration (weeks) intervals on the morphology of ovarian histology. Secondly, we did not address the aspect relating to the impact of these stressors on the hormonal profiles and reproductive outcome of the female rats. Notwithstanding, the observed ovarian changes following exposure to restraint or intruder stress or have a synergy tothe findings of Nwogueze et al. [31, 48]which reported elevationin serum corticoster one and altered oxidative stress markers. Hence, this could be responsible for such alterationsin the morphologyof the ovary histology as basis for estimating stress related infertility.
Conclusion
Changes in the body and ovary organ weights and ovarian histology in female rats was influenced by exposure to different stressors with variations in intensities. Findings from this study have shown that exposure to restraint or intruder stressor proved to have more severe stress-induced morphological impact on the ovarian histology and the loss in organ/or body weights of the female Wistar rats. In all, exposure of the rats for up to 3weeks caused cellular degeneration in the ovarian histology evaluated;however, equivalent exposure of the rats to mirrored stressor did not cause any observable degenerative changes in the ovary. Therefore, further study is required to understand the mechanistic relationship behind the altered ovarian histoarchitecture of rats exposed to intruder or restraint stressor and reproductive success in female rats.
Acknowledgement
We would like to acknowledge the contributions and technical support of Emma Maria Laboratory, Abraka. Also, we would like to acknowledge and gracefully appreciate all individuals and organization that had an input for this study, most especially the supervisors of this research and Kpankpan Mary.
Conflict of Interests
The authors declare that they have no competing interests.
Funding Source
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- Merwe, E. Infertility-Related Stress and Specific Aspects of the Marital Relationship, University of Stellenbosch, Master Thesis, in press, 2010; 9-13.
- Akter, B. Stress in Marital Disruption as a Function of Education Level in the Socioeconomic and Cultural Context of Bangladesh. Journal of the Indian Academy of Applied Psychology,2008; 34(1): 41-46.
- Davis, M., Eshelman, E.R. and Mc Kay, M. The Relaxation & Stress Reduction Workbook. (6th ed). New Harbinger Puplications, Inc.2008.
- Evans MD, Dizdaroglu M, Cooke MS.Oxidative DNA damage and disease: induction, repair and significance.Mutat Res.; 2004; 567(1):1-61.
CrossRef - Fink, G. Stress: Concepts, Definition and History: Reference Module in Neuroscience and Biobehavioral Psychology, 2017; 1–9
CrossRef - Joseph, G., Schenker, D.M. and Eran, S. Stress and human reproduction. European Journal of Obstetrics & Gynaecology and Reproductive Biology, 1991; 45: 1-8.
CrossRef - Davies, KJ. Oxidative stress: the paradox of aerobic life. Biochemical Society Symposium 1995; 61: 1–31.
CrossRef - Bhat, M.S. and Yajurvedi, H.N. Stress induced alterations in pre-pupertal ovarian follicular development in rat. Stress Physiol and Biochem, 2011;7: 51-68.
- Wu, L.M., Liu, Y.S., Tong, X.H., Shen, N., Jin, R.T., Han, H., Hu, M.H., Wang, W. and Zhou, G. Inhibition of Follicular Development Induced by Chronic Unpredictable Stress Is Associated with Growth and Differentiation Factor 9 and Gonadotropin in Mice. Biology 521 of Reproduction, 2011; 86(121): 1–7.
CrossRef - Joseph, G., Schenker, D.M. and Eran, S. Stress and human reproduction. European Journal of Obstetrics & Gynaecology and Reproductive Biology, 1991; 45: 1-8.
CrossRef - Colvin, CW and Abdullatif, H. “Anatomy of female puberty: The clinical relevance of developmental changes in the reproductive system”. Clinical Anatomy. 2013; 26(1): 115–129
CrossRef - Eunice, KS. “What are some signs of pregnancy?”National Institute of Child Health and Human Development, 2013; 2(1): 1-6
- Lu, J., Wang, Z. and Cao, J.A. Novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol2018; 16:
CrossRef
- Ishikawa M. Oxygen radicals-superoxide dismutase system and reproduction medicine. Nihon Sanka Fujinka Gakkai Zasshi.1993; 45:842–848.
- Agarwal A. Role of oxidative stress in endometriosis. Reprod BioMed Online,2006; 13:126–134.
CrossRef - Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J Soc Gynecol Investig.2001; 8: 40–42
CrossRef - Sabatini L, Wilson C, Lower A, Al-Shawaf T, Grudzinskas JG. Superoxide dismutase activity in human follicular fluid after controlled ovarian hyperstimulation in women undergoing in vitro fertilization. Fertil Steril.1999;72:1027–1034.
CrossRef - Suzuki T, Sugino N, Fukaya T, Sugiyama S, Uda T, Takaya R, Yajima A, Sasano H. Superoxide dismutase in normal cycling human ovaries: immunohistochemical localization and characterization. Fertil Steril. 1999;72:720–726
CrossRef - El-Mouatassim S, Guérin P, Ménézo Y.Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol Hum Reprod.1999; 5(8):720-5.
CrossRef - Bhat, M.S. and Yajurvedi, H.N. Stress induced alterations in pre-pupertal ovarian follicular development in rat. Stress Physiol and Biochem 2011; 7: 51-68.
- Wu, L.M., Liu, Y.S., Tong, X.H., Shen, N., Jin, R.T., Han, H. et al. Inhibition of Follicular Development Induced by Chronic Unpredictable Stress Is Associated with Growth and Differentiation Factor 9 and Gonadotropin in Mice. Biology of Reproduction, 2011; 86(121): 1–7.
CrossRef - Whirledge, S. and Cidlowski, J.A. Glucocorticoid, stress, and fertility. Minerva Endocrinologica 2010; 35:109-125.
- Saraswathi, C.D, Sreemantula, S. and Prakash, W.S. Effect of chronic cold restraint and immobilization stress on estrous cycle in rats. Pharmacol Online 2010; 2:151-160.
- Zhang, S.Y., Wang, J.Z., Li, J.J., Wei, D.L., Sui, H.S., Zhang, Z.H. et al. Maternal restraint stress diminishes the developmental potential of oocytes. Biology of Reproduction 2011; 84: 672-681.
CrossRef - Gao, Y., Chen, F., Kong, Q.Q., Ning, S.F., Yuan, H.J., Lian, H.Y. et al. Stresses on female mice impair oocyte developmental potential: Effects of stress severity and duration on oocytes at the growing follicle stage. Reproductive sciences 2016; 23(9):1148-1157.
CrossRef - Spiers, J.G., Chen, H.C, Sernia, C. and Lavidis, N.A. Activation of the hypothalamic-pituitary adrenal stress axis induces cellular oxidative stress. Frontiers in Neuroscience. 2015; 8:
CrossRef - Ward, I.D., Zucchi, F.C., Robbins, J.C., Falkenberg, E.A., Olson, D.M. and Benzies, K. Transgenerational programming of maternal behaviour by prenatal stress. BMC Pregnancy Childbirth. BioMed Central Ltd. 2013; 13:9.
CrossRef - Gupta, S., Ghulmiyyah, J., Sharma, R., Halabi, J. and Agarwal, A.Power of proteomics in linking oxidative stress and female infertility. Biomed Res Int: 2014; 916212.
CrossRef - Lian, H.Y., Gao, Y., Jiao, G.Z., Sun, M.J., Wu, X.F., Wang, T.Y. et al. Antioxidant supplementation overcomes the deleterious effects of maternal restraint stress-induced oxidative stress on mouse oocytes. Reproduction 2013; 146: 559-568.
CrossRef - Nwogueze B.C, Ojieh, A.E, Aloamaka C.P, Igweh J.C and Onyesom, I. Changes In Antioxidant Enzymes Activities And Lipid Peroxidase Levels In Tissues Of Stress-Induced Wistar Rats: Iranian Journal of Toxicology; 2020 (In Press)
- Nwogueze B.C, Ojieh, A.E, Aloamaka C.P, Igweh J.C and Onyesom, I. Organ and Body Weights Changes in Female Wistar rats exposed to different stressors: Nigeria Journal of Science and Environment; 2020, 18(1): 135-144
- Bancroft,D. and Gamble, M.R. Theory and practice of histological techniques. 5th Ed. Edinburgh. Churchill Livingstone Pub. 2002; 593-620.
- Agarwal, A., Aponte-Mellado, A., Premkumar, B.J., Shaman, A. and Gupta, S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 2012; 10: 49.
CrossRef - Tremellen, K. Oxidative stress and male infertility – a clinical perspective. Reprod. Update 2008; 114: 243-258.
CrossRef - Anil, K., Gurleen, K. and Puneet, R. Buspirone along with melatonin attenuates oxidative damage and anxiety-like behavior in a mouse model of immobilization stress.Chinese Journal of Natural Medicines, 2014; 12(8): 582-589.
CrossRef - Rai D, Bhatia G, Sen T, Palit G. Anti-stress effects of ginkgo biloba and panax ginseng: a comparative study. Pharmacol. Sci., 2003; 93(4): 458 -464
CrossRef - Berton, O., Agucore, S., Sarrieau, A., Mormede, P. and Chaouloff, F. Differential effects on social stress in central serotonergic activity and emotional reactivity in Lems and spontaneously hypertensive rats. Neuroscience 1998; 82:147-159.
CrossRef - Razzoli, M., Carboni, L. and Arban, R. Alterations of behaviour and endocrinological activity induced by 3 brief defeats in rats: relevance of human psychopathology. Psychoneuroendocrinology, 2009; 34: 1405-1416.
CrossRef - Nagaraja, H. and Jeganathan, P. Forced swimming stress-induced changes in the physiological and biochemical parameters in Albino Wistar rats. Indian Journal of Physiology and Pharmacology, 1999; 43(1): 53-59.
- Nayanatara, A.K., Nagaraja, H.S. and Anupama, B.K. The effect of repeated swimming stress on organ weights and lipid peroxidation in rats. Thai Journal of Physiological Sciences 2005; 18:3-9.
- Liu, G.H., Dong, Y.L., Wang, Z.X., Cao, J. and Chen, Y.X. Restraint stress alters immune parameters and induces oxidative stress in the mouse uterus during embryo implantation stress. The International Journal of Stress 2014;17: 494-503.
CrossRef - Nancy, E.E., Paul, W.S., Keith, L.B., Brad, B., Dianne, M.C., George, L.F. et al. Interpreting stress responses during routine toxicity studies: A review of Biology, Impact and Assessment. Toxicological Pathology; 2013; 41(4), 560-614.
CrossRef - Bo, L, De-Li W, Ya-Nan C, Hong-Jie Y, Juan L, Xiang-Zhong C Restraint Stress Impairs Oocyte Developmental Potential in Mice: Role of CRH-Induced Apoptosis of Ovarian Cells, Biology of Reproduction, 2013; 89(3): 64, 1–12
CrossRef - Agarwal, A., Gupta, S. and Sharma, R.K. Role of oxidative stress in female reproduction. Reproductive biology and endocrinology: RB&E, 2005; 3:28
CrossRef - Iwa M, Nakade Y, Pappas TN, Takahshi T. Electro-acupuncture improves restraint stress-induced delay of gastric emptying via central glutaminergic pathways in conscious rats. Lett. 2006; 399(1-2): 6-10
CrossRef - Devine, P.J., Perreault, S.D. and Luderer, U. “Roles of reactive oxygen species and antioxidants in ovarian toxicity,” Biology of Reproduction, 2012; 86(2): 27
CrossRef - Khalaf, H.A., Elmorsy, E., Mahmoud, E.M. The role of oxidative stress in ovarian toxicity induced by haloperidol and clozapine a histological and biochemical study in albino rats. Cell Tissue Res2019; 378: 371–383.
CrossRef - Nwogueze BC, Aloamaka CP, Igweh JC.Serum Corticosterone Levels Following Exposure to Stressors of Varying Nature and Stress-Induced Withdrawal in Rats: W J Biomed Res,2020; 7(1):55-62.