Swathi Putta1*, Kotaiah Silakabattini1 and Jagadeesh Kumar T2
1A.U. College of Pharmaceutical Sciences, Andhra University, Visakhapatnam, Andhra Pradesh, India
2Santhi Healthcare India Pvt Ltd., Medchal, Hyderabad, Telangana, India
Corresponding Author E-mail: swathidbmp@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2050
Abstract
The objective of the study is to evaluate the ethanolic leaf extract of Tylophora indica (ELTI) on pancreatic and hepatic oxidative stress in streptozotocin (STZ) induced diabetic rats. The serum blood glucose and liver enzymes (AST, ALT and ALP) were estimated in all the groups. The elevated blood glucose levels and liver enzymes were found to be decreased with ELTI in STZ induced diabetic rats. The activities of antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-S- transferase (GST) and the levels of reduced glutathione (GSH) were also decreased, while an increase in the levels of thiobarbituric acid reactive substances (TBARS) were observed in pancreas and liver with ELTI treatment in STZ induced diabetic rats. Histopathology reveals that the protective effect of ELTI over STZ induced oxidative damage in both liver and pancreas. These results indicated that ELTI has more potential antioxidant effects on diabetic-induced oxidative stress.
Keywords
Oxidative Stress: Streptozotocin; Tylophora Indica
Download this article as:| Copy the following to cite this article: Putta S, Silakabattini K, Kumar T. J. Protective Effect of Tylophora Indica Against Streptozotocin Induced Pancreatic and Liver Dysfunction in Wistar Rats. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Putta S, Silakabattini K, Kumar T. J. Protective Effect of Tylophora Indica Against Streptozotocin Induced Pancreatic and Liver Dysfunction in Wistar Rats. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/3s8EICv |
Introduction
Chronic hyperglycemia is a key feature of diabetes mellitus resulted to lack of insulin secretion and / or production. An estimated sufferer of 346 million diabetics worldwide and the number was found to increase two fold during 2015 to 2030. The incidence and death rate of diabetes was associated with poor glycemic index over long period.1 The human body undergoes many physiological and biochemical process respective to the function. Chronic metabolic syndrome such as diabetes mellitus generates number of free radicals like superoxide, hydroxyl, hydrogen peroxide and nitrite etc. Free radicals are generated as products of metabolism even directly through the environmental stimuli. 2 However in adverse situation or disease condition large effluxes of oxygen and nitrogen free radicals are produced. Oxidative stress results from imbalance between radical generating and radical scavenging systems that is increased free radical production or reduced activity of antioxidant defenses or both these phenomenon. 3 Various non enzymatic and natural antioxidants such as ascorbic acid, glutathione, calciferol, anthocyanins, carotenoids and phenolic compounds etc., were are also acts as free radical scavengers. 4
The diabetes induced oxidative stress leads to protein oxidation and protein glycation, which lead to lipid peroxidation. 5 Lipid peroxidation is resulting from reactive oxygen species and disturbances in antioxidant defense system has been encountered in the diabetic patients. Severe hyperglycemia was showed altered enzyme levels, impaired glutathione metabolism and decreased ascorbic acid levels. 6 Therapy with antidiabetic agents have generally been shown to decreased levels of serum biomarkers of pancreatic and hepatic injury, however some these agents have shown to produce serious side effects are insulin and insulin analogues, biguanides, sulphonylureas, thiazolidinediones, gliptines and incretins etc. Now a day, people relay on natural remedies to avoid adverse effects of allopathic medicine, thereby increased demand for the consumption of natural antioxidants which are rich in fruits and vegetables. Many phytoconstituents are already reported for their potential health benefits due to safer, effective alternatives and availability at low cost even their biological active compounds is unknown.
Complementary and alternative therapies using herbal drugs are gaining interest due to possibility of better glycemic control and developing insulin sensitivity. 7 Since, extensive studies are required for herbal drug usage to establish mechanism of action, safety profile, dosage adjustments in diabetic patients and phytochemical responsible for antidiabetic action.
Alkaloids reported for antidiabetic activity might be due to multifaceted targeted action such as berberine, catharanthine, vindoline, aegelin, cryptolepine, harmane, jambosine etc. 8
Tylophora indica, belongs to of the family Asclepiadaceae has been used widely in traditional medicine for cough, dysentery and asthma. The leaves of Tylophora indica were reported for alkaloids such as tylophorine and tylophorinine, tannins and flvaniods majorly. 9 Tylophora indica been reported to posses antiasthamatic, immunomodulatory, anticancer and antitumor and diuretic activities. 10,11 The leaves of T. indica are reported to have antioxidant activity and antihyperglycemic and antihyperlipidemic activity.12 Hence, this study was designed to evaluate the ameliorator effect of T.indica on pancreatic and hepatic oxidative stress in diabetes rats induced by Streptozotocin.
Materials and Methods
Tylophora indica ethanolic leaf extract (batch no: 6979) obtained from the AMSAR private Ltd., Indore, Madhya Pradesh. Streptozotocin obtained from the Sigma Aldrich, USA. All other chemicals and kits were obtained from the laboratory scale and analytical grade.
Experimental animals
Albino Wistar rats weighing 150 to 200gm were obtained from the central animal house facility (743/abc) of PRIST University, Tanjavuru. Animals are maintained with chow diet (pellet diet) and water ad libitum. They were placed in cages at 22 to 240C and under 12hr light/dark cycle. The present study was approved by institutional animal ethics committee PRIST/IAEC/M. Pharm/PH.COLOGY/05/2009-2010.
Diabetes induction
All the rats are acclimatized for a period of 2 weeks and were fasted before 18hr prior to experiment. Diabetes was induced with streptozotocin and nicotinamide combined model. Diabetes was induced with nicotinamide at the dose of 100mg/kg, i.p before to STZ induction. STZ at 55mg/kg., i.p was injected after nicotinamide induction. Blood was withdrawn from retro-orbital puncture after 72 hrs of STZ induction. The rats with blood glucose levels over 250mg/dL were only included in this study.
Acute toxicity studies
Healthy adult albino mice of either sex, starved overnight were divided into five groups (n=6). They were orally fed with the ELTI extract in increasing dose levels of 5, 50, 300, 2000 mg/kg b.wt. The animals were observed continuously for 72 h for any signs of behavioral, neurological and autonomic profile, toxicity and mortality. The 1/10th and 1/20th dose of safe dose level 2000mg/kg were considered in this study.
Experimental Procedures
Once after induction of diabetes, total 30 rats were divided into 6 groups of each 6 rats. Group 1 treated as normal control, group 2 served as diabetic control, group 3 with standard gliclazide (1mg/kg), group 4 and 5 treated with ELTI 200mg/kg and 400mg/kg bd.wt respectively for 6 weeks. All the treatments were given once daily singly dose using oral route administration.
Serum biochemical parameters
The animals were kept under overnight fasting at the end of 6 weeks study period. Blood was collected using retro orbital plexus and serum was separated at 4000rpm for 10 min for the estimation of serum biochemical parameters. The fasting blood glucose levels were estimated at o day, 2nd, 4th and 6th week of study using GOD POD method as described by Trinder et al., 1969 13 , serum glycosylated haemoglobin, SGOT, SGPT and ALP were estimated according to the procedures recommended in commercially available kits (Span diagnostics Ltd, India)
Enzyme estimations in pancreas and liver
After the estimation of serum analysis, all the animals were dissected using mild pentobarbitol sodium 30mg/kg i.p. the pancreas and liver tissue were separated and stored in -200c until further tissue analysis. Antioxidant enzymes such as Superoxide dismutase using NBT/riboflavin method described by Kakkar et al., 198414; catalase was based on H2O2 oxidation the method as prescribed Sinha et al., 1972 15 ; Glutathione peroxidase estimation was followed by Rostruck et al., 1984 16 ; Glutathione -S- Transferase was estimated by the method habig et al., 1974 17 ; reduced glutathione measured by method of Jollow et al., 1974 18 and melanoldehyde was measured by the method of Ohkawa et al., 1979 19 .
Histopathology
The animals of different groups were sacrificed under light anesthesia (pentobarbitone) 1 day after the end of the experiment. A small piece of liver and hepatic tissues were removed for histological analysis. The tissues liver and pancreas were fixed in 10% formalin (diluted to 10% with normal saline) and embedded in paraffin wax. The tissues were dehydrated in graded concentrations of xylene, embedded in molten paraffin wax, and sectioned at 5 μ. Tissue sections were fixed on glass slides and stained with hematoxylin and eosin for light microscopy at 400x.20 The histological changes were evaluated in nonconsecutive, randomly chosen x 200 histological fields using light microscope, NIKON ECLIPSE E200.
Statistical Analysis
All the data were expressed as mean±SEM. Statistical analysis was carried out using one way ANOVA followed by Dunnet’s multiple comparison test and two way ANOVA followed by Bonferroni’s post test.
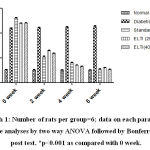 |
Graph 1: Number of rats per group=6; data on each Parameter Were analyses by two way ANOVA followed by Bonferroni’s post test, *p<0.001 as compared with 0 week. |
Results
Blood glucose
The treatment STZ showed elevated blood glucose levels. Treatment with the ethanolic leaf extract of Tylophora indica (ELTI) showed significant (p<0.05) reduction in the elevated blood glucose levels during 2nd , 4th and 6th week in STZ induced diabetic rats (Figure 1).
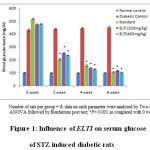 |
Figure 1: Influence of ELTI on serum glucose of STZ induced diabetic rats |
Liver enzymes
STZ induced diabetic rats showed elevated levels of liver enzymes SGOT, SGPT and ALP. Treatment with ELTI showed dose dependent reduction of SGOT, SGPT and ALP significantly at p<0.05 compared with diabetic control (Figure 2).
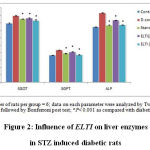 |
Figure 2: Influence of ELTI on liver enzymes in STZ induced diabetic rats |
Antioxidant enzymes
The diabetic rats induced with STZ showed reduction in antioxidant enzymes namely SOD, CAT, GST and GPx and non enzymatic antioxidant GSH and elevation of MDA in both pancreatic and liver homogenates. Treatment with ELTI showed significant elevation of SOD, CAT, GST, GPx and GSH at P< 0.01, P< 0.001 in a dose dependent manner in pancreatic and liver homogenates compared with diabetic control rats. The MDA levels were found to be reduced significantly in STZ induced diabetic rats of both pancreatic and liver homogenates (Table 1 and Table 2).
Table 1: Effect of ELTI on antioxidant enzymes and MDA in pancreas of STZ induced diabetic rats.
| Groups | SOD | CAT | GPx | GST | GSH | MDA |
| Control | 0.89±0.09* | 48.03±1.96* | 6.91±0.26* | 0.46±0.06* | 22.99±0.43* | 0.34±0.05* |
| D.Control | 0.43±0.06 | 24.36±2.01 | 3.98±0.44 | 0.21±0.06 | 13.42 ±0.22 | 0.82±0.09 |
| Gliclazide (1mg/kg) | 0.88±0.02* | 42.98±1.97** | 6.66±0.13* | 0.43±0.09*** | 18.36±0.98** | 0.36±0.06*** |
| ELTI (200mg/kg) | 0.64±0.09** | 32.05±2.16* | 5.69±0.35* | 0.36±0.02** | 16.33±0.46* | 0.48±0.13* |
| ELTI (400mg/kg) | 0.81±0.19*** | 40.36±2.00*** | 6.13±0.61*** | 0.41±0.05*** | 21.09±0.74*** | 0.40±0.09** |
Number of rats per group = 6; data on each parameter were analyzed by one-way ANOVA followed by Dunnet’s test; *P< 0.05, **P< 0.01, *** P< 0.001 as compared with diabetic control
SOD expressed as mM /min/mg of protein CAT expressed as mM of hydrogen peroxide decomposed/min/mg of protein; GPx expressed as mM of glutathione oxidized/ min/mg of protein; GST expressed as U/min/mg of protein, GSH expressed as mg/g of protein, MDA expressed as nmol MDA/mg protein.
Table 2: Effect of ELTI on antioxidant enzymes and MDA in liver of STZ induced diabetic rats.
| Groups | SOD | CAT | GPx | GST | GSH | MDA |
| Control | 2.93±0.23* | 85.53±1.06* | 12.36±0.65* | 0.98±0.09* | 31.98±0.36* | 0.69±0.10* |
| D.Control | 1.23±0.09 | 56.99±2.03 | 07.16±0.36 | 0.57±0.06 | 21.06±0.43 | 1.48±0.03 |
| Gliclazide (1mg/kg) | 2.68±0.13** | 83.69±1.98*** | 11.66±0.87** | 0.92±0.03*** | 29.33±0.59*** | 0.72±0.09** |
| ELTI (200mg/kg) | 2.20±0.09* | 76.28±1.59** | 08.97±0.85* | 0.68±0.04* | 25.66±0.54** | 0.86±0.07* |
| ELTI (400mg/kg) | 3.06±0.20*** | 82.06±2.11*** | 11.06±0.91** | 0.89±0.07** | 28.95±0.39*** | 0.65±0.11*** |
Number of rats per group = 6; data on each parameter were analyzed by one-way ANOVA followed by Dunnet’s test; *P< 0.05, **P< 0.01, *** P< 0.001 as compared with diabetic control
SOD expressed as mM /min/mg of protein CAT expressed as mM of hydrogen peroxide decomposed/min/mg of protein; GPx expressed as mM of glutathione oxidized/ min/mg of protein; GST expressed as U/min/mg of protein, GSH expressed as mg/g of protein, MDA expressed as nmol MDA/mg protein.
Histopathology
Histological studies are known to provide further evidence of oxidative damage induced by STZ in diabetic rats. Pathologically, In general liver and pancreatic histological structure was normal in healthy control groups however, the STZ induced diabetic rats were shown to poses massive fatty changes and necrosis (Fig. 3& 4).
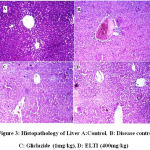 |
Figure 3: Histopathology of Liver A:Control, B: Disease control, C: Gliclazide (1mg/kg), D: ELTI (400mg/kg) |
In liver tissues in the normal control group (Group I), normal lobular architecture is observed (Fig.3A). In Group II Liver cell necrosis, inflammation (due to excess generation of ROS) and fatty changes in centrilobular region is observed (Fig. 3B). In Group III a mild fatty change in centrilobular portions is observed (Fig. 3C) and finally in Group IV and V, ELTI prevented the pathological conditions (presence of most of normal hepatocytes, the structure of nuclei is normal) and no considerable fatty change was observed along with absence of cell necrosis and inflammation (Fig. 3D).
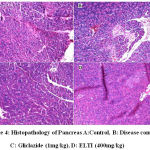 |
Figure 4: Histopathology of Pancreas A:Control, B: Disease control, C: Gliclazide (1mg/kg), D: ELTI (400mg/kg) |
In the pancreatic oxidative stress studies the histopathology of control group (Group 1) revealed that the normal structure of the exocrine and endocrine parts of the pancreas (Fig. 4A). In Group II, it was observed that there was altered structure of both the exocrine and endocrine portions with significant decrease in the number of secretary cells (Fig.4B), particularly the pancreatic lobules were covered with acini. However treatment with Gliclazide (Group III) and ELTI (Group IV and V) were found to prevent the degenerative changes in the pancreas of STZ induced diabetic rats (Fig. 4C & 4D).
Discussion
Underlying mechanism for initiation of diabetes is majorly oxidative stress. 21 This study was designed to evaluate the beneficial effect of ethanolic leaf extract of Tylophora indica (ELTI) on pancreatic and hepatic oxidative stress in diabetic rats. The experimental model suitable to stress induced diabetes is streptozotocin. The STZ showed direct cytotoxic effect on pancreatic β cells through the generation of reactive free radicals and by inducing diabetes in experimental animals. 22 Thereby it enhances lipid peroxidation and its byproducts TBARS, depletion of antioxidant enzymes in both plasma and tissues during diabetes. The degree of induction and structural damage depends on the imbalance of antioxidant defense system. The hyperglycemia leads to stimulation CYP450 by enhancing NADPH resulting from the glucose metabolism. 23 STZ with nicotinamide model was used to induce the diabetes in the present study. Nicotinamide was used to control sudden pancreatic damage by STZ through inhibition of poly ADP ribose polymerase which involved in DNA repair. STZ induced diabetic rats showed significant elevation of blood glucose levels and was found to be normalized with ELTI treatment at both dose levels. The same results were observed in hyperglycemic activity of Tylophora indica in the alloxan induced diabetic rats. 24 It was observed that decreased glucose utilization with excessive production of glucose was observed in liver and cause tissue damage. It leads to both physiological and histological changes in the liver. 25 The same was observed in STZ induced diabetic rats by way of elevated liver enzymes (AST, ALT and ATP). Treatment with ELTI showed significant control over the STZ induced toxicity by normalizing the serum levels of AST, ALT and ALP in diabetic rats. This might be due to preventive effect on ELTI protecting the hepatocytes thereby preventing the leakage of hepatic enzymes into circulation.
The raised oxidative stress accompanied with elevated stress markers and diminished antioxidant enzymes in clinical and preclinical diabetic models. 26 Increased events of cellular damage resulted to excessive formation of lipid peroxyl and hydrogen peroxide with diminished activities of SOD, CAT and GPx. 27 These events cause excessive production of super oxide peroxyl and hydroxyl radicals could inhibit the GPx activity thereby disturbs the glutathione system. 28 The depleted GSH content in the tissues leads to lipid peroxidation and subsequent structural disruption. It is observed that a marked depletion in the GSH content in liver was observed in diabetic control rats. Furthermore, treatment with ELTI showed a significant restoration in antioxidant enzymes GSH content and MDA levels in the tissues of pancreas and liver at p<0.001(Table 1 and 2). While, increased the level of SOD, CAT, GPx, GSH and decreased lipid peroxidation (MDA) levels were observed after ELTI administration may point to a reduction in free radical production. The diminished antioxidant enzyme levels in pancreas was found to be more compared to that of liver indicating the susceptibility of pancreas to stress induced diabetes. The lesser antioxidant enzymes in pancreas when compared with liver suggest the increased susceptibility of pancreatic tissue to oxidative damage during development of diabetes. The liver was found to be more resistant to oxidative stress due to high amount of fatty acids.
Histopathological studies indicating that the ELTI showed ameliorating effect on pancreatic damage by protecting the islets, acinar cells and increasing number of secretary cells. The hepatoprotective activity of ELTI evidenced by regeneration of hepatocytes and inflammation in STZ induced diabetic rats. The results obtained were similar to Gujrati V et al., that Tylophora indica can able to protect liver from steatosis and necrosis in ethanol induced hepatotoxicity. 29
It is reported that several major alkaloids such as tylophorine and tylophorinine, tylophrinidine 30, 31 have been isolated from the leaves T. indica may responsible for the observed pharmacological activities like antioxidant activity and hepatoprotective activity, The same might be responsible for increasing the hepatic antioxidant enzymes and lowering the serum liver enzyme levels with T.indica against hepatic and pancreatic oxidative stress in STZ induced diabetic rats.
Conclusions
The propensity of Tylophora indica to have shown protective activity on pancreatic and hepatic dysfunction in diabetic rats might be due to the presence of active phytochemicals like alkaloids, tannins and flavanoids. The antidiabetic activity observed could be due to amelioration of β-cell damage and necrosis of liver, thus leading to reduction of oxidative stress by treatment with Tylophora indica.
References
- Fekadu G, Bula K, Bayisa G,Turi E, Tolossa T, and Kasaye Challenges And Factors Associated With Poor Glycemic Control Among Type 2 Diabetes Mellitus Patients At Nekemte Referral Hospital, Western Ethiopia. J Multidiscip Health., 12: 963–974 (2019).
CrossRef - Adwas AA, Elsayed ASI, Azab AE, Quwaydir FA. Oxidative stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng., 6(1):43‒47 (2019).
CrossRef - Patlevic P, Vaskova J, Svorc P, Vasko L, Svorc Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integrative Medicine Research., 5(4):250-25 (2016).
CrossRef - Lobo V, Patil A, Phatak A. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev., 4(8):118–126 (2010).
CrossRef - Felix R, Valentao P, Andrade PB, Felix C, Novais SC, Lemos ML. Evaluating the In Vitro Potential of Natural Extracts to Protect Lipids from Oxidative Damage. Antioxidants., 9:231 (2020).
CrossRef - Ullah A, Khan A, Ismail Diabetes mellitus and oxidative stress—A concise review, Saudi Pharmaceutical Journal., 24(5):547-553 (2016).
CrossRef - Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, Ern An update on natural compounds in the remedy of diabetes mellitus: A systematic review, J Tradit Complement Med., 8(3): 361–376 (2018).
CrossRef - Switi BG, Krishna Mohan G and Sandhya Rani M. Phytochemicals for Diabetes Management, Pharmaceutical Crop.s, 5(1):11-28 (2014).
CrossRef - Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi CSIR. (1956).
- Chia-Mao W, Cheng WY, Yue ZE. Tylophorine arrests carcinoma cells at G1 phase by downregulatingcyclin A2 expression. Bio Chem Bio Phy Res Com., 386 (1):140-145 (2009).
CrossRef - Meera R. Evaluation of Diuretic activity from Tylophora indica leaves extract. J Phar Sci Res., 1(3):112-116(2009).
- 12 Swathi P, Eswar Kumar K, Jagadeesh Kumar T. Evaluation of anti hyperglycemic and anti hyperlipidemic activity of ethonolic extract of Tylophora indica in alloxan induced diabetic rats. Int J Curr Pharm Res., 4(1): 25-31(2012).
- Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem., 6(24) 1969.
CrossRef - Kakkar P, Das B, Viswanathan PA. Modified method for assay of superoxide dismutase. Ind J Biochem Biophys., 21:131-132 (1984).
- Sinha AK. Colorimetric assay of catalase. Anal Biochem,. 47(2):389-94 (1972).
CrossRef - Rotruck JT, Pope AL, Ganther H. Selenium biochemical role as a component of glutathione peroxidase. Science., 179, 588 – 590 (1973).
CrossRef - Habig WH, Pabst MJ, Jakoby WB. GlutathioneS-transferases. The first enzymatic step in mercaptouric acid formation. J Biol Chem., 249: 7130-7139 (1974).
CrossRef - Jollow DJ, Mitchell JR, Zampaglione N. Bromobenze induced liver necrosis: protective role of glutathione and evidence for 3,4bromobenzene oxide as the hepatotoxic metabolite. Pharmacology., 11:151-169 (1974).
CrossRef - Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric reaction. Anal Bio chem., 95: 351-358 (1979).
CrossRef - Kiernan JA. Histological and Histochemical Methods: Theory and Practice, Pergamon Press, Oxford, UK. (1981).
- Swathi P and Eswar Kumar K. Advanced glycated end products: A Review. J Global Trends Phar Sci., 5(2):1729–37 (2014).
- Kim MJ, Ryu GR, Chung JS. Protective effect of epicatechin against the toxic effects of STZ on rat pancreatic islets: in vivo and in vitro. Pancreas., 26:292–299 (2003).
CrossRef - Jain SK. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem., 264:21340–5 (1989).
CrossRef - Swathi P, Eswar Kumar K., Jagadeesh Kumar T. Evaluation of anti hyperglycemic and anti hyperlipidemic activity of ethonolic extract of Tylophora indica in alloxan induced diabetic rats. Int J Curr Pharm Res., 4(1): 25-31(2012).
- Yazdi HB, Hojati V, Shiravi A, Hosseinian S, et al. Liver Dysfunction and Oxidative Stress in Streptozotocin-Induced Diabetic Rats: Protective Role of Artemisia J Pharmacopuncture., 22(2): 109–114 (2019).
- Jordan D. An Offering of Wine. Doctoral Thesis. The Department of Semitic Studies, Univeristy of Sidney. 2002.
- Laight DW, Carrier MJ and Anggard EE. Antioxidants, diabetes, and endothelial dysfunction. Cardiovasc Res., 47:457-464 (2000).
CrossRef - Escobar JA, Rubio MA, Lissi EA. SOD and catalase inactivation by singlet oxygen and peroxyl radicals. Free Rad Biol Med., 20: 285-290 (1996).
CrossRef - Gujrati V, Patel N, Rao V, Nandakumar K, Gouda TS, Shanta Kumar SM. Hepatoprotective activity of alcoholic and aqueous extracts of leaves of Tylophora indica (Linn.) in rats. Ind J Pharmacol., 39(1):43-47 (2007).
CrossRef - Sarita S, Pawan KK, Shakti Kumar, Ranjeet Kumar and Abdulqader A A.Tylophorine, a phenanthraindolizidine alkaloid isolated from Tylophora indicaexerts antiangiogenic and antitumor activity by targeting vascular endothelial growth factor receptor 2–mediated angiogenesis. Mol Cancer., 12: 82 (2013).
CrossRef - Dhiman M, Naik V, Kshirsagar R, Chandrakant DD, Manju S.L. Antioxidant activity of hydrochloride salt of tylophorinidine and tylophorinine isolated from aerial parts of Tylophora indica, Int J Res Ayur Phar.,3(1):121-124 (2012).








