Manuscript accepted on :31-Oct-2020
Published online on: --
Plagiarism Check: Yes
Reviewed by: Rajajeyakumar Manivel
Second Review by: PP Mohanty
Final Approval by: Ayush Dogra
D. Barathane*, K. Manimekalai and Kartik J Salwe
Department of Pharmacology, Mahatma Gandhi Medical College and Research Institute,
Sri Balaji Vidyapeeth, Puducherry-607403, India.
Corresponding Author E-mail: barathane20@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2067
Abstract
Objective: To evaluate the Hypolipidemic& Antioxidant effect of Garcinia gummi-gutta barks on Atherogenic Diet-Induced Hyperlipidemic Rats. Materials and methods: Bark extract was prepared using a Soxhlet apparatus. 30 Wistar albino rats of either sex was divided into 5 groups (n=6). Atherogenic diet (AD) was administered to all the rats except group 1 for 4 weeks. Following induction, Group 3 & 4 were treated with bark extracts at 100 & 200mg/kg respectively and group 5 with atorvastatin at 10mg/kg for the next 4 weeks along with AD. Weekly estimation of lipid profile was done and, in the end, all the animals were sacrificed and the liver and heart were sent for histopathological examination. MDA levels were estimated by measuring the TBARS activity. Results were expressed in the Mean ± standard error of the mean. Statistical analysis was done using one-way ANOVA followed by post hocTukey test. p<0.05 is considered statistically significant. Result: There was a significant decrease in the mean cholesterol, triglyceride, low-density lipoproteins&very-low-density lipoproteins and an increase in High-density lipoproteins when compared to the AD group. Barks extracts at both doses (100mg/kg & 200mg/kg) showed a significant reduction in lipid profile wherein the extract at higher dose was found to be better and there was no significant difference when compared to atorvastatin. Conclusion: The study showed that bark extract of Garcinia gummi-guttapossess significant hypolipidemic & antioxidant activity. Further studies are warranted for its clinical use in humans.
Keywords
Atherogenic Diet; Barks; Cholesterol; Dyslipidemia; Garcinia Gummi-Gutta; Lipid Profile
Download this article as:| Copy the following to cite this article: Barathane D, Manimekalai K, Salwe K. J. Hypolipidemic and Antioxidant effect of Garcinia gummi-gutta barkson Atherogenic Diet-Induced Hyperlipidemic Rats. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Barathane D, Manimekalai K, Salwe K. J. Hypolipidemic and Antioxidant effect of Garcinia gummi-gutta barkson Atherogenic Diet-Induced Hyperlipidemic Rats. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/36lP4pU |
Introduction
Cholesterol, which is the substrate for many body functions, have now been considered as the Satan of modern health. Elevated lipid levels, oxidative stress, and abnormal lifestyles have contributed to the emergence of atherosclerosis and associated diseases like hepatic steatosis Stroke and myocardial infarction [1]. Hyperlipidemia associated with elevated cholesterol, triglycerides, and LDL with low levels of HDL increases the risk of CVD and CAD by several folds [2]. Atherosclerosis as a result of hyperlipidemia, wherein the deposition of lipids and associated inflammation narrows the vascular pathway resulting in poor perfusion, ischemia, and necrosis of cells distal to the block [3].
Although various drugs like HMG- CoA reductase inhibitors, Nicotinic acid, Fibrate derivatives & bile acid sequestrants are already available to combat the situation, they are not always free from side effects. Myopathy, hyperuricemia, peripheral neuropathy, impotence, gynecomastia, hepatitis, impotence, skin rashes,etc. are some to be noted [4]. The risk of myopathy and rhabdomyolysis is typically increased when given in combinations. For ages, there has been always a search for an alternative to synthetic drugs with maximal efficacy, safety at a minimal cost.
Many Indian medicinal plants are known for their therapeutic value and most of them are used in our day to day life. They lack scientific validation and hence they are not recognized. Garcinia gummi-gutta, also known as G. cambogia is popular over the web as a holy grail for weight loss. It is long been used in some south Indian cuisines as a flavoring agent & carminative [5]. Traditionally it is used in the treatment of parasitic infestations, hemorrhoids, rheumatism, and menstrual abnormalities [6]. Many studies have been done on the plant to explore its therapeutic potential were mainly for its anti-obesity activity [7]. Very few studies have explored its lipid-lowering potential. Hence this study has been taken up to scientifically validate the hypolipidemic potential of Garcinia gummi-gutta barks using animal models.
Materials and Methods
Identification and collection of plant materials
Barks of Garcinia gummi-gutta were collected during the summer season from Thiruvananthapuram district, Kerala. The materials were authenticated by a local botanist from science college and a voucher specimen of the same was submitted. 4kg of barks were collected discarding parts that arediseased, decayed, andunfit for analysis. They were later cleaned, shade dried, powdered using a mechanical grinder and stored in airtight glass containers until the extraction process.
Preparation of The Extract
Extraction was done using the process elaborated by Mahanta and Mukherjee [8]. The Soxhlet instrument was filled with 45g of the bark powder and the extraction was done continuously using 80% ethanol refluxing at 50-70°C.The Yield was collected in a porcelain cup and was dried using a hot air oven at 45°C for 24h. The final yield was around 20% (~9g) per cycle.
Chemicals
All the Solventsand reagents used in the study were of analytical grade and purchased from Sigma Aldrich, Bengaluru.
Ethical Clearance
Institutional Animal Ethics Committee approval was obtained before the commencement of the study.
Animals
Adult Wistar albino rats of either sex weighing 180±20g were procured from TANUVAS Chennai. They were housed in the central animal house of MGMCRI, one week before the commencement of the study for acclimatization. Animals were kept in polypropylene cages with paddy husk bedding fed with standard pellet diet and water ad libitum. The room temperature was maintained at 26±2°C with a 12:12h dark and light cycle. The animals were cared for & maintained according to CPCSEA guidelines [9].
Acute Toxicity Testing
Acute toxicity was tested using two doses at 1000mg/kg and 2000mg/kg body weight with 3 animals for each dose respectively. The animals were observed continuously for the first 4h and then occasionally for the next 4h. Animals were observed for signs of tremor, ataxia, opisthotonos, catatonia, spasticity, ptosis, and respiration. The animals were monitored up to 72 h for any signs of lethality/death [10].
Dose Determination
Since the animals did not show any signs of toxicity, a dose of 200 and 100mg/kg of the extract which was 1/10th and 1/20th of 2000mg/kg respectively was taken up for the main study. Atorvastatin at 10mg/kg was taken as a standard control.
Atherogenic Diet (AD)
The atherogenic diet was prepared by mixing commercially available feed powder along with atherogenic constituents. It contains Cholesterol (5%), Sucrose (20%), Hydrogenated vegetable oil (20%), Sodium cholate (2%), Lactose (20%), Choline chloride (0.4%), Thiouracil (0.15%) with equal quantity of commercially available feed powder (w/w) [11].
Experimental Design
30 adult Wistar albino rats were used in the study and the experiment was performed following 1-week acclimatization. Duration of study is for 8weeks and all the procedures were done under strict aseptic precautions in the Central Animal House, MGMCRI. A baseline lipid profile was determined before the commencement of the main study. For the elimination of any possible bias, the rats were numbered and caged using random numbers generated using computer software.
Induction of Hyperlipidemia
The study includes 30 adult Wistar rats randomly divided into five groups with six in each group (n=6). Except for group 1 which received standard pellet diet and water ad libitum, the remaining four groups received an atherogenic diet for four weeks. (Table. 1)
Induction of hyperlipidemia was confirmed by estimating the lipid profile at the end of four weeks Induction was followed with the administration of extracts & standard drug (p.o) along with the daily atherogenic feedfor the next four weeks between 9 and 10 am suspended in 1% carboxymethyl cellulose.
Table 1: Experimental Design
|
Group (n=6) |
Purpose | Treatment |
| Group 1 | Normal control | Standard pellet diet+1% CMC |
| Group 2 | AD control | AD + 1 % CMC |
| Group 3 | BEGG 100 | AD+ Bark extract of G. gummi‑gutta at 100 mg/kg |
| Group 4 | BEGG 200 | AD+ Bark extract of G. gummi‑gutta at 200 mg/kg |
| Group 5 | Standard control | AD+Atorvastatin at 10 mg/kg |
AD: Atherogenic diet, BEGG100: Bark extract of Garcinia gummi-gutta at 100 mg/kg, BEGG200: Bark extract of Garcinia gummi-gutta at 200 mg/kg, G. gummi-gutta: Garcinia gummi-gutta, CMC: Carboxymethylcellulose
Lipid Level Estimation
Lipid profile was estimated following blood samples collected form lateral tail vein [12]. Samples were taken at day 0 (baseline), 4th week, 6th week, and 8th week was centrifuged at 3000 rpm for 10 min and the supernatant was collected and stored at -20°C until further analysis.Lipid profile was estimated using the following methods (Table. 2) [13-16].
Table 2: Lipid Profile Estimation Method
| Lipid fraction | Estimation method | Unit |
| Total cholesterol (TC) | Enzymatic method [13] | mg/dl |
| Triglycerides (TG) | Converted to glycerol and estimated using glycerol kinase enzyme-based kinetic method [14] | mg/dl |
| Low-density lipoproteins (LDL) | Friedewald’s equation [15] | mg/dl |
| Very low-density lipoproteins (VLDL) | [15] | mg/dl |
| High-density lipoproteins (HDL) | Polyanion precipitation [16] | mg/dl |
All the animals were sacrificed at the end of the 8th week using the carbon dioxide euthanasia chamber. Liver and heart tissues were dissected, washed in the physiological salt solution, and then fixed using 10% formalin.
Histopathology
Liver and heart tissues fixed in formalin were washed, dehydrated using isopropyl alcohol, and finally rinsed with xylene. Tissue specimens were embedded with molten paraffin wax and sections of 5μm thickness were cut using a microtome. The cut specimens were then fixed in a glass slide and stained with hematoxylin& eosin and viewed under a light microscope for changes [17].
Thiobarbituric acid reactive substances (TBARS) assay
1 ml of serum was mixed with 500 μl of 15% trichloroacetic acid in 0.25 N hydrochloric acids (HCl) and 500 μl of 0.375% thiobarbituric acid in 0.25 N HCl. The contents were mixed thoroughly and incubated in a waterbath at 95°C for 15min. Cooling down to room temperature the contents were centrifuged at 1000 r.p.m for 10 min and a pink supernatant was collected. Absorbance was determined using a spectrophotometer set to a wavelength of 535nm. The concentration of malondialdehyde (MDA) in serum was determined using the molar extinction coefficient of 1.56 M/cm, and the results were expressed in ηmol/ml of plasma [18].
Statistical Analysis
Data were collected and maintained in Microsoft excel 2016 and analyzed using SPSS v.17. Results were expressed as Mean ± SEM as tables. Statistical analysis was done using one-way ANOVA followed by post hoc Tukey’s test. p-value<0.05 was considered statistically significant.
Results
Phytochemical Analysis
Preliminary phytochemical analysis of Garcinia gummi-guttabark extract showed the presence of flavonoids, terpenoids, phenols, tannins, cardiac glycosides, carbohydrates, saponins, phlobatannins, sterols, coumarin and some amino acids like arginine. asparagine, glutamine, threonine, glycine, proline.
Acute toxicity study and dose selection
Acute toxicity studies revealed that the administration of bark extract of Garcinia gummi-gutta at a dose of 2000mg/kg did not produce any lethality or significant change in the behavior of the animals. The results of the preliminary toxicity study showed that a single dose of the plant extract (2000mg/kg, p.o.) had no adverse effects on rats, which indicated that the median lethal dose (LD50) could be greater than 2000mg/kg body weight. So, a dose of 200mg/kg, p.o. and 100mg/kg, p.o. which was 1/10th and 1/20th of LD50 was chosen for the main study.
Effect of Bark extract on lipid profile
Following induction, there was a significant increase in the serum lipids, especially TC, TG, LDL, VLDL, and a decrease in HDL as a result of the administration of the atherogenic diet in all the test groups compared to normal control.
Compared to the AD controlgroup, administration of bark extracts of Garcinia gummi-guttaon the test groups at a dose of 100mg/kg and 200mg/kg had a significant impact by reducing the TC, TG, VLDL and LDL and a concomitant increase in HDL levels. There was a dose-dependent reduction in lipid levels wherein BEGG at 200mg/kg was found to be better. Significant improvement in the HDL levels was also observed with bark extracts at higher doses (Tables. 3 -7).
Compared to the standard drug Atorvastatin, BEGG at 100mg/kg failed to produce a significant effect, whereas with BEGG at 200mg/kg there was no significant difference between the groups. This shows that BEGG at 200mg/kg was significantly better than the lower dose and in fact, can produce the same effect as that of the standard drug atorvastatin.
Table 3: Effect of the Bark Extract of G. gummi-gutta on the Total Cholesterol Levels
| Groups | Total cholesterol levels mg/dl | |||
| Induction of hyperlipidaemia | Drug administration | |||
|
Day 0 Baseline |
Day 30 | Day 45 | Day 60 | |
|
Group 1 Normal control |
62.67±1.50 | 61.67±1.73 | 64.50±1.38 | 65.50±0.81 |
|
Group 2 AD control |
64.00±0.97 | 128.83±1.08 | 135.67±0.84## | 141.33±1.23## |
|
Group 3 BEGG 100 |
63.00±1.29 | 131.50±0.99 | 128.17±1.47**,## | 117.33±1.50**,## |
|
Group 4 BEGG 200 |
64.83±0.91 | 135.67±1.05 | 118.17±1.14**,## | 100.17±1.54**,# |
|
Group 5 Standard control |
65.83±1.66 | 129.00±1.18 | 106.00±0.97** | 93.67±0.80** |
(AD – Atherogenic diet, BEGG 100 – Bark extract of Garcinia gummi-gutta at 100mg/kg, BEGG 200 – Bark extract of Garcinia gummi-gutta at 200mg/kg. Standard control – Atorvastatin 10mg/kg)
Results expressed as Mean ± SEM. One Way ANOVA followed by post hoc Tukey test, significance at *p<0.05, **p<0.001 as compared to AD control. #p<0.05, ##p<0.001 as compared to standard control.
Table 4: Effect of the Bark Extract of G. gummi-gutta on the Triglyceride levels
| Groups | Triglyceride levels mg/dl | |||
| Induction of hyperlipidaemia | Drug administration | |||
|
Day 0 Baseline |
Day 30 | Day 45 | Day 60 | |
|
Group 1 Normal control |
79.83±1.25 | 79.67±1.28 | 80.50±0.96 | 82.33±1.09 |
|
Group 2 AD control |
83.83±0.87 | 182.50±1.18 | 197.83±2.95## | 204.00±3.22## |
| Group 3
BEGG 100 |
84.17±1.35 | 183.67±1.12 | 172.50±1.26**,## | 153.50±2.05**,## |
| Group 4
BEGG 200 |
82.50±1.45 | 182.83±1.40 | 156.83±1.08**,## | 117.33±1.02**,# |
| Group 5
Standard control |
83.50±0.76 | 180.50±0.76 | 120.17±1.30**,## | 106.17±1.28** |
(AD – Atherogenic diet, BEGG 100 – Bark extract of Garcinia gummi-gutta at 100mg/kg, BEGG 200 – Bark extract of Garcinia gummi-gutta at 200mg/kg. Standard control – Atorvastatin 10mg/kg)
Results expressed as Mean ± SEM . One Way ANOVA followed by post hoc Tukey test, significance at *p<0.05, **p<0.001 as compared to AD control. #p<0.05, ##p<0.001 as compared to standard control.
Table 5: Effect of the Bark Extract of G. gummi-gutta on the High-Density Lipoprotein levels
| Groups | HDL levels mg/dl | |||
| Induction of hyperlipidaemia | Drug administration | |||
| Day 0
Baseline |
Day 30 | Day 45 | Day 60 | |
| Group 1
Normal control |
28.17±0.87 | 28.17±0.54 | 28.50±0.76 | 30.00±0.63 |
| Group 2
AD control |
25.00±0.58 | 27.00±1.26 | 23.83±1.08## | 24.83±1.72## |
| Group 3
BEGG 100 |
27.50±1.23 | 29.50±0.92 | 32.67±0.56**,## | 34.33±0.88**,## |
| Group 4
BEGG 200 |
26.67±0.92 | 29.33±0.92 | 35.33±0.49**,## | 43.33±0.84**,† |
| Group 5
Standard control |
26.00±0.86 | 30.00±1.13 | 41.67±0.88** | 42.17±1.22** |
(AD – Atherogenic diet, BEGG 100 – Bark extract of Garcinia gummi-gutta at 100mg/kg, BEGG 200 – Bark extract of Garcinia gummi-gutta at 200mg/kg. Standard control – Atorvastatin 10mg/kg)
Results expressed as Mean ± SEM. One Way ANOVA followed by post hoc Tukey test, significance at *p<0.05, **p<0.001 as compared to AD control. #p<0.05, ##p<0.001 as compared to standard control.
Table 6: Effect of the Bark Extract of G. gummi-gutta on the Low -Density Lipoprotein levels
| Groups | LDL levels mg/dl | |||
| Induction of hyperlipidaemia | Drug administration | |||
| Day 0
Baseline |
Day 30 | Day 45 | Day 60 | |
| Group 1
Normal control |
18.53±2.06 | 17.57±1.98 | 19.90±1.45 | 19.03±0.86 |
| Group 2
AD control |
22.23±1.17 | 63.83±2.17 | 72.27±0.31## | 75.70±1.41## |
| Group 3
BEGG 100 |
18.67±0.81 | 65.27±1.67 | 61.00±1.47**,## | 51.70±2.16**,## |
| Group 4
BEGG 200 |
21.67±1.52 | 69.77±1.10 | 51.47±1.47**,## | 35.37±2.00**,† |
| Group 5
Standard control |
23.13±1.67 | 62.90±1.77 | 40.30±0.95** | 30.27±1.61** |
(AD – Atherogenic diet, BEGG 100 – Bark extract of Garcinia gummi-gutta at 100mg/kg, BEGG 200 – Bark extract of Garcinia gummi-gutta at 200mg/kg. Standard control – Atorvastatin 10mg/kg)
Results expressed as Mean ± SEM. One Way ANOVA followed by post hoc Tukey test, significance at *p<0.05, **p<0.001 as compared to AD control. #p<0.05, ##p<0.001 as compared to standard control.
Table 7: Effect of the Bark Extract of G. gummi-gutta on the Very Low -Density Lipoprotein levels
| Groups | VLDL levels mg/dl | |||
| Induction of hyperlipidaemia | Drug administration | |||
| Day 0
Baseline |
Day 30 | Day 45 | Day 60 | |
| Group 1
Normal control |
15.97±0.25 | 15.93±0.26 | 16.10±0.19 | 16.47±0.22 |
| Group 2
AD control |
16.77±0.17 | 36.50±0.24 | 39.57±0.59## | 40.80±0.64## |
| Group 3
BEGG 100 |
16.83±0.27 | 36.73±0.22 | 34.50±0.25**,## | 31.30±0.41**,## |
| Group 4
BEGG 200 |
16.50±0.29 | 36.57±0.28 | 31.37±0.22**,## | 23.47±0.20**,# |
| Group 5
Standard control |
16.70±0.15 | 36.10±0.15 | 24.03±0.26** | 21.23±0.26** |
(AD – Atherogenic diet, BEGG 100 – Bark extract of Garcinia gummi-gutta at 100mg/kg, BEGG 200 – Bark extract of Garcinia gummi-gutta at 200mg/kg. Standard control – Atorvastatin 10mg/kg)
Results expressed as Mean ± SEM. One Way ANOVA followed by post hoc Tukey test, significance at *p<0.05, **p<0.001 as compared to AD control. #p<0.05, ##p<0.001 as compared to standard control.
Effect of Bark extracts on MDAlevels
Lipid peroxidation as a result of long-standing high lipid levels results in endothelial inflammation and damage due to the generation of free radicals. Malondialdehyde which is formed as a result of lipid peroxidation can be detected using TBARS assay.
Group 2 treated with atherogenic dietshowed a significant rise in the TBARS value (3.98±0.14 ηmol/ml) indicative of ongoing lipid peroxidation.Compared to the AD control, a significant reduction in TBARS was observed in groups treated with the standard drug and bark extracts at both doses. The TBARS value in BEGG 200 (1.22±0.02ηmol/ml) was significantly lower when compared with other groups. This reveals that the bark extract has significant antioxidant potential in effectively combating the effects of free radical damage due to lipid peroxidation.
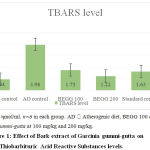 |
Figure 1: Effect of Bark extract of Garcinia gummi-gutta on Thiobarbituric Acid Reactive Substances levels. |
Effect of bark extracts on Histopathology
Liver
Histology of normal control showed normal liver histologyillustrating portal vein surrounded by well-defined hepatocytes with prominent nucleus radiating like spokes of a wheel, from the central vein and branches of hepatic artery around the lobular periphery. Hepatic sinusoids were visible. Kupffer cells are seen lying in the periportal region (Fig. 2a). Group treated with atherogenic diet showed hepatocytes with micro fatty deposits, distortion in morphology, and early feathery degeneration (Fig. 2b). Rats treated with bark extract at 100mg/kg showed distortion in architecture, granular degeneration with apoptotic changes, and micro fatty vacuolation of hepatocytes (Fig. 2c). On the other hand, rats treated with BEGG 200 showed only mild congestion and periportal inflammation with well-preservedliver architecture (Fig. 2d).The group which received AD along with atorvastatin showed only mild periportal congestion with overall preservation of normal liver morphology (Fig. 2e).
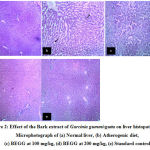 |
Figure 2: Effect of the Bark extract of Garcinia gummi-gutta on liver histopathology. Microphotograph of (a) Normal liver, (b) Atherogenic diet, (c) BEGG at 100 mg/kg, (d) BEGG at 200 mg/kg, (e) Standard control |
Heart
Histopathology of Group which served as normal control showed myocardial cells with oval-elongated nucleus with homogenous cytoplasm (Fig. 3a). Leucocytic infiltrates with mild congestion were observed with rats treated with an atherogenic diet (Fig. 3b). All other remaining groups did not show any significant changes in preserving the overall myocardial integrity (Fig. 3c-e).
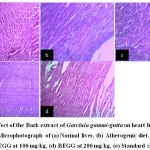 |
Figure 3: Effect of the Bark extract of Garcinia gummi-gutta on heart histopathology. Microphotograph of (a) Normal liver, (b) Atherogenic diet, (c) BEGG at 100 mg/kg, (d) BEGG at 200 mg/kg, (e) Standard control |
Histopathology of Group which served as normal control showed myocardial cells with oval-elongated nucleus with homogenous cytoplasm (Fig. 3a). Leucocytic infiltrates with mild congestion were observed with rats treated with an atherogenic diet (Fig. 3b). All other remaining groups did not show any significant changes in preserving the overall myocardial integrity (Fig. 3c-e).
Discussion
Cardiovascular disease (IHD & Stroke) is the leading cause of death worldwide, with dyslipidaemia contributing significantly to atherosclerosis. Around 17.3 million deaths per year occur due to CVD and the number is expected to grow to more than 23.6 million by 2030 [19]. Due to high morbidity associated with CVD, there is a continuous need for safe hypolipidemic drugs which made researchers look for safer alternatives, especially herbal therapies.
In the present study,the hypolipidemic and antioxidant activity of Garcinia gummi-gutta barks were investigated in atherogenic diet-induced hyperlipidemic animal models. Administration of bark extract at both doses showed a dose-dependent reduction in lipid levels along with a reduction in MDA.
At the end of 4 weeks, the experimental groups (2-5) showed a rise in serum total cholesterol, triglycerides, LDL, and VLDL levels, with a corresponding decrease in HDL levels following administration of atherogenic diet. But at the end of 8weeks following administration of bark extract at 100 and 200mg/kg, there was a significant reduction in the TC,TG, VLDL, and LDL with a corresponding increase in HDL. A high dose of bark extract was significantly better in reducing the serum lipids compared to group 2 and near equal results with standard drug atorvastatin.
The hypolipidemic activity of the bark extract of Garcinia gummi-guttacan be attributed to the collective effect of various organic acid like (-) hydroxy citric acid, malic acid, and other derivatives like guttiferone E,garcinol, isogarcinol , flavonoids, and rheediaxanthone A [20].
The key enzyme ATP citrate lyase which is inhibited by (-) HCA leads to an effective reduction of two carbon moiety required for fatty acid and cholesterol synthesis [21].Flavonoids on the other hand can significantly reduce the activities of glucose -6-phosphate dehydrogenase and isocitrate lyase which is also responsible for the biosynthesis of cholesterol. Moreover, flavonoids are also known to enhance the activity of lipoprotein lipase and plasma lecithin cholesterol acyltransferase which effectively clears peripheral pooling of lipoprotein fractions [22].
Various studies reveal that the potential risk associated with CVD is due toelevation in thelevels of LDL associated with lipid peroxidation with a concurrent reduction in HDL levels. In our study,a significant reduction in LDL levels was observed following the administration of BEGG at both the doses with higher doses comparable to that of standard control. This may be due to the presence of (-) HCA and other derivatives especially flavonoids.
Administration of bark extracts at both doses significantly increased the levels of HDL which is now considered to the therapeutic targetto curb lipid peroxidation and enhance reverse cholesterol transport thereby preventing atherogenesis [23]. A higher dose of bark extract was found to be better than the standard control in elevating the levels of HDL.
Most of our study findings are consistent with previous studies conducted on various parts of the plant. Ramoset al in his study found out that administration of (-) HCA has a significant impact on lipid levels. Following continuous administration of (-) HCA, there was a significant reduction in LDL levels with a proportionate increase in HDL-CH [24]. Koshyet alin their study concluded that flavonoids in the plant possess significant lipid-loweringeffects mainly by increasing lipid metabolism and decreasing lipid biosynthesis [22]
Peroxidation of cellular and circulating lipids which leads to the generation of MDA is a result of oxidative stress and free radical damage associated with the atherogenic diet. MDA quantifying assay (TBARS) may be considered as an indirect measure of ongoing oxidative damage and proper functioning of the inherent antioxidant mechanism. Our study showed that the bark extract at both doses significantly reduced the TBARS level thus revealing their potent antioxidant effect. Strong antioxidant activity than any other group was evident in bark extract at higher doses. This may be related to the presence of flavonoids and other polyisoprenylated products like garcinol &guttiferone E. Similar results were shown by Kolodziejczyket al. who concluded that polyisoprenylated benzophenones such as garcinol and guttiferone K possess significant antioxidant potential in combating lipid peroxidation and oxidative stress in dyslipidaemic conditions [25].
The histopathological findings were no way different from the obtained biochemical results. Bark extract at both doses showed a significant reduction in periportal inflammation, leucocytic infiltration, microfatty deposits, and signs of degeneration. A higher dose was found to be better in effectively controlling the pathological changes associated with the atherogenic diet and showed signs of hepatocellular recovery and regeneration.Except for the AD group showing signs of inflammation all other groups (3, 4, 6, and 7) failed to show significant pathological changes. This may be due to the fact that myocardial involvement in hyperlipidemiamay be a terminal phenomenon and in our study,the bark extracts were effective in protecting the myocardial cells from the probable oxidative stress due to lipid peroxidation.
Conclusion
The present study has confirmed the potential hypolipidemic and antioxidant activity of Garcinia gummi-gutta barks. This was evident by a dose-dependent reduction in lipid levels with an increase in HDL even with the daily administration of the atherogenic diet.
The presence of (-) HCA, Flavonoids, and other phytochemical derivativesin the extract may be responsible for the reduction of lipid fractions and MDA, producing near equal results with that of the standard drug. Further studies may be required to isolate the active principle for effective use in humans.
Acknowledgement
We would like to thank Dr. Dhananjay Kotasthane, Professor and Head of Department Pathology and Dr. Vaishali Kotasthane for helping out with the pathological specimens. We are also thankful for the help rendered by the technicians and attenders of pharmacology, biochemistry and pathology department.
Conflict of interest
There are no conflict of interest
Funding source
Mahatma Gandhi Medical College & Research Institute , Sri Balaji Vidyapeeth, Puducherry.
References
- Saravanan R, Rajendra Prasad N, Pugalandi KV. Effect of Piper beetle leaf extract on alcoholic toxicity in the rat brain. J. Med. Food. 2003;6: 261-5.
CrossRef - Davey Smith G. Cholesterol lowering and mortality: the importance of considering initial level of risk. Int. Med. J. 1993; 306: 1367-1373, Correction: 1648.
CrossRef - Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: Process, Indicators, Risk Factors and New Hopes. Int J Prev Med. 2014;5(8):927-946.
- Speight TM. In: Avery’s Drug Treatment Principles and Practice of clinical pharmacology and therapeutics.3 Edition, ADIS press Ltd, 1987: 599.
- J. Stohs, H. G. Preuss, S. E. Ohia, et al., “Safety and efficacy of hydroxycitric acid derived from Garcinia cambogia—a literature review,” Herbal Gram. 2010; 85: 58–63.
- Tharachand Selvaraj I, Avadhani M. Medicinal properties of Malabar Tamarind [Garcinia cambogia (Gaertn.) Desr.]. Int J Pharm Sci Rev Res. 2013; 19:101–07.
- Oz Calls Garcinia a Weight-Loss “Holy Grail” [Internet]. 2012 [cited 2015 Sep 23]. Available from: http://www.naturalproductsinsider.com/blogs/global-evolutions/2012/10/dr-oz-calls-garcinia-a-weight-loss-holy-grail.aspx.
- Mahanta M, Mukherjee AK. Neutralisation of lethality, myotoxicity and toxic enzymes of Najakaouthia venom by Mimosa pudica root extracts. J Ethnopharmacol. 2001; 75:55-60.
CrossRef - CPCSEA Guidelines for laboratory animal facility. Indian J Pharmacol. 2003 Jul 1;35(4):257.
- Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA), OECD Guidelines for testing of chemicals, revised draft guidelines 425: Acute oral toxicity – Acute toxic class method, revised document. India: Ministry of social justice and empowerment; 2000.
- Anbukkarasi M, Sundararajan M, Venkadeswaran K, Ruban V, Anand T, Geraldine P. Antihypercholesterolemic, antioxidative and anti-inflammatory potential of an extract of the plant Tabernaemontanadivaricata in experimental rats fed an atherogenic diet. Biocatalysis and Agricultural Biotechnology. 2019;19:101115.
CrossRef - Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D et al. A Good Practice Guide to the Administration of Substances and Removal of Blood, Including Routes and Volumes J. Appl. Toxicol. 2001;21: 15-23.
CrossRef - Wybenga DR, Pileggi VJ, Dirstine PH, Giorgio JD. Direct Manual Determination of Serum Cholesterol with Single Stable Reagent. Clin Chem. 1970 Dec 1;16(12):980-4.
CrossRef - Bucolo G, David H. Quantitative determination of Serum Triglycerides by the use of Enzymes. Clin Chem. 1973 May 1;19(5):476-82.
CrossRef - Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without Use of Preparative Ultracentrifuge. Clin Chem. 1972 Jun 1;18(6):499-502.
CrossRef - Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res.1970 Nov;11(6):583-95.
- Carleton H. Histological Techniques. 4th ed. New York, USA, Toronto, London: Oxford University Press; 1979. p. 267
- Nichans WH, Samuelson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968; 6:126-30.
CrossRef - Laslett LJ, Alagona Jr P, Clark III BA, Drozda Jr JP, Saldivar F, Wilson SR, et al. The Worldwide Environment of Cardiovascular Disease: Prevalence, Diagnosis, Therapy, and Policy Issues: A Report from the American College of Cardiology. J Am Coll Cardiol. 2012 Dec 25;60(25, Supplement): S1–49.
CrossRef - Chuah L, Ho W, Beh B, Yeap S. Updates on Antiobesity Effect of Garcinia Origin (−)-HCA. Evidence-Based Complementary and Alternative Medicine. 2013;2013:1-17.
CrossRef - Altiner A, Ates A, EsenGursel F, Bilal T. Effect of The Antiobesity Agent Garcinia Cambogia Extract On Serum Lipoprotein (A), Apolipoproteins A1 And B, And Total Cholesterol Levels In Female Rats Fed Atherogenic Diet. The Journal of Animal & Plant Sciences. 2012;22(4):872-77.
- Koshy A, Anila L, Vijayalakshmi N. Flavonoids from Garcinia cambogia lower lipid levels in hypercholesterolemic rats. Food Chemistry. 2001;72(3):289-294.
CrossRef - Yokozawa T, Cho EJ, Sasaki S, Satoh A, Okamoto T, Sei Y, et al. The protective role of Chinese prescription Kangen-Karyu extract on diet-induced hypercholesterolemia in rats. Biol Pharm Bull 2006;29:760-5.
CrossRef - Ramos RR, Saenz JF, Aguilar MCFA. Control of obesity with Garcinia cambogia Invest Med Int. 1996; 22:97–100.
- Kolodziejczyk J, Masullo M, Olas B, Piacente S, Wachowicz B. Effects of garcinol and guttiferone K isolated from Garcinia cambogiaon oxidative/ nitrative modifications in blood platelets and plasma. Platelets. 2009;20(7):487-92.
CrossRef







