Deepika Purohit1, Manisha Saini2, Nisha Pathak3, Ravinder Verma4, Deepak Kaushik4, Prashant Katiyar5, Pawan Jalwal6 and Parijat Pandey6*
1Department of Pharmaceutical Sciences, Indira Gandhi University, Meerpur, Rewari – 123401, India.
2School of Medical and Allied Science, GD Goenka University, Sohna-Gurgaon Road, Sohna -122103 Haryana, India.
3Faculty of Engineering (Biotechnology), Lunds University, Lund, P.O. Box 118, 22100, Sweden.
4Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak -124001, India.
5Department of Biochemistry and Biochemical Engineering, SHUATS, Naini, Pryagraj-211007, UP-India.
6Shri Baba Mastnath Institute of Pharmaceutical Sciences and Research, Baba Mastnath University, Rohtak – 124001, India.
Corresponding Author E-mail: parijatpandey98@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2054
Abstract
A novel threat to mankind by novel coronavirus infection occurred in December 2019. According to the World Health Organization (WHO) Situation Report-141, 7,039,918 confirmed cases and 404,396 death cases were observed till 9 June 2020 in the different regions of world. Therefore, this article aims to summarize and share the update on the present status of the outbreak and possible treatment options. The present review focuses on latest statistics, diagnostic and preventive measures under study and the future planning of the researchers to discover an effective cure for this threat to the mankind. For carrying out this review, literature searches were performed on Clinicaltrials.gov, official website of WHO, Centers for Disease Control and Prevention, PubMed, Google scholars, etc. Data from these searches was collected and evaluated for getting the available literature on COVID-19 outbreak and drugs under study. The details of history, virology, epidemiology, possible therapeutic options, associated risk factors and preventive measures related to COVID-19 are compiled here in this review. Along with this, some ongoing clinical trials have also been included in this review in order to conclude the efforts of researchers towards controlling this outbreak. The trajectory and severity of this outbreak can’t be predicted at present, but immediate actions are required to be taken in order to develop and implement an effective treatment against the global threat.
Keywords
Coronavirus; COVID-19; 2019-nCoV; Outbreak; SARS-CoV-2; Treatment
Download this article as:| Copy the following to cite this article: Purohit D, Saini M, Pathak N, Verma R, Kaushik D, Katiyar P, Jalwal P, Pandey P. COVID-19 ‘The Pandemic’: An Update on the Present Status of the Outbreak and Possible Treatment Options. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Purohit D, Saini M, Pathak N, Verma R, Kaushik D, Katiyar P, Jalwal P, Pandey P. COVID-19 ‘The Pandemic’: An Update on the Present Status of the Outbreak and Possible Treatment Options. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/2HQYBMf |
Introduction
As of late, a worldwide danger to human wellbeing has risen as Coronavirus Disease 2019 (COVID-19) in December 2019, which is an outbreak of the respiratory. It has been reported to be caused by a novel virus, named coronavirus (SARS-CoV-2 or 2019-nCoV) having structural similarity with the virus causing severe acute respiratory syndrome (SARS). According to the reports of World Health Organization Situation Report-141 (WHO), till 9 June 2020, the different regions of world have reported cases of COVID-19 was 7,039,918 (108,918 cases within one day) and 404,396 deaths (3,539 within one day) globally [1]. The timeline of early outbreak of COVID-19 has been shown in the Fig. 1 [2].
 |
Figure 1: Timeline showing early phases of COVID-19 outbreak. |
The study reported that the Human coronaviruses (HCoVs) as one of the viruses that has evolved most rapidly due to its high rates of recombination and genomic nucleotide substitution [3].
Still, researchers have named six HCoVs, namely HCoV-NL63, HCoV-229E, HCoV-HKU1, HCoV-OC43, middle east respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV). Among these HCoVs, four HCoVs (HCoVNL63, HCoV-229E, HCoV-HKU1 and HCoV-OC43) have been accounted to be circulated in the humans around the globe and have been found to contribute towards about one-third of the infections due to cough and cold in humans [4]. When infection gets severe, these four reported HCoVs were responsible for causing life-threatening disease like bronchiolitis and pneumonia especially, noticed in immune incompetent patients, children, and elderly [5, 6]. According to the WHO report, the first trial for vaccine started within 60 days after receiving the viral genetic sequence shared by China. For ensuring the clear evidence of which treatments are most effective, WHO and its accomplices have composed an enormous worldwide examination, called the Solidarity Trial in many countries to differentiate the diagnosing the treatment methodology [7] against the COVID-19. On the basis of information available from the literature survey and research experience, that is, vital for treatment of other viral infection like Ebola, Malaria, MERS and SARS, while the drugs being used for treating COVID-19 include anti-inflammable and anti-viral drugs, anti-malaria drugs and other agents [8]. Several efforts are also being made all around the world for understanding the clinical efficacy of hydroxychloroquine as postexposure prophylaxis of COVID-19 [9]. Recently, plasma therapy is emerging as a new hope towards treatment of COVID-19 patients [10]. In this review, the authors aim to summarize the details regarding history and outbreak of COVID-19 along with various diagnostic and therapeutic approaches effective and preventive in this early stage of the outbreak.
History and Virology of 2019-nCoV
Initially, it was thought that palm civets were the natural reservoir for coronaviruses. Though, in later phylogenetic studies, it has been reported that bat is the origin of SARS-CoV. Similar clinical manifestations were observed during the MERS-CoV epidemic, it affects the Saudi Arabia in the year 2012. However, the MERS-CoV transmission is geographically limited with cases within the Middle Eastern countries; it is not the case with SARS-CoV [11]. The historical backdrop of human coronaviruses (HCoVs) started in the year 1965, when the two scientists “Tyrrell and Bynoe” observed that they could passage a virus named as B814. This viral infection was found in the tracheal organ and their culture is collected from the respiratory tract of an adult suffering from common cold. They also demonstrate the infectious agent existence by inoculating the culture intranasal in human volunteers. Prior investigation reports suggested that the occurrence of flu upon infection with virus, but previously, the Tyrrell and Bynoe couldn’t culture the infective agent [12].
At about a similar time, in another examination, the researchers Hamre and Procknow gathered the examples from clinical understudies experiencing colds and effectively developed a virus having uncommon properties in tissue culture of those samples and named them as 229E. The report suggested that the both B814 and 229E viruses, were sensitive to ether and that’s why, they probably required a lipid-containing coating surfaces required for infectivity [13].
In another study, McIntosh et al., who were working at the National Institutes of Health in Robert Chanock’s laboratory, reported that the recovery of multiple strains of ether-sensitive agents residing in the human respiratory tract. For this recovery purpose, they were used the similar technique as used by Tyrrell and Bynoe, therefore they named these viruses “OC”, where OC reflects the culture of viruses in organ [14].
In the late 1960s, Tyrrell leads a group of virologists working simultaneously with various animal viruses and the human strains. Their study includes an infectious transmissible gastroenteritis virus of swine, mouse hepatitis virus and bronchitis virus, having similar morphological and physiological character as revealed after carrying out intensive observation study in electron microscopy. This novel group of viruses was termed as a Coronavirus (the term corona meant appearance like a crown of the surface projections), that was, later officially announced as another family of infections [15, 16]. Since 2003-present, five newly identified Human Coronaviruses species come into existence, as listed in a (Table 1).
Table 1: Originated Human Coronaviruses (HCoVs) species
| Virus | Location | Year | Reference |
| SARS | China | 2003 | [17-19] |
| NL63 | Netherlands | 2004 | [20] |
| NL | Netherlands | 2004 | [21] |
| HCoV-NH* | New Haven | 2005 | [22] |
| HKU1 | Hong Kong | 2005 | [23] |
| COVID-19 | China | 2019 | [24] |
Structurally, Coronaviruses can be described as a group of RNA enveloped viruses belong to Coronaviridae family [25]. Related to Roniviridae and Artierivirdae, Coronaviridae is likewise arranged under the same order Nidovirale [26, 27]. The structure of coronavirus has been examined under the electron microscope and was found to be roughly spherical with spike protein forming distinct “club-like” projections [28, 29]. The interior of the virion has been found to contain a positive sense, single-stranded RNA viral genome enclosed in a helically symmetrical nucleocapsid with size (26-32 kilobases) [27]. The positive-sense messenger RNA (mRNA), containing a 51 terminal cap structure and a 3’poly-A tail. The Apo form of protease enzyme present at viral envelop, present in protein data bank (PDB ID: 6M03), Fig. 2 [30].
![Figure 2: Crystal structure of protease COVID-19 (PDB ID: 6M03) [30].](https://biomedpharmajournal.org/wp-content/uploads/2020/10/Vol_13_No_4_Cov_Deep_Fig2-150x150.jpg) |
Figure 2: Crystal structure of protease COVID-19 (PDB ID: 6M03) [30]. |
COVID-19 and SARS-CoV-2 – Epidemiology and Pathology
All CoVs identified till now, a pleomorphic RNA virus with a very high rate of recombination on account of continuously developing RNA dependent-RNA Polymerase. COVID-19 mainly attacks on the respiratory system by following the entry into the lungs. Coronavirus reaches to lungs alveoli, and starts damaging them [31]. Normally, human lung alveoli have two types of pneumocytes, distinguished as Type-1 Pneumocytes and Type-2 Pneumocytes. The functional role of Type-1 pneumocytes and Type-2 pneumocytes, incorporates the gaseous exchange of CO2 and O2, and also had role in the production of surfactants, respectively. [32]. This virus affects the Type-2 pneumocytes after entering into the lungs. This virus contains a S-Spike protein and a single stranded RNA as a genetic material. These S type-spike proteins are attached with the Angiotensin Converting Enzyme-2 (ACE-2) receptors. These receptors permits the viral infection to enter inside the host cell, which leads to a release of single-stranded RNA (ss-RNA), and binds to the ribosomal machinery of the host cell and initiates the protein synthesis of viral structural proteins. Its ss-RNA help to synthesize the additional RNA molecules in the presence of RNA-Dependent RNA-polymerase (RDRP), that has a role to synthesized the viral structural protein components such as Spikes, Capsids, and various kinds of cellular enzymes etc. All of these structural components and synthesized RNA together constitutes the virus cellular machinery. This process of RNA replication repeatedly occurred to make up the viral copy in a huge amount [31, 33].
Following a different route of entry inside the host cells, Coronavirus specifically, damaged the Type-2 pneumocytes, leading to a decreased or with no surfactants production, resulting into an increased surface tension. Because of increased surface tension, it causes a collapsing of lung alveoli, raised the detrimental effect of hypoxia (in which less gaseous exchange occurred). Due to this event, neutrophils become in active mode, initiates the proteases to activate the reactive oxygen species (ROS) to kill the virus and infective species. Activated ROS, also damaged the normal Type-1 and Type-2 pneumocytes raising the critical complications of lesser exchange of O2 in respect to CO2, which ultimately decrease the oxygen concentration in a blood. Meanwhile, cellular signaling component, i.e. cytokines secreted from lung alveoli and transmit their signals to the hypothalamus, which stimulates the release of prostaglandin, a hormone, which raised the body temperature [31] of an infected host. A schematic diagram of pathophysiology pathway of COVID-19 is displayed in a Fig. 3.
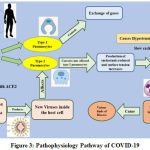 |
Figure 3: Pathophysiology Pathway of COVID-19. |
SARS-CoV-2 displayed the high rate of mutation, that’s why, diversified in nature, it is found in a diverse array of animals and humans with different state of clinical manifestations ranging from asymptomatic infections to need of hospitalization, leading to series of infections aroused in neurological, gastrointestinal, respiratory and hepatic systems [34].
COVID-19 Outbreak in China
Wuhan, Hubei province, China, recognized a sudden outbreak of pneumonia among the people. The reported unknown cases in China and across the world had attracted the world. These cases of pneumonia were later demonstrated to be related with a novel coronavirus which was named as 2019-nCoV, that was identified by scientists in China by 7 January 2020, from infected patients in Wuhan. By January 30, 2020, the reported number of confirmed cases was reached to 9692 out of 15,238 suspected cases, it has prevalence in around 31 cities and prominently exists in a China. Out of 15,238 confirmed cases, about 1527 cases were severe. The disease existence is because of contact and respiratory droplets as reported by National Health Commission of People’s Republic of China. The 2019-nCoV genetic sequence empowered the advancement of point-of-care real-time diagnostic tests explicit for COVID-19 at a beginning phase of the outbreak [35-37]. The COVID-19 Cases were no longer limited to Wuhan city. A great increment in COVID-19 Cases has been reported till date, already reported by WHO (28th May 2020), a region wise details of which has been summarized in the Table 3 below. At present the United States of America has been found to have the maximum number of COVID-19 cases (3,366,251 cases) till now, reported by WHO [1]. Globally situation of COVID-19 cases existing is presented in a Table.3.
Table 3: The reported active and death cases of COVID-19 cases globally (Data as of 9th June 2020).
| S. No. | Region | Total reported cases (new cases in last 24 hours) | Total deaths (new deaths in last 24 hours) |
| 1. | Africa | 140,498 cases (5,086) | 3 352 deaths (116) |
| 2. | Americas | 3,366,251 cases (54,864) | 183,950 deaths (2,146) |
| 3. | Eastern Mediterranean | 658,614 cases (17,185) | 14,913 deaths (311) |
| 4. | Europe | 2,303,361 cases (16,801) | 184,671 deaths (551) |
| 5. | South-East Asia | 378,118 cases (13,922) | 10,376 deaths (406) |
| 6. | Western Pacific | 192,335 cases (1,060) | 7,121 deaths (9) |
Clinical Manifestations for COVID-19 cases
Doctors and hospitals all around the world are testing the decades-old antimalarial drugs for the treatment of COVID-19 patients by applying drug repurposing technique on already existing therapies in a race for developing an effective treatment option. Antimalarial drugs chloroquine phosphate and hydroxychloroquine have given an early indication of progress in manifestations of patients with COVID-19 positive status, in light of the reports by specialists and scientists of South Korea, France and China, and other U.S Physicians are also using the drugs [38]. On the basis of currently available epidemiological information, the initial symptoms after infected with COVID-19 includes cough, fever, dyspnea, sputum production, fatigue, hemoptysis and headache [39]. while less common symptoms of red or irritated eyes, no taste or sore throat sense, no smell sensation was seen, even diarrhoea, stuffy and running nose with aches [40] was not clearly observed in positive patients The currently available data suggested that the age of disease onset in pediatric patients has been 1.5 months, that is, ranging to 17 years [41, 42]. Children infected with 2019-nCoV may have asymptomatic infection or may have minor symptoms of fever, dry cough, and fatigue, or in some cases upper respiratory symptoms can also registered in some cases, which includes the running nose with nasal congestion [43]. But in some cases, this disease may also cause the lower respiratory tract infection. The reported data of COVID-19 severe cases obtained from adult patients has revealed that, predominantly observed dyspnea symptoms arises within one week after the onset of disease. In such cases, if it is remained untreated then it may lead to a rapid progression of refractory metabolic disorders like acidosis, septic shock, coagulation dysfunction and acute respiratory distress syndrome (ARDS) [44]. And it characterized the COVID-19 clinical manifestations elaborately in a Table 4 [45].
Table 4: Clinical manifestations of COVID-19
| S. No. | Clinical manifestation | Description |
| 1. | Silent or asymptomatic infection | Positive tests for COVID-19, but clinical chest imaging findings are normal |
| 2. | Acute infection in upper respiratory tract | Only pharyngeal pain, headache, fever, fatigue, nasal congestion, cough, discomfort, etc., with no signs of pneumonia or chest imaging. |
| 3. | Mild pneumonia | Children may or may not have respiratory symptoms like fever, cough, and indication of pneumonia chest imaging. |
| 4. | Severe pneumonia | Patients with any of the following symptoms
1) Increased respiratory rate: ≥ 50 times/min (≥ 1 year) ≥ 70 times/min (< 1 year) 2) Hypoxia: assisted breathing 3) Oxygen saturation < 92% 4) Difficulty in feeding or food refusal, with signs of dehydration 5) Disturbance of consciousness: coma, convulsion, or somnolence |
| 5. | Critical cases | Patients meeting the following criteria and need ICU:
1) Failure of respiratory system and requirement of mechanical ventilation 2) Shock in association with failure of other organs system |
Auxiliary Examinations of Infected with COVID-19
The auxiliary examinations for COVID-19 include both laboratory examination and chest imaging examination. Both these examinations helped in determining the disease severity and recommended treatment commenced, in order to, save the life of the patients.
Laboratory Examination [41]
Fig. 4. depicts the laboratory examination at different stages of COVID-19.
![Figure 4: Laboratory examination demonstrate the COVID-19 different stages [41]](https://biomedpharmajournal.org/wp-content/uploads/2020/10/Vol_13_No_4_Cov_Deep_Fig4-150x150.jpg) |
Figure 4: Laboratory examination demonstrate the COVID-19 different stages [41]. |
Recommended Potential Treatments Upon Infected with COVID-19
Unfortunately, a very little or no treatment options are available today, for suddenly occurring viral diseases and being the similar case, still no effective treatment or vaccine developed to prevent from the infection caused by 2019-nCoV. Several molecules are being studied in-vitro, they are proven to effectual for the treatment of COVID-19, but these are also based upon the previous trials on human MERS-Cov and SARS-CoV infections [46]. Some featured medications are accounted for in this examination, as an antiviral medication, Remdesivir has been effectively actualized in first announced instance of COVID-19 found in the United States of America, albeit, more contextual investigations are expected to show the medication efficacy to affirmed as a treatment against the COVID-19 [46, 47] infection. Some of the drugs/molecules are still being to be evaluated as treatment of COVID-19, that is, already summarized in a Table 5. Furthermore, additional observational and clinical studies are being conducted to illustrate a potential treatment against the COVID-19 infection. Table 6, summarized a few clinical trials are being conducted at different stages in order to develop the possible effective therapy against this pandemic as obtained from clinicaltrials.gov [48].
Plasma Therapy
Recently, plasma therapy appears to be a promising development in the race to signify a treatment against the viral infection. Researchers all around the globe tested this experimental therapy, depicting the transfusion of antibody-rich blood serum of recovered COVID-19 patients into those people who are struggling with this serious illness. In the underlying preliminary period of the investigation around 5,000 seriously ill patients have received the blood plasma transfusions, and it was noted during the plasma therapy there is less chances of side effects occurred occasionally while observing the significant improvement signs [10].
Table 5: Studies conducted to demonstrate the clinical treatment for COVID-19.
| S. No. | Type of report | Category of drug | Drugs applied or suggested | Outcomes | Ref |
| 1. | Clinical observation | Anti-inflammatory and antimalarial | Glucocorticoids, Janus kinase (JAK) inhibitors, IL-6 antagonist, and hydroxychloroquine/ chloroquine | Improvement in clinical outcomes | [49] |
| 2. | Open label non-randomized clinical trial with 6 patients suffering from COVID-19 | Antiviral plus anti-inflammatory | Hydroxychloroquine plus azithromycin for 6 days | An improved efficacy for eradicating the virus | [50] |
| 3. | In vitro cytotoxicity and antiviral tests | Antimalarial, anti-inflammatory | Hydroxychloroquine and chloroquine | Efficacy and safety of Hydroxychloroquine against COVID-19 | [51] |
| 4. | Opinion paper | Antiviral, Antimalarial, and others | Hydroxychloroquine chloroquine and several other therapeutic agents | Efficacy and safety of Hydroxychloroquine over chloroquine | [52] |
| 5. | Open label clinical trial | Antiviral | Favipiravir vs control (lopinavir or ritonavir). Interferon alfa | Efficacy of favipiravir over control | [53] |
| 6. | In vitro murine TH17 cell study | Anti-inflammatory | JAK2 inhibitor fedratinib | Effectiveness of fedratinib in reducing cytokine storm associated with COVID-19 | [54] |
| 7. | In vitro infected vero cells | Anti-malarial
|
Hydroxychloroquine and chloroquine
|
Efficacy of Hydroxychloroquine over chloroquine | [55] |
| 8. | Case study; COVID-19 induced pneumonia on a hemodialysis patient | Antiviral | Lopinavir plus ritonavir | Improved clinical symptoms | [56] |
| 9. | Cell culture and pangolin coronavirus modelling | Anti-inflammatory/
antineoplastic, antiparasitic, |
Cepharanthine, selamectin and mefloquine | Complete inhibition of cytopathic effects in cell culture by all three drugs | [57] |
Table 6: List of ongoing clinical trials on COVID-19 cases.
| S. No. | Trial ID | Age range | Title | Phase | Interventions | Outcome measures | Ref |
| 1 | NCT04324489 | 18-70 Yrs | DAS181 for Severe COVID-19: Compassionate Use | Recruitment completed | DAS181 | · Improved clinical status
· Discharge · Death |
[58] |
| 2 | NCT04343768 | Adults | An investigation into beneficial effects of interferon beta 1a, compared to interferon beta 1b and the base therapeutic regiment in moderate to severe COVID-19: A randomized clinical trial | Phase II | Hydroxychloroquine
Interferon Beta-1A Interferon Beta-1B Lopinavir / Ritonavir
|
· Time to clinical improvement
· Mortality · Duration of hospitalization |
[59] |
| 3 | NCT04244591 | Adults | Glucocorticoid therapy for COVID-19 critically ill patients with severe acute respiratory failure | Phase II
Phase III |
Methylprednisolone therapy
Standard care |
· The difference of PaO2/FiO2 between two groups
· Mechanical ventilation support · Clearance of SARS-CoV-2 · All-cause mortality |
[60] |
| 4 | NCT04291729 | 18-75 Yrs | Evaluation of Ganovo (Danoprevir) combined with ritonavir in the treatment of SARS-CoV-2 infection | Phase IV | Ganovo + ritonavir +/- Interferon nebulization | · Time to recovery
· Rate of no fever, no cough, no dyspnea, and no requirement of supplemental oxygen · Rate of undetectable new coronavirus pathogen nucleic acid · Rate of mechanical ventilation, ICU admission and serious adverse event |
[61] |
| 5 | NCT04261517 | 18 years | Efficacy and safety of hydroxychloroquine for treatment of COVID-19 | Phase III | Hydroxychloroquine | · The virological clearance rate of throat swabs, sputum, or lower respiratory tract secretions at day 3, day 5, and day 7
· The mortality rate of subjects at weeks 2 |
[62] |
| 6 | NCT04378712 | 18-75 Yrs | Hydrogen/Oxygen mixed gas inhalation for Coronavirus Disease 2019 (COVID-19) | Recruitment completed | Hydrogen Oxygen Generator with Nebulizer | · The proportion of patients with improved disease severity at day 2, 3 and at the day before hospital discharge
· The change from baseline in oxygen saturation, dyspnea scale, cough scale, chest distress scale and chest pain scale at day 2, 3, and at the day before hospital discharge |
[63] |
| 7 | NCT04358614 | 18-75 years | Baricitinib Therapy in COVID-19 | Phase II
Phase III |
Baricitinib 4mg Oral Tablet | · Impact of baricitinib in terms of clinical, laboratory, respiratory parameters
· ICU admission and discharge rate |
[64] |
| 8 | NCT04276688 | Adult
Older Adult |
Lopinavir/Ritonavir, Ribavirin, and IFN-beta combination for the treatment of COVID-19 | Phase II | Lopinavir/ritonavir
Ribavirin Interferon Beta-1B |
· Time to clinical improvement
· Hospitalization · Mortality |
[65] |
Based on the ailments of the people suspected to have the disease, disconnection must be done in a solitary room or self-confinement at home be followed according to the specialists’ recommendation. The patients with confirmed infections can be admitted in the same isolation ward as for suspected patients. While, the reported critical cases should be immediately admitted to an ICU as earliest as possible. The general and symptomatic treatments presently reported for COVID-19 already described in a Fig. 5 [66].
 |
Figure 5: Details of general and symptomatic treatments available for COVID-19. |
The specialists everywhere throughout the world have distinguished the treatment or discover alternatives treatment and adjuvant treatments which can be utilized and are being utilized for the treatment of COVID-19 in this beginning stage. These are discussed here in this study report:
Oxygen Therapy
In COVID-19 cases where hypoxia appears, the patient should be given effective oxygen therapy immediately using mask oxygen and nasal catheter. In extreme cases, obtrusive or non-intrusive mechanical ventilation and nasal high-stream oxygen treatment ought to be given if important. [67].
Antiviral Therapy: Lopinavir and Remdesivir
Lopinavir or Litonavir are the recommended anti-viral drug, tried to evaluate its effectiveness against COVID-19 in adult patients, but no successful data has been reported yet [47, 68]. Recent report declares the Japanese organization (Gilead sciences) had approved, a drug named Remdesivir to manufacture in the country, a first officially authorized drug to tackle the disease. On account of this, Japan had reached the decision within three days after the US drug organization has filed for fast-track approval of the drug [69].
Ivermectin
Ivermectin is an anti-parasitic drug with antiviral potential against a number of viruses like dengue, HIV, influenza and Zika. The drug acts by inhibiting the interaction of viral protein with importin α/ß1 heterodimer which normally participates in nuclear transport of viral protein. In a study conducted by Kylie Wagstaff of Monash Biomedicine Discovery Institute, a single dose of drug was found to significantly reduce the viral RNAs stability within 48 hours [70, 71].
Another antiviral drug Darunavir, known to have HIV protease inhibitor observed during the clinical trials evaluation against coronavirus infection. This drugs functionally acts by blocking the viral proteases by forming the hydrogen bonds. Due to which, it generates an enzyme-inhibitor complex which further prevents the cleavage of polyproteins, thus producing non-infectious and immature viral particles [72]. A combination of Darunavir and Cobicistat are being prescribed to COVID-19 patients under clinical trials considering it to be an efficacious treatment option [73].
Interferon‑α
Interferon-α have been found to decrease the viral load at the initial stage of infection and thus, it helps in relieving the symptoms and to reduce the disease progression. Based on accessible information on clinical examination and the prior consequences of utilizing interferon-α for the treatment of intense viral contamination of upper respiratory, pneumonia, SARS, bronchiolitis, hand foot mouth sickness, and different diseases brought about by the infection in youngsters, the recommended interferons practices are suggested [74].
Other Agents or Drugs: Anti‑influenza Drugs
Arbidol, an anti-influenza drug can be prescribed for adult patients suffering from COVID-19; however, the safety and efficacy of the drug is not much clear yet. Some other drugs of this category like Oseltamivir can be applied and tested for their efficacy [75].
Humanized Monoclonal Antibody
Human monoclonal antibodies are also among the drugs being evaluated for the treatment of COVID-19. A Tocilizumab, a humanized monoclonal antibody, an immune suppressant drug, utilized for the treatment of rheumatoid arthritis by binding specifically to interleukin-6 (IL-6) receptors. This drug has been suggested by China for the treatment of COVID-19 just because of its safety and efficacy, as shown in clinical practices. The drug has been found to be effective in some severe COVID-19 cases reported in Naples and Italy [72]. Another known drug Siltuximab, having reaction mechanism similar to Tocilizumab, has also been evaluated in a several reported clinical studies and found to be effective against the COVID-19 [76].
Other Recommended Treatment and Precautions
Psychotherapy
For a proper recovery from this disease, a psychological counseling could be effectively useful strategy. Psychotherapy is required in cases where the patients showing symptoms of psychological disorders, fear, or mood swing and for this active psychological intervention can also recommended for treatment [77].
Release and Discharge Criteria
The patients with confirmed infection can be released from isolation wards or if required, they can transfer to the related departments to treat other similar kind of diseases. This can be done only if the patient meets all the following inclusion criteria [78, 79]
Improvement in respiratory symptoms
Normalization of the body temperature longer than 3 days
The test reports should be negative for respiratory pathogenic nucleic acid for two times in a row with sampling interval of at least 1 day.
Prevention
COVID-19 is a novel communicable outbreak that has affected the human globally. This infection has been categorized as category-B infectious disease legally but being managed as category A. It is very important that infection control practices must be implemented for controlling the source of disease, blocking the route of transmission, and protecting the immune incompetent populations [79].
Risk Factors
As this chronic infection has emerged as a new disease, along these lines, there is restricted data with respect to hazard factors for serious illness. The currently available scientific reports and clinical expertise suggested that those individuals who are affected by infection are older aged adults or people of any ages and they have serious underlying medical conditions might be at higher risk of severe illness from COVID-19 [80]. The major risk factors observed are as follows
Age >65 years old
Open exposure to artificial environment
Patients with medical conditions (chronic lung disease or moderate to severe asthma, rheumatic diseases [81], serious heart conditions, immune incompetent patients like HIV & AIDS infections [82], severe obesity, diabetes, chronic kidney disease.
Risk of Infection in Pets and Other Animals
The Centre for disease control and prevention (CDC) announced the alert of a small number of pets worldwide, including cats and dogs, and expected to be infected with the virus that causes COVID-19, mostly after close contact with those peoples who are infected with COVID-19. Thus, no such literature is available to date, describing the animals risk and COVID-19 transmission to people is considered to be low. It was notified in some study that the COVID-19 virus can cause and spread infection from people to animals. So, just to avoid this, pets should also be treated as other human family members, should not be allowed to come in contact with suspected people or animals outside the household. If a particular individual inside the household carried an infection, that individual should be take care of all family members and remain isolated from everyone else in the family, including pets [83].
Discussion
The coronavirus has affected a large portion of population worldwide and have taken up lives of so many people across the globe. This is a rapidly evolving situation; so much information and clarity on the aspects are still not available. The most common clinical manifestations associated with COVID-19 involves cough, fever, sputum production, fatigue, sore throat, shortness of breath, headache, conjunctivitis, pleuritic pain, etc. [84, 85]. Washing hands, wearing masks, and surface disinfection have been found contribute towards decreasing the risk of infection [86]. The countries around the globe have increased the rate of testing for potential cases and isolating the infected people in any possible way to prevent the spread of COVID-19 [87]. In acute respiratory infection, reverse transcriptase based-PCR is routinely used. In this circumstance, more swab testing to distinguish infections in the respiratory discharges is required which may help in early screening of the presumed cases [88].
It is very important to identify proper treatment against COVID-19. A number of studies for SARS-CoV-2 have sprung up since the outbreak of this epidemic COVID-19. In-depth of understanding the SARS-CoV-2 has an underlying pathogenic mechanism that is target based therapy of COVID-19. Based on the currently available literature it has been observed that different phases are associated with COVID-19 including infection, invasion and viral replication and then immune response of human body, up to hyper-inflammation. As the disease is highly complex in nature, a multidisciplinary approach entangled towards its treatment can be best option. From the beginning of this outbreak, a lot of information is being published every day regarding diagnosis and treatment of COVID-19. All this information is mainly based on preliminary experiments and experiences gained from retrospective studies. Among all the proposed treatment options for SARS-CoV-2 infection, antimalarials, antivirals, biotechnological molecules, corticosteroids, interferons anticoagulants and convalescent plasma are commonest [89].
For COVID-19, different curative factors have been recommended and evaluated, but none of factors shown proper effective in treatment. Intravenous Remdesivir, tested in 1063 COVID patients for 10 days. This study noted the time to recovery engaged either in hospitalization upon infection or discharge from the hospital. Remdesivir was only the drug, which proof to be effective in terms of time shortening to recovery [90]. The Food and Drug Administration has also made Remdesivir available under an emergency-use authorization to treat the patients with severe SARS-CoV-2 infection [91]. In a study, conducted by Wang et al. the effectiveness of remdesivir was evaluated against SARS-CoV-2. For this, time-of-addition assay was used by implementing Vero E6 cells and remdesivir showed effectiveness as treatment option on administration after 2 hours of infection, but not as prophylactic administered prior to the SARS-CoV-2 infection [92]
Recently, intravenous immunoglobulin, plasma therapy, emapalumab and etoposide have shown effectiveness for the treatment of this infection as a rescue therapy [93, 94]. A clinical trial has also been registered recently in which the efficacy and safety of emapalumab and anakinra have been evaluated in COVID-19 [95]. Targeting IL-1 with monoclonal antibodies like Canakinumab is another approach which has been approved by the Italian drug agency for the treatment of COVID-19 pneumonia. A phase 2 clinical trial is ongoing to evaluate the effectiveness of Canakinumab in the treatment of COVID-19 pneumonia [96].
The effectiveness of plasma therapy is associated with its low rate of causing serious adverse effects which makes is advantageous to be used over other unproven therapies for SARS-CoV-2 infection; however more evidences related to its efficacy are still required [97]. The use of immunosuppressants such as corticosteroids is very controversial, and it may be appropriate for some to mitigate the impact of the SARS-CoV-2 mediated cytokine storm. Dexamethasone is showing to reduce death rates and has shown efficacy particularly in critically ill patients . There were 2104 patients who received a dose of 6 mg per day in this study. The risk of death in ventilated patients was decreased by one third in the dexamethasone arm [98].
Another effective drug has been found to be hydroxychloroquine alone or in combination with azithromycin which has shown effectiveness in various in vitro pre-clinical studies. Although in vitro results have been found to be efficacious, the translational value of these pre-clinical studies to clinical stage is yet to be established at a great level [99].
The experts suggested that the if no effective vaccine and drugs are widely implemented at early stage of this pandemic and it is expected that by the year 2022, COVID-19 cases might affect 90% of the global population and more than 40 million individuals may lose their life [100, 101]. Therefore, effective and safe treatment options for COVID-19 are required to be developed as early as possible. The sooner the treatment identified and developed, the better will be the outcomes. Early diagnosis, quarantine, and supportive treatments are playing a crucial role in curing the patients. In this current study, authors aim to review and focused the available context relevant to disease parameters including the epidemiology, prevention and diagnosis, and current treatment options for this infection. Alongside that, some clinical preliminaries exploring treatment alternatives for COVID-19 have likewise been examined.
Acknowledgement
Authors thanks to Mr. Dhaval Desai and Mr. Udit Malik for their valuable suggestions and assistance in the preparation of the manuscript.
Ethical Approval
As this is a review of literature, no such approval is required.
Funding Source
No Funding resource supports this study.
Conflict of Interest
No conflict of interest is declared by any of the authors of the study.
References
- World Health Organization [homepage on the internet]. Coronavirus Disease (COVID-19) Situation Report-128 [updated 2020 June 9; cited 2020 May 10]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200518-covid-19-sitrep-119.pdf?sfvrsn=4bd9de25_4.
- World Health Organization [homepage on the internet]. WHO Timeline COVID-19. [cited 2020 May 4]. Available from: https://www.who.int/news-room/detail/27-04-2020-who-timeline—covid-19.
- Vijgen L, Keyaerts E, Moës E, et al. Development of one-step, real-time, quantitative reverse transcriptase PCR assays for absolute quantitation of human coronaviruses OC43 and 229E. J. Clin. Microbiol. 43:5452-5456, 2005.
CrossRef - Raoult D, Zumla A, Locatelli F, et al. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress [serial on the internet]. 2020 [cited 2020 May 15]. Available from: https://doi: 10.15698/cst2020.04.216.
CrossRef - Gorse GJ, O’Connor TZ, Hall SL, et al. Human coronavirus and acute respiratory illness in older adults with chronic obstructive pulmonary disease. J. Infect. Dis. 199:847-857, 2009.
CrossRef - Walsh EE, Shin JH, Falsey AR. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J. Infect. Dis. 208:1634-1642, 2013.
CrossRef - World Health Organization [homepage on the internet]. WHO Director-General’s opening remarks at the media briefing on COVID-19 – 18 March 2020. [updated 2020 March 18; cited 2020 May 3]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—18-march-2020.
- Rabby MII. Current drugs with potential for treatment of COVID-19: A Literature Review. J. Pharm. Sci. 23:58-64, 2020.
CrossRef - S. National Library of Medicine [homepage on the internet]. ClinicalTrial.gov [cited 2020 May 9]. Available from: https://clinicaltrials.gov/ct2/show /NCT04308668.
- Convalescent plasma is safe to treat COVID-19: nationwide study [homepage on the internet] [cited 2020 May 19]. Available from: https://www.nbcnews.com/health/health-news/convalescent-plasma-safe-treat-covid-19-nationwide-study-n1206126.
- Funga SY, Yuen KS, Ye ZW, et al. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerging Microbes. Infect. 9:558-570, 2020.
CrossRef - Tyrrell DA, Bynoe ML. Cultivation of viruses from a high proportion of patients with colds. Lancet. 1:76-77, 1966.
CrossRef - Hamre D, Procknow JJ. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 121:190-193, 1966.
CrossRef - McIntosh K, Becker WB, Chanock RM. Growth in suckling-mouse brain of “IBV-like” viruses from patients with upper respiratory tract disease. Proc. Natl. Acad. Sci. USA. 58(6):2268-2273, 1967.
CrossRef - McIntosh K, Dees JH, Becker WB, et al. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc. Natl. Acad. Sci. USA. 57:933-940, 1967.
CrossRef - Witte KH, Tajima M, Easterday BC. Morphologic characteristics and nucleic acid type of transmissible gastroenteritis virus of pigs. Arch. Gesamte. Virusforsch. 23:53-70, 1968.
CrossRef - Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976, 2003.
- Ksiazek TG, Erdman, D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966, 2003.
CrossRef - Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 361:1319-1325, 2003.
CrossRef - van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJM, Wolthers KC, et al. Identification of a new human coronavirus. Nat. Med. 10:368-373, 2004.
CrossRef - Fouchier RA, Hartwig NG, Bestebroer TM, et al. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. USA. 101:6212-6216, 2004.
CrossRef - Esper F, Weibel C, Ferguson D, et al. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J. Infect. Dis. 191:492-498, 2005.
CrossRef - Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79:884-895, 2005.
CrossRef - Huang WH, Teng LC, Yeh TK, et al. 2019 novel coronavirus disease (COVID-19) in Taiwan: Reports of two cases from Wuhan, China. J Microbiol Immunol Infect [serial on the internet]. 2020 [cited 2020 May 16]. Available from: https://doi.org/10.1016/j.jmii.2020.02.009.
CrossRef - Tyrrell DA, Almeida JD, Cunningham CH, et al. Coronaviridae. Intervirol. 5:76-82, 1975.
CrossRef - Masters PS. The molecular biology of coronaviruses advances in virus research. Academic Press: Massachusetts, MA, USA. 193-292, 2006.
CrossRef - McBride R, Fielding BC. The role of severe acute respiratory syndrome (SARS)-coronavirus accessory proteins in virus pathogenesis. Viruses. 4:2902-2923, 2012.
CrossRef - Marsolais G, Berthiaume L, DiFranco E, Marois P. Rapid diagnosis by electron microscopy of avian coronavirus infection. Can. J. Comp. Med. 35(4):285-288, 1971.
- Kolesnikova L, Slenczka W, Brodt H. Electron microscopy in diagnostics of SARS case. Microsc. Microanal. 9:438-439, 2003.
CrossRef - Protein Data Bank [homepage on the internet]. The crystal structure of COVID-19 main protease in apo form (PDB ID: 6M03) [updated 2020 March 11; cited 2020 May 5]. Available from: https://www.rcsb.org/structure/6m03.
- Mundhe S. Covid-19: Pathophysiology at a Glance [updated 2020 April 25; cited 2020 June 5]. Available from: https://timesofindia.indiatimes.com/readersblog/pharmdiansblog/covid-19-pathophysiology-at-a-glance-13903/.
- Ferng A. Alveoli [cited 2020 June 5]. Available from: https://www.kenhub.com/en/library/anatomy/alveoli.
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clinical Immunology. 215:108427-108434, 2020.
CrossRef - Sahin AR, Erdogan A, Agaoglu PM, et al. 2019 novel coronavirus (COVID-19) outbreak: A review of the current literature. Eurasian J. Med. Oncol. 4(1):1-7, 2020.
CrossRef - US Centers for Disease Control and Prevention [homepage on the internet]. First travel-related case of 2019 novel coronavirus detected in United States. Atlanta, GA: US Centers for Disease Control and Prevention, 2020 [cited 2020 May 5]. Available from: https://www.cdc.gov/media/releases/2020/p0121-novel-coronavirus-travel-case.html.
- World Health Organization [homepage on the internet]. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected [cited 2020 May 6]. Available from: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
- Beijing: China National Health Commission, 2020 [homepage on the internet] [cited 2020 May 6]. Available from: http://www.nhc.gov.cn/xcs/yqfkdt/202001/c5da49c4c5bf4bcfb320ec2036480627.shtml
- Doctors turn to malaria drugs as potential coronavirus treatment [homepage on the internet]. [cited 2020 May 7]. https://www.wsj.com/articles/doctors-turn-to-malaria-drugs-as-potential-coronavirus-treatment-11584729626.
- Li Z, Liu T, Yang N, et al. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 1-9, 2020.
CrossRef - World Health Organization [homepage on the internet]. Coronavirus disease (COVID-19) [updated 2020 June 8; cited 2020 May 10]. Available from: pandemichttps://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update-24-immuniy-n-clinical-manifestations.pdf?sfvrsn=7c84a6bf_4
- Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person to-person transmission: a study of a family cluster. Lancet. 395:514-523, 2020.
CrossRef - Shen K, Yang Y, Wang T, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J Pediatr [serial on the internet]. 2020 [cited 2020 May 16]. Available from: https://doi.org/10.1007/s12519-020-00343-7.
CrossRef - Chen NS, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 6736(20):30211-30217, 2020.
CrossRef - World Health Organization [homepage on the internet]. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected [cited 2020 May 16]. Available from: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
CrossRef - World Health Organization [homepage on the internet]. Coronavirus disease (COVID-19) pandemic [cited 2020 May 18]. Available from: https://www.who.int/healthtopics/coronavirus#tab=tab_1.
- Centers for Disease Control and Prevention [homepage on the internet]. Information for Clinicians on Investigational Therapeutics for Patients with COVID-19 [cited 2020 May 17]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html.
- Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of Remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 11:222-235, 2020.
CrossRef - S. National Library of Medicine. [homepage on the internet]. ClinicalTrials.gov. [cited 2020 May 17]. Available from: https://clinicaltrials.gov/ct2/results?cond=COVID19&Search=Apply&recrs=e&age_v=&gndr=&type=&rslt=.
- Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe corona virus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 214:108393-108412, 2020.
CrossRef - Gautret P, Lagiera JC, Parolaa P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open label non-randomized clinical trial. Int. J. Antimicrob. Agents. 105949-105972, 2020.
- Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6:16-29, 2020.
CrossRef - Sahraei Z, Shabani M, Shokouhi S. Aminoquinolines against Coronavirus Disease 2019 (COVID-19): Chloroquine or Hydroxychloroquine. Int. J. Antimicrob. Agents. 55(4):105945-105959, 2020.
CrossRef - Cai Q, Yang M, Liu D, et al. Experimental treatment with Favipiravir for COVID-19: An open-label control study. Engineering [serial on the internet]. 2020 [cited 2020 May 16]. Available from: https://doi.org/1016/j.eng.2020.03.007.
CrossRef - Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microb. Immunol. Inf. [serial on the internet]. 2020 [cited 2020 May 17]. Available from: https://doi.org/10.1016/j.jmii.2020.03.005.
CrossRef - Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Inf. Dis. [serial on the internet]. 2020 [cited 2020 May 17]. Available from: https://doi.org/: 1093/cid/ciaa237.
CrossRef - Tang B, Li S, Xiong Y, et al. Coronavirus disease 2019 (COVID-19) pneumonia in a hemodialysis patient. Kidney Med. [serial on the Internet]. 2020 [cited 2020 May 17]. Available from: https://doi.org/11016/j.xkme.2020.03.001.
- Fan HH, Wang LQ, Liu WL, et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus (2019-nCoV) related coronavirus model. Chinese Med. J. [serial on the internet]. 2020 [cited 2020 May 17]. Available from: https://doi.org/10.1097/CM9.0000000000000797.
CrossRef - S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT04324489 (accessed 9 May 2020).
- S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04343768.
- S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT04244591.
- S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT04291729.
- S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT04261517.
- S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT04378712.
- S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT04358614.
- S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT04276688.
- S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://www.gov.uk/government/publications/covid-19-stay-at-home-guidance/stay-at-home-guidance-for-households-with-possible-coronavirus-covid-19-infection.
- S. National Library of Medicine [homepage on the Internet]. Clinical management of suspected or confirmed COVID-19 disease [cited 2020 May 18]. Available from: http://www.nicd.ac.za/wpcontent/uploads/2020/03/Clinical_management_of_suspected_or_acute_COVID_V1.1_13.03.20_updated.pdf.
- Baden LR, Rubin EJ. Covid-19 – The search for effective therapy. N. Eng. J. Med. The Editorial. 1-2, 2020.
CrossRef - Japan approves Gilead Sciences’ Remdesivir as COVID-19 drug [homepage on the Internet]. [cited 2020 May 19]. Available from: https://in.reuters.com/article/health-coronavirus-japan-remdesivir/japan-approves-gilead-sciences-remdesivir-as-covid-19-drug-idINKBN22J1UT.
- Yang SN, Atkinson SC, Wang C. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 177: 104760, 2020.
CrossRef - Wagstaff K.M., Sivakumaran H., Heaton S.M. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 443:851-856, 2012.
CrossRef - Manhas S, Anjali A, Mansoor S, et al. Covid-19 Pandemic and Current Medical Interventions. Arch. Med. Res. 2020 (In Press) (https://doi.org/10.1016/j.arcmed.2020.05.007).
CrossRef - S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 June 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT04252274.
- All about Cuba’s ‘wonder drug’ being pitched against coronavirus [homepage on the Internet]. [cited 2020 May 19]. Available from: https://www.theweek.in/news/health/2020/03/24/all-about-Cubas-wonder-drug-being-pitched-against-coronavirus.html.
- Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Pan. Am. J. Public Health. [serial on the internet]. 2020 [cited 2020 May 19]. Available from: https://doi.org/26633/RPSP.2020.40
- S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 June 8]. Available from: clinicaltrials.gov/ct2/show/NCT04329650.
- Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Military Med. Res. 7(4):1-23, 2020.
- Adhikari S, Meng S, Wu Y, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 9:29-40, 2020.
CrossRef - Novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – sixth update [homepage on the Internet]. [cited 2020 May 20]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/RRA-sixth-update-Outbreak-of-novel-coronavirus-disease-2019-COVID-19.pdf.
- Centers for Disease Control and Prevention [homepage on the Internet]. Coronaovirus Disease 2019 (COVID-19) [cited 2020 May 20]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html.
- Gabriel FP, Adrian CO. Are my patients with rheumatic diseases at higher risk of COVID-19? Annals Rheumatic Dis. [serial on the internet] 2020. [cited 2020 May 17]. Available from: https://doi.org/10.1136/annrheumdis-2020-217322.
CrossRef - S. National Library of Medicine [homepage on the Internet]. COVID-19 Infection in Patients Infected with HIV and/or on PrEP (COVIDHIVPrEP) [cited 2020 May 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT04379245.
- Centers for Disease Control and Prevention [homepage on the Internet]. If you have pets [cited 2020 May 20]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/pets.html.
- Kui L, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 133:1025-1031, 2020.
CrossRef - Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 395:507-513, 2020.
CrossRef - Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J. Hosp. Infect. 104:246-251, 2020.
CrossRef - Vallurupalli M, Berliner N. Emapalumab for the treatment of relapsed/refractory hemophagocytic lympho histiocytosis. Blood. 134(21):1783-1786, 2019.
CrossRef - Zhai P, Ding Y, Wub X, et al. The epidemiology, diagnosis and treatment of COVID-19. J. Antimicrob. Agents. 55:105955, 2020.
CrossRef - Galluccio F, Ergonenc T, Martos AG, et al. Treatment algorithm for COVID-19: A multidisciplinary point of view. Clinical Rheumatology. 1-8, 2020.
CrossRef - https://clinicaltrials.gov/ct2/show/NCT04280705.
- https://www.fda.gov/media/137566/download
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30:269-271, 2020.
CrossRef - Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH2004 study. Blood. 130(25):2728–2738, 2017.
CrossRef - S. National Library of Medicine [homepage on the Internet]. ClinicalTrials.gov [cited 2020 May 18]. Available from: https://clinicaltrials.gov/ct2/show/NCT04324021.
- Chakraborty I, Maity P. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci Total Environ. 728:138882 ,2020.
CrossRef - https://clinicaltrials.gov/ct2/show/NCT04362813
- Devasenapathy N, Ye Z, Loeb M, et al. Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and meta-analysis. Canadian Med. Asso. J. 1:1-7, 2020.
CrossRef - https://clinicaltrials.gov/ct2/show/NCT04381936
- Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: Results of a randomized clinical trial. medRxiv BMJ Yale. 2020 (In Press) (https://doi.org/10.1101/2020.03.22.20040).
CrossRef - MRC Centre for Global Infectious Disease Analysis [homepage on the internet] London IC. Report 13: Estimating the number of infections and the impact of nonpharmaceutical interventions on COVID-19 in 11 European countries 2020 March 30 [Available from: https://www.imperial.ac.uk/mrc-global-infectious-diseaseanalysis/covid-19/.]
- Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the post pandemic period. Science. 368(6493):860-868, 2020.
CrossRef








