Mousa K. Magharbeh, Tayel A. Al-Hujran, Saied M. I. Al-Dalaen and Abdul-Wahab R. Hamad
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Mu’tah University, 61710 Al-Karak, Jordan.
Corresponding Author E-mail : magharbeh@mutah.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/2049
Abstract
Urinary calculi are stones (urolithiasis) that can form anywhere in urinary tract outside of the kidneys and mostly composed of calcium oxalate and phosphate, additionally with elevated throughout the last two decades in the world. Chemical composition plays a major part in nephrolithiasis. Therefore, the high concentrations of lithogenic substances in urine enhance the crystallization method in urine tract system. The most kidney stones form from calcium oxalate, the present study was inspected the effect of the crude aqueous extract as well as the fractionated methanol extract (ethyl acetate, isopropanol, acetone and methanol residue) of paronychia argentea on the crystallization of calcium oxalate salts. The effect of aqueous extract and fractionated methanol extract on the size, number, type of calcium oxalate crystals. Paronychia argentea both the crude aqueous and the fractionated extract, especially ethyl acetate fraction have antiurolithic activity via reducing crystal size as well as activate the formation of calcium oxalate dihydrate (COD) crystals out from calcium oxalate monohydrate (COM) with increasing concentration of extract. The shifting of crystallization process to producing calcium oxalate dihydrate (COD) rather than oxalate monohydrate (COM) and the reducing the crystal size and calcium ion concentration, in addition to the diuretic action of extract plays an important role in controlling urolithiasis.
Keywords
Calcium Oxalate; Prosopis Farcta; Urinary Stone Antioxidant; Urolithiasis
Download this article as:| Copy the following to cite this article: Magharbeh M. K, Al-Hujran T. A, Al-Dalaen S. M. I, Hamad A. W. R. Assessment of Paronychia Argentea Extraction on Kidney Stone By Using Calcium Oxalate Method. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Magharbeh M. K, Al-Hujran T. A, Al-Dalaen S. M. I, Hamad A. W. R. Assessment of Paronychia Argentea Extraction on Kidney Stone By Using Calcium Oxalate Method. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/3n75ic9 |
Introduction
Nephrolithiasis has no longer considered as isolated condition, because the hazard has been raised for chronic kidney disease. Kidney stones turn into one of the most prevalent disease and many studies have been shown the ratio incremented universal throughout the twenty years.
Also, kidney stones consider as markers of metabolic imbalance, presaging other comorbidities, due to their linke to the metabolic syndrome, hypertension, coronary artery disease, and reduced bone mineral density. (Lada Beara-Lasic1 and David S. Goldfarb 2019). Therefore, prevention of stone recurrence offers possibility to keep away from painful, high priced stone episodes, while enhancing standard health via nutritional and pharmacologic interventions.
Kidney stones, urinary stones (aka urolithiasis) become one of the main diseases that affects 10-12% of population in developed countries (Sowers et al., 1998).
The prevalence of the kidney stone, after an initial episode of nephrolithiasis of the first stone, showed that the chance for recurrence the second stone within a year is about 15% and within ten years about 50%. Unlikely, nephrolithiasis literatures showed incremented in the number of individuals suffering from kidney stone in future decades. Due to, the cost of treatment will be raised globaly.(Luyckx et al., (2020)) is predicted to spend five billion dollars by 2030 on its treatment in USA. (Akram, M. and Idrees, M., 2019). (Antonelli et al., 2014).)
Many factors play a major roles in increasing the risk of formation kidney stone, such as lifestyle, gender and geographic region, environmental and genetic factors contribute to their formation , and dietary, for example, type of food up take if it rich with protein continent the high risk to formation kidney stones. Also type of vegetable or fruits have been taken. A having a strong family history of urolithiasis, increase the risk of formation kidney stones (Luyckx et al., (2020).
In other hand the gender factor showed that men have the high risk symptomatic stones compare with women. (Priti et al., 2017).
There are several types of kidney stones based on the type of crystals of which are consist. The majority are calcium oxalate stones, observed by way of calcium phosphate stones. More rarely, Struvite stones (MgNH4PO4) are produced by urea splitting bacteria in people with urinary tract infections, and people with certain metabolic abnormalities may produce uric acid stones or cystine Stones. (Dyer and Nordin, 1967) and Singh et al., 2010).
In general, it is recommended to have a dietary abundant with calcium and lower with oxalte, potassium and sodium contain to decrease the danger of having kidney stone. In contrast, excessive animal protein intake is the principle supply of acid in the human frame and lowers urine pH, which then will increase the risk for uric acid stones.
Herbal medicine spread through the world rapidly like Europe, United States, China and Hong Kong (Gohel and Wong, 2006; Yasui et al., 1999). Many medicinal plants showed good effective antiurolithic activity such as Phyllanthus niruri, Aerra lanata, Crataeva nurvala, Herniaria hirsuta and indigenous plant (Kulaksizoglu et al., 2008; Atmani et al., 2003; Freitas et al., 2002; Selvam et al., 2001; Varalakshmi et al., 1990; Patankar, 2013).
In this study, we will investigate the medicinal effect of Prosopis farcta in the treatment of kidney stones as alternative to surgically or ultrasound treatment of kidney stone, so to characterize effect of Prosopis farcta on kidney stone. In this paper study the effect of an aqueous extract of Prosopis farcta on calcium oxalate crystallization induced in vitro.
In different regions on the world, traditional medicinal have been used plants to cure urolithiasis. Herbel plants are containe different mixture of organic compounds, so, we must be diggening deep by doing more researches to isolation and characterization of the active compounds and elucidation of the relationship between structure and activity. Combination of modern technology and traditional knowledge can produce invective cure for many diseases such as kidney Stones. (Patankar, 2013).
The aim of the study is to investigate the effect of crude aqueous extract and fractionated methanol extract of paronychia argentea on kidney stone.
Materials and Methods
General Experimental Procedures
Retsch Laboratory Mill model 5657 (Haan, Germany) was used to grind Paronychia argentea. Thermo Electro Corporation forma orbital shaker equipped with HEPA Filter (Massachusetts, USA) was used to prepare the total methanol extract that was thereafter fractionated using Soxhlet apparatus. UV/VIS double beam Spectrophotometer SPUV-26 (Staffordshire, UK) was used to measure the absorbance of the samples. Flame Photometer Models PFP7 (JENWAY, UK) was used to determine the concentration of calcium ion. Krüss microscope connected to a Krüss optronic camera (Hamburg, Germany) was used to investigate the size and shape of calcium oxalate crystals.
Plant Material
The aerial parts of Paronychia argentea was collected entire from the local Jordanian market within Karak governorate in May, 2019. The collected Paronychia argentea was cleaned then dried at 45ºC overnight and afterwards ground to powder.
The plant was authenticated by Prof. Dr. Saleh Al-Qur’an at Department of Botany, Faculty of Science, Mu’tah University and a voucher specimen coded PA-201905 was kept at Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Mu’tah University.
Preparation of Plant Extract and Subsequent Fractions
Through reflux method, 50 g of the dried ground the aerial parts of Paronychia argentea was exhaustively defatted with 400 ml n-hexane for 8 hr. The n-hexane extract was filtered out and concentrated under reduced pressure. The defatted plant was again refluxed using 400 ml methanol for 8 hr. The total methanol extract was concentrated till dryness under vacuum. Part of methanol extract residue was then fractionated against three different solvents, namely, ethyl acetate, acetone and isopropanol using Soxhlet apparatus. The different fractions together with the residual methanol extract were filtered out, concentrated, and dried under reduced pressure. Afterwards, they were all resuspended in distilled water, filtered through a 0.45 µm filter and used to prepare 1 mg/ml solution from each fraction in distilled water, also 100 mg/ml of aquous solution for crude methanol extract prepared and different concentrations from it preapared as follow (1, 4, 8,16,32,64,80 and 100mg/ml).
Phytochemical screening of the plant extract and subsequent fractions
Testing for Carbohydrate
Molisch’s test
To a 2 ml portion of the total extract and its subsequent fractions, 2-3 drops of α-naphthol solution in alcohol was added, shaken for 2 min and 1 ml of concentrated sulphuric acid was added slowly on the sides of the test tube. A deep violet colour at the junction of two layers indicates the presence of carbohydrates in all samples.
Testing for Flavonoids
NaOH test
A portion of the total extract and its subsequent fractions was separately treated with few drops of sodium hydroxide solution. Formation of intense yellow colour, which becomes colourless on addition of dilute acid, indicates the presence of flavonoids in all samples except isopropanol fraction.
Testing for Saponins
Froth test
An aliquot of 30 mg from each tested fraction was dissolved in 2 ml distilled water by sonication then was vigorously shaken for 5 min each in graduated test tube. The solution is then allowed to stand for 15 min and the persistent forth height was then measured and taken as qualitative and quantitative indicator of the saponin content for each tested fraction, all fractions gave positive indication , according to the following order from the highest to lowest (ethyl acetate, acetone, methanol and isopropanol fraction).
Testing for Tannins and Phenols
Ferric chloride test
To a 3 ml aliquot of the total extract and its subsequent fractions, 3 ml of 5% (w/v) ferric chloride solution was added. The resulting blue-black colour indicates the presence of tannins and phenols.
Lead acetate test
To a 3 ml aliquot of the total extract and its subsequent fractions, 3 ml of 1% (w/v) lead acetate solution was added. The formed precipitate was filtered, dried and weighed where it given an indication about the presence of tannins and phenols.
As a result for above two tests all fractions showed presense of tannins and phenols except isopropanol fraction.
Crystallization Assay in Urine
A human urine samples were obtained from a 30-year old a healthy person in polypropylene bottles refrigerated immediately after collection. Urine sample was centrifuged at 5000 rpm for 8 min and the supernatant was decanted in clean test tube. After that Urine sample was divided into 14 portions (2 ml each); one as negative control, standard drug (cystone) , ethyl acetate fraction, acetone fraction, isopropanol fraction and the residual methanol extract, and another eight portion for each concentration of crude extract as follow (1, 4, 8, 16, 32, 64, 80 and 100 mg/ml), to each urine sample was added 50 µl from the prevuously prepared 1 mg/ml solutions from each fraction and each concetration of crude extract as follow (1, 4, 8, 16, 32, 64, 80 and 100mg/ml), followed by addtion 50 µl of 0.1 M sodium oxalate and 50 µl of 0.1 M CaCl2 to each test tube also. The experiment was conducted in triplicate. All samples were then incubated with shaking at 37ºC for 2 hours. Then, optical density (OD) to each concentration of crude solution was measured at 578 nm.13 Also, a 10 µl aliquot of the filtrate from all 14 samples was applied on a slide and examined under KRUSS microscope 151 and equipped with KRUSS optronic camera (Hamburg, Germany).
The percentage of the dissolution (inhibition) produced by the herb extracts was calculated using the following formula:
The description of calcium oxalate crystals with respect to hydration, size and shape, i.e. calcium oxalate monohydrate (COM) and calcium oxalate dehydrate (COD) were recorded. The concentration of soluble calcium in the samples was measured by using two methods as follows:
(a) Flame Photometric determination: To draw the calibration curve a serial dilution of a standard 100 mg/dl stock solution of unhydrous calcium chloride in deionized water was measured by using Flame Photometer JENWAY Models PFP7 (Staffordshire, UK). By measuring the emmission of calcium ions, the concentrations of calcium ions for control and standard (cystone) and each fraction were determined by using calibration curve equation and correlation coefficient. The results shown in Table (1)
(b) Spectrophotometric determination of calcium ion: in order to measure calcium ions in the samples. To each sample was added arsenazo III which was forming a colored complex that can be measured by UV/VIS double beam Spectrophotometer SCOTech SPUV-26 (Dingelstadt, Germany) at 650 nm using a Kit (Calcium-Arsenazo Biosystems). The concentrations of calcium ions in control and standard (cystone) and each fraction determined by measuring the absorbance, then comparing it with the standard in the kit . The results shown in Table(1).
Antioxidant activity of plant extracts using DPPH radical scavenging assay
The DPPH (2,2-Diphenyl-1-picrylhydrazyl) radical assay was carried out spectrophotometrically as described by Tepe and co-workers in 2005(Tepe et al., 2005). A 50μl from the various concentrations of each fraction extracts was added 5 mL of 0.004% methanolic solution DPPH. The samples were incubated for 30 min. at room temperature, the absorbance was determined using methanol as blank at 517 nm. All mesurements were done in triplicate. The percentage of the Inhibition free radical scavenging activity was calculated by using the following equation:
inhibition (%) = (Abs. control – Abs. sample)/Abs. control × 100 (eq2)
where Abs. control is the absorbance of the control reaction (containing all reagents except the tested compound) and Abs. sample is the absorbance of the tested compound with all other reagents. Extract concentration providing 50% inhibition (IC50) was determined from a graph plotting percentage inhibition against extract concentration. Trolox® [(±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid] (final concentration 0 to 1.5 µg/ml) was used as a standard antioxidant drug for the construction of the calibration curve, and the DPPH radical-scavenging activities were expressed as mg Trolox® equivalents per gram of plant extract(Barros et al., 2003). The results shown in Table(2).
Results and Disscussion
The in vitro kidney stone which composed from calcium oxalate salt, was artificially prepared by mixing equall moles of sodium oxalate and calcium chloride.
Analysis of calcium oxalate by using dissolution method
The calcium concentrations were determined by two methods spectrophotometric by using calcium kit and flame photometric, as shown in Table 1. The statistical analysis by using anova single factor, to the result of both methods showed that no significant difference between two methods of measuring calcium concentration in Flame Photometry or in Spectrophotometry, because Fcalc less than Fcrit, as shown in (Table 3 ).
Table 1: Concentration of calcium ion in each fraction and cystone produced from dissolution of calcium oxalate measured by flame photometer and spectrophotometer.
| Fraction | Concentration Ca+2 (ppm)
Flame Photometry |
Concentration Ca+2(ppm) Spectrophotometry |
| Methanol | 357.926±1.427 | 353.582±0.048 |
| Acetone | 360.829±0.755 | 363.911±0.039 |
| Ethyl acetate | 381.819±0.713 | 373.129±0.099 |
| Isopropanol | 343..095±0.756 | 335.404±0.195 |
| Oxalate | 246.699±2.854 | 241.629±0.179 |
| Cystone | 360.397±2.854 | 364.595±0.147 |
The concentration calcium dissolved from calcium oxalate salt which was considered as control approximately around 246 ppm. Mean while the standard drug cystone showed 310 ppm after treatment with artificial calcium oxalate crystals.
The aerial parts of Paronychia argentea was extracted by different solvent. These extracts were used to dissolute the calcium oxalate crystals. Ethyl acetate fraction was observed to have the highest rate of dissolution; because ethyl acetate fraction showed the maximum calcium ion concentration around 381ppm, Table 1. Which means the dissolution is the highest rate. The less amount of calcium oxalate dissolution was observed in isopropanol extract, which showed the calcium ion concentration around 343 ppm. Even though the isopropanol extract shows the less dissolution rate compared with other solvent extract of the aerial parts of Paronychia argentea, it shows higher dissolution rate compared with the standard drug cystone which shows less dissolution rate compared with isopropanol extract. In other hand the calcium ion concentration of both methanol and acetone extracts 358ppm, this result exhibited methanol and acetone extracts have approximately the same dissolution rate.
Phytochemical screening
Phytochemical screening of the different fractions of the total methanolic extract prepared from Paronychia argentea areal parts revealed that all the fractions possessed carbohydrates among their contents depend on Molisch test results. All the fraction give positive results for tannins, phenols and flavonoids tests ecxept isopropanol fraction. Interestingly, ethyl acetate fraction displayed the highest and the most persistent froth indicating its relative high content of saponins compared to other fractions. Atmani.,et al, 2006 found that the fraction rich in saponins of methanolic extract has the highest potency on treatment of kidney stones in vitro and in vivo. While other studies showed that the fractions of the total extract has high potency in treatment of kidney stones (calcium oxalate and calcium phosphate ) mainly fractions contanis non polar and semipolar compounds of this plant( Tork. et al, 2018).
DPPH radical scavenging assay
The in vitro antioxidant (DPPH) activity of the extracts of Paronychia argentea areal parts were determine by measuring the DPPH radical scavenging activity at 517nm, as shown in Table 2. acetone and ethylacetate fraction showed the highest ability to scavenge the DPPH radical with IC50 values of 34 mg/mL and 42mg/mL respectively. In other hand, both methanolic fraction and standard drug cystone have lowest ability to scavenge the DPPH radical with IC50 of values 466 mg/mL and 297 mg/mL respectively, than acetone, ethyl acetate and isopropanol extracts.
Table 2: In vitro antioxidant (DPPH) assay results.
| Fraction of coffee seeds and control and standard | IC50 (mg/ml) |
| Acetone | 34.22 |
| Methanol | 465.93 |
| Ethyl acetate | 41.82 |
| Isopropanol | 99.08 |
| Oxalate | No inhibition |
| Cystone | 297.33 |
Table 3: Statistical analysis (ANOVA single factor)
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| Column 1 | 6 | 2032.25 | 338.7083 | 2429.324 | ||
| Column 2 | 6 | 2047.862 | 341.3103 | 2302.381 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Between Groups | 20.31121 | 1 | 20.31121 | 0.008585 | 0.928007 | 4.964603 |
| Within Groups | 23658.52 | 10 | 2365.852 | |||
| Total | 23678.83 | 11 | ||||
| F crit | < | F calc | ||||
| 4.964603 | 0.008585 | |||||
| 0.928007 | P-value |
Nucleation
In this study, it was found that the extracts of areal parts of Paronychia argentea increased the crystallization process of calcium oxalate salt in a concentration dependent manner (Fig.1, R2 = 0.9822). The rusalt showed the turbidity of the solutions directly proportional to concentrations of extract, as a result of that more but smaller size of (COD) produced from dissolving of (COM) crystals as shown in (Fig. 2).
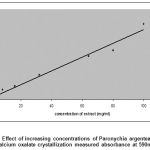 |
Figure 1: Effect of increasing concentrations of Paronychia argentea extract on calcium oxalate crystallization measured absorbance at 590nm. |
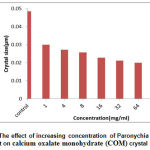 |
Figure 2: The effect of increasing concentration of Paronychia argentea extract on calcium oxalate monohydrate (COM) crystal size. |
It is well known scientific fact that kidney stone formation process is a result of crystallization that happened in supersaturated urine (Barros et al., 2003), so to treat and forbid of urolithiasis, is necessary to change and control the crystallization process using some substances in order to modification the products that affect on kidney stone formation (Veronika B and Khan SR., 2009). The medicinal plants that traditionally used are the best alternative substances for treatment of kidney stone. Insertion of the extract of Paronychia argentea in the crystallization process of calcium oxalate in the human urine has shown influence effect on the calcium oxalate crystal size and number. The extract has the ability to preventing urinary stone formation by inducing large number but smaller size calcium oxalate crystals which were easy to excretion from the kideny and prevent them from retention in the urinary tract.
Furthermore, the extract enhance the induce (COD) by dissolving of (COM) crystals. This property of Paronychia argentea extract lead to preventing kideny stone formation. Because the COD crystal less bounded to epithelial cell comper with COM crystal (Wesson et al., 1998).
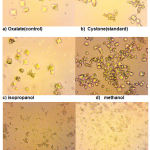 |
Figure 3: Light microscopy of calcium oxalate crystals induced in vitro, (a) control(oxalate) (b) standard(cystone) (c) isopropanol (d) methanol (e) acetone (f) ethyl acetate at 400x magnification |
It is known that control urine sample contains mixture of COD and COM calcium oxalate crystals (Fig. 3). Incorporation of Paronychia argentea extract has shown a good effect by changing the morphology of crystals from COM form to COD form that change the ratio in the direction to get less COM that has high affinity for cell membrane damages the epithelial cells than COD and high activation effect on kidney stone formation (Yamaguchi et al., 2005).
Paronychia argentea areal parts extract was significantly more effective in inhibiting the nucleation and aggregation of calcium oxalate monohydrate (COM) crystals than was cystone. Moreover, the extract induced more calcium oxalate dehydrate (COD) crystals, with a significant reduction in the number and size of COM crystals.
Microscopic results (Fig. 3) indicated that the crystals in the control oxalate had the hexagonal shape related to COM. When the different fractions were added, the COM crystals lost their crystalline nature and they were converted to octahedral shape, as indicated by the dispersed, lesser, very smaller octahedral shape COD particles compared with control in 400× magnification. By comparing different fractions of methanol extract of Paronychia argentea areal parts extract, it was found that ethyl acetate fraction (Fig. 3) have the the highest ability to reduce the size and shape of calcium oxalate monohydrate crystals compared wih other fractions and standard drug cystone under the same magnification power. This notion reflects the influence of the natural products in ethyl acetate fraction that inhibited the crystal growth of calcium oxalate monohydrate, which according to the phytochemical screening results might be attributed to saponins and polyphenolics such as flavonoids and tannins featuring potent antioxidant activities.
Conclusion
The present study domonstrated that extract of Paronychia argentea areal parts showed an ability to inhibite the calcium oxalate crystallization process in vitro and possese ahigh antioxidant activity. Baside that, the Paronychia argentea areal parts extract has the ability to reduce the crystal size and chang the morphology of calcium oxalate monohydrate (COM) from hexagonal to tetrahydral a calcium oxalate dihydrate (COD) crystal. That might be attributed to its high polyphenolic contents including saponins mainly. However, further in vivo studied using animal models of urolthiasis is needed to evalute the potential antiurolithic activity. Future work will be continued to characterization and isolation of the major active compounds from this plant.
Acknowledgments
The authors are expressing their appreciations to all those who have assisted in the research, namely the staff of Pharmacy College, the Mutah’ University, and researchers at the Medical Research Centre at College of Pharmacy, Mutah’ University, Jordan.
References
- Lada Beara-Lasic and David S. Goldfarb. Recurrent Calcium Kidney Stones. Clin J Am Soc Nephrol, 14 (9) :1388-1390(2019).
CrossRef - Sowers, M. R., Jannuasch, M., Wood, C., Pope, S. K., Lachance, L. L. and Peterson, B. Prevalence of renal stones in a population-based study with dietary calcium oxalate and medication exposures. Am. J. Epidemiol; 147(10): 914-920 (1998).
CrossRef - Luyckx,V. A.; David Z.I. Cherney, D. Z.I.; and Bello A.K. Kidney International Reports 5, 263–277 (2020).
CrossRef - Antonelli, J. A., Maalouf, N. M., Pearle, M. S. & Lotan, Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur. Urol; 66(4): 730–731(2014).
CrossRef - Priti Arya, Savita Pandey, Vipin Verma. Kidney stones formation and use of medicinal plants as antiurolithiatic agents, Univers. J. Pharm. Res; 2( 4): 36 – 41 (2017).
CrossRef - Dyer, R. and Nordin, B. E., 1967. Urinary crystals and their relation to stone formation. Nature; 215: 751-752 (1967).
CrossRef - Sinha, M. R. and Tagore, R. N. Effect of acid-hydrolyzation of fruits juice of apple (Malus domestica), moushmi (Citrus medica) and amla (Emblica officinalis) on solubility of urinary stones. Asian J. Chem; 22: 4840-4846 (2010).
- Khan, S. R. and Kok, D. J. Modulators of urinary stone formation. Frontiers in Bioscience; 9: 1450-1482 (2004).
CrossRef - Sinha, M. R., Dev, A., Prasad, A., Ghosh, M. and Tagore, R. N. Experimental study of solubility of urinary stones in juice of Chikku (Achras zapota) fruit. J. Chem. Pharm. Res; 3: 231-237 (2011).
- Yasir, F. and Waqar, M. A. Effect of indigenous plant extracts on calcium oxalate crystallization having a role in urolithiasis. Urology Research; 39: 345-350( 2011).
CrossRef - Leusmann, D. B., Niggemann, H., Roth, S. and von Ahlen, H. Recurrence rates and severity of urinary calculi. Scand J Urol Nephrol; l29: 279-283(1995).
CrossRef - Atmani, F., Slimani, Y., Mimouni, M., Aziz, M., Hacht, B. and Ziyyat, A. Effect of aqueous extract from Herniaria hirsuta L. on experimentally nephrolithiasic rats. J Ethnopharmacol; 95: 87–93(2004).
CrossRef - McHarg, T., Rodgers, A. and Charlton, K. Influence of cranberry juice on the urinary risk factors for calcium oxalate kidney stone formation. BJU Int; 92: 765–768(2003).
CrossRef - Araújo Viel, T., Diogo Domingos, C., da Silva Monteiro, A. P., Riggio Lima-Landman, M. T., Lapa, A. J. and Souccar, C. Evaluation of the antiurolithiatic activity of the extract of Costus spiralis Roscoe in rats. J Ethnopharmacol; 66: 193–198(1999).
CrossRef - Grases, F., Ramis, M., Costa-Bauzá, A. and March, J. G. Effect of Herniaria hirsuta and Agropyron repens on calcium oxalate urolithiasis risk in rats. J Ethnopharmacol, 45: 211–214(1995).
CrossRef - Afifi, F. U., Al-Khalidi, B. and Khalil, E. Studies on the in vivo hypoglycemic activities of two medicinal plants used in the treatment of diabetes in Jordanian traditional medicine following intranasal administration. J Ethnopharmacol; 100: 314–318(2005).
CrossRef - Gohel, M. D. I. and Wong, S. P., 2006. Chinese herbal medicines and their efficacy in treating renal stones. Urology Research; 34: 365-372.
CrossRef - Kulaksizoglu, S., SoWkerim, M., Cevik, C . In vitro effect of lemon and orange juices on calcium oxalate crystallization. Int Urol Nephrol; 40: 589–594(2008).
CrossRef - Atmani, F., Slimani, Y., Mimouni, M. and Hacht, B.,. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU International; 9: 137-140(2003).
CrossRef - Freitas, A. M., Schor, N. and Boim, M. A.,. The effect of Phyllanthus niruri on urinary inhibitors of calcium oxalate crystallization and other factors associated with renal Stone formation. BJU International; 89: 829–834(2002).
CrossRef - Selvam, R., Kalaiselvi, P., Govindaraj, A., Bala, M. V. and Kumar, A. S. S.,. Effect of Aerva lanata leaf extract and vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol. Res; 43: 89–93(2001).
CrossRef - Varalakshmi, P., Shamila, Y. and Latha, E., , Effect of Crataeva nurvala in experimental urolithiasis. J Ethnopharmacol; 28: 313– 321(1990).
CrossRef - Suresh Balkrishna Patankar; patent Novel herbal composition for the treatment of kidney stone and other urinary tract disorders, US 2013/0337057 A1, United States Patent, Dec. 19, 2013.
- Tepe, B., Sokmen, M., Akpulat, H. A. and Sokmen, A.,. In vitro antioxidant activities of the methanol extracts of five Allium species from Turkey. Food Chemistry 92: 89-92(2005)
CrossRef - Barros, M. E., Schor, N. and Boim, M. A. Effects of an aqueous extract from Phyllanthus niruri on calcium oxalate crystallization in vitro. Urology Research; 30: 374-379(2003).
CrossRef - Tork, A., Hosseinabadi, T., Fasihzadeh, S., Sadeghimanesh, A., Wibowo, J. P. and Lorigooini.,. Solubility of Calcium Oxalate and Calcium Phosphate Calcium Crystallization in the Presence of Crude Extract and Fractions from Kelussia odoratissima Mozaff. Pharmacol. Res; 10(4): 379-384(2018).
CrossRef - Veronika B, Khan SR.,. Herbal medicines in the management of urolithiasis:alternative or complementary. Planta Med; 75: 1095–1103(2009).
CrossRef - Wesson, J. A.; Worcester E. M.; Wiessner, J. H.; Mandel, N. S.; Kleinman, J. G . Control of cacium oxalate structure and cell adherence by urnary macromolecules. Kidney Int.; 53: 952-957(1998).
CrossRef - Yamaguchi S, Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS.,.Study of a rat model for calcium oxalate crystal formation without severe renal damage in selected conditions. Int J Urol; 12: 290–298(2005).
CrossRef








