Jacobus Jeno Wibisono1,2, Syahrul Rauf3, Mochammad Hatta4* , Rosdiana Natsir5, Muh Nasrum Massi4, M. Husni Cangara6, Rina Masadah6, Ilham Jaya Pattelongi7 and Jonathan Salim2
, Rosdiana Natsir5, Muh Nasrum Massi4, M. Husni Cangara6, Rina Masadah6, Ilham Jaya Pattelongi7 and Jonathan Salim2
1Post Graduate Program of Medical Science, Faculty of Medicine, University of Hasanuddin, Makassar, Indonesia.
2Department of Obstetrics and Gynecology, Faculty of Medicine, Pelita Harapan University and Siloam Hospitals Lippo Village, Tangerang, Indonesia
3Department of Obstetrics and Gynecology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
4Molecular Biology and Immunology Laboratory, Faculty of Medicine, University of Hasanuddin, Makassar, Indonesia
5Department of Biochemistry, Faculty of Medicine, University of Hasanuddin, Makassar, Indonesia
6Department of Pathological Anatomy, Faculty of Medicine, University of Hasanuddin, Makassar, Indonesia
7Department of Biostatistics, Faculty of Medicine, University of Hasanuddin, Makassar, Indonesia
Corresponding Author E-mail: hattaram@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1977
Abstract
Background and Aim: Cervical cancer is the global 2nd most prevalent cancer with ± 266 000 related deaths. Yin Yang 1 (YY1) is a ubiquitous and multifunctional zinc-finger transcription factor in controlling cell cycle. Similarly, p53 suppresses tumors by selectively inhibiting carcinogenesis. The interventional study aims to evaluate YY1 and p53 gene expressions in cervical cancer, serving for further treatment and intervention. Methods: The study measured YY1 and p53 mRNA gene expressions by quantitative real-time PCR through blood sample, which was analyzed by t-test and correlation. Moreover, demographics and medical history were acquired through interviews and medical records. 20 samples are selected by random sampling from July to September 2016. Results: YY1 and cervical cancer stage has negative correlation (r: -0.5) due to its inhibition of cell growth until a certain phase of cancer cell development. While, positive correlation between p53 and cancer stage (r: 0.47) was found because of p53 gene dysregulation. YY1 and p53 correlation are negative (r: -0.9) due to their conflicting functions. Conclusion: The study describes the correlation between YY1 and p53 gene expressions in cervical cancer stages. It was found that the downregulated YY1 expression in cervical cancer as the stage progress is directly opposed to the p53 gene trend. The higher YY1 and lower p53 gene expressions dictate the likelihood of surgery. Therefore, YY1 and p53 can be utilized as a cervical cancer progression indicator and surgery indicator.
Keywords
Cervical Cancer; mRNA Expression; p53 Gene; Staging; YY1
Download this article as:| Copy the following to cite this article: Jacobus J. W, Rauf S, Hatta M, Natsir R, Massi M. N, Cangara M. H, Masadah R, Pattelongi I. J, Salim J. YinYang 1 (YY1) and P53 Gene Expression Analysis in Cervical Cancer and Its Relationship with Cancer Staging. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Jacobus J. W, Rauf S, Hatta M, Natsir R, Massi M. N, Cangara M. H, Masadah R, Pattelongi I. J, Salim J. YinYang 1 (YY1) and P53 Gene Expression Analysis in Cervical Cancer and Its Relationship with Cancer Staging. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/3iFvyI8 |
Introduction
Cervical cancer is the second most common cancer worldwide, with 15% of all cancers in women amounting to 600 000 annual global incidence rate and prevalence in some countries as high as 20-30%.1 In developing countries, cervical malignancy is the second leading cause of mortality with up to 266 000 related deaths.2
HPV (Human Papilloma Virus) is the main etiology of cervical cancer, with intertwined risk factors including multiple sexual partners and age of sexual activity.3 HPV infection stimulates carcinogenesis in cervical epithelial cells through inhibition of tumor suppressor gene by E6 and E7 HPV-Encoded viral oncoproteins.4 HPV-Encoded E6 oncoprotein has the ability to bind directly to p53 and cause degeneration via the E6-AP-mediated ubiquitination pathway.5
Yin Yang 1 (YY1) is a ubiquitous zinc-finger transcription factor, which has an important role in the controlling the cell cycle.6 YY1 has a regulatory role in cell growth, development, and differentiation by influencing MDM2 and p53 levels with its role in increasing the MDM2-p53 interaction, leading to the ubiquitination and degradation of p53.7,8 Based on several studies over the past 2 decades, YY1 is gene transcription activator, repressor, and initiator that regulate the human genes.9 While examining the tumor’s YY1 expression, Zaravinos explained that YY1 has a role in inhibiting p300, a co-activator of p53.9 Moreover, Wang portrayed that YY1 overexpression was associated with low-grade to high-grade cervical intraepithelial neoplasia (CIN) progression.10 In turn, YY1 also able to bind to the HPV-18 upstream regulatory region (URR) to regulate viral oncogenes E6 and E7 transcription.11,12
In HPV induced cervix cancer, p53 has an essential role as a tumor suppressor.13 Wild type p53, which is unstable and only can last for 20-30 minutes, can act as cell cycle negative control and genome guardian; in which will then be degraded to p53-E6 complex or mutated p53.13 Thereafter, p53 also utilized as a molecular prognostic indicator for pre-cancer lesion development or cancer treatment viability.14
According to the author’s knowledge, there are many comprehensive works dedicated to the research of cervical cancer. However, there is little to none published analysis of the intergenic factors affecting cervical cancer carcinogenicity of YY1 and p53. Therefore, it is sensible to modify the material and methods in order to improve and fully realize the potential of YY1 and p53 in cervical cancer.
Materials and Methods
The study encompasses 20 cervical cancer patients in Siloam Teaching Hospital, Tangerang in July–September 2016 respondents who have signed the written informed consent. Ethical consideration was obtained from Hasanuddin University medical ethics committee with the references of 901/H4.8.4.5.31/PP36-KOMETIK/2017.
The interventional study observes age, occupation, education, income, religion, contraception method, family history, smoking, cancer operability, medical history, chemotherapy, and cancer stage relations to cervical cancer gene expression, where blood samples are used for laboratory parameter analysis. YY1 and p53 mRNA gene expression were measured by real-time PCR assay and analyzed using Bio‑Rad CFX Manager 3.1.
The study utilizes YY1 and p53 specifically targeted oligonucleotide primary gene, as well as ß-actin and GAPDH for internal control.15-19. Table 1 shows YY1, β-actin, p53, and GAPDH primer sequences.
Table 1: Primary Gene Specific Oligonucleotide
| Gene | Forward Sequence | Reverse Sequence |
| YY119 | 5′-GCTTCGAGGATCAGA TTC TCATCC-3′ | 5′-GACTACATTGAACAAACG CTGGTC-3′ |
| β-actin19 | 5′-CGCCCAGCACGATGAAA-3′ | 5′-CCGCCGATCCACACAGA-3′ |
| p5323 | 5’-AGAGTCTATAGGCCCACC CC-3’ | 5’-GCTCGACGCTAGGATCTG AC-3’3′ |
| GADPH23 | 5’-CATGGGGAAGGTGAAGGT CGGA-3’ | 5’-TTGGCTCCCCCCTGCAAA TGAG-3’ |
In detecting mRNA YY1 and p53 gene expression quantitative real-time PCR was used. The study utilized a protocol which is optimized for CFX Connect system instruments, Biorad Laboratories, Real Time PCR 96 well 0.1 ml, USA; including the adaptation of diluting agents according to recommended factory manuals for the RT-PCR program.15-19
The protocol involves initial DNA denaturation with 94oC for 60 seconds, then 32 times cycles loop of 40 seconds 94oC and 30 seconds 54oC. In addition, qRT-PCR employs the one step Green QRT-PCR master kit.
The study sample was taken by random sampling and calculated by 49.65% prevalence, 0.883 proportion difference, 5% alpha and 80% power through
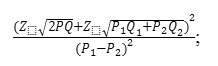
while the results analyzed through t-test and correlation.
Results and Discussion
Within the research period, there are 20 eligible cervical cancer patients comprising 80% housewives, 40% elementary school students, and 70% Islamic people. Other contraceptive methods than pills, injections, and implants are used by 9 respondents. Incidentally, 90% of the respondents do not have a family history of cancer and do not smoke. Currently, 45% of the patients are not treated and 25% are in the IB cancer stage. Table 2 and 3 portray the respondents’ demographic and medical history.
Table 2: Demographic Characteristics
| Variable | Freq. | Percentage |
| Occupation | ||
|
16 | 80 |
|
4 | 20 |
| Education | ||
|
8 | 40 |
|
4 | 20 |
|
6 | 30 |
|
2 | 10 |
| Income (IDR)* | ||
|
7 | 38.89 |
|
6 | 33.33 |
|
5 | 27.78 |
| Religion | ||
|
14 | 70 |
|
2 | 10 |
|
3 | 15 |
|
1 | 5 |
| Contraception | ||
|
4 | 20 |
|
6 | 30 |
|
1 | 5 |
|
9 | 45 |
*2 respondents choose not to answer
Table 3: Respondents’ Medical History
| Variable | Freq. | Percentage |
|
2 | 10 |
|
2 | 10 |
|
10 | 50 |
Previous Medical History
|
9 |
45 |
|
5 | 25 |
|
2 | 10 |
|
2 | 10 |
|
1 | 5 |
|
1 | 5 |
Cancer Stage
|
1 |
5 |
|
5 | 25 |
|
3 | 15 |
|
4 | 20 |
|
1 | 5 |
| IIIB | 5 | 25 |
|
1 | 5 |
Chemotherapy*
|
4 |
33.33 |
|
8 | 66.67 |
*8 respondents choose not to answer
Among the cervical cancer patients, the average distribution and standard deviation of age and gene concentration were measured by real-time PCR. YY1 gene expression was 10.4 ± 2.86 fold change (fc) ranging from 6.173 to 15.743 fc; meanwhile, p53 expression has an average of 9.113 ± 1.776 fc from 5.682 to 12.762 fc. Table 4 portrays YY1 and p53 gene expression distribution based on cervical cancer stage.
YY1 effects on ovarian and cervical carcinoma have contradicting results. In 2019, Kun Qiao et al. showed a positive correlation with YY1 expression and cancer proliferation;20 however, Meliala and Hosea et al. found YY1 expression has a better survival prognosis in cancer.21 Similarly, many studies portray pro-tumor qualities of YY1 overexpression in breast cancer and primary tumors due to AP2 inducing the Erbb2 oncogene.22 Baritaki et al explained that YY1 overexpression was also found in cervical cancer patients rooted from HPV-18 or HPV-16 infections.22
Numerous research demonstrated that the majority of p53 mutations in cancers is missense mutation on the nucleus DNA binding domain.23 In its application, p53 gene infusion into cancer cells, which beforehand has to lose its endogenic p53, display tumorigenesis reduction while vice versa for mutated p53 infusion.24,25 The p53 levels also determined to be null or decremented on 71.05% blood samples and 72.73% of cervical cancer patients. Based on the cancer staging, 20% overexpression happens on stage I, 20% on stage II, 25% on stage III, and 66% on stage IV.26
Table 4: YY1 and p53 Expression According to Cervical Cancer Stage and Age
| Variable | Freq. | Mean ± SD (Fold Change) | Range (Median) |
| Age | 20 | 49.900 ± 10.760 | 34 – 74 (46.5) |
| YY1 Expression | 20 | 10.404 ± 2.859 | 6.173 – 15.743 (10.234) |
|
1 | 11.210 ± 0 | |
|
5 | 12.690 ± 1.855 | |
|
3 | 11.790 ± 3.570 | |
|
4 | 8.890 ± 2.640 | |
|
1 | 6.990 ± 0 | |
|
5 | 9.849 ± 2.356 | |
|
1 | 6.173 ± 0 | |
| p53 Expression | 20 | 9.113 ± 1.776 | 5.682 – 12.762 (9.286) |
|
1 | 9.436 ± 0 | |
|
5 | 7.905 ± 1.308 | |
|
3 | 8.002 ± 1.904 | |
|
4 | 10.271 ± 2.443 | |
|
1 | 10.935 ± 0 | |
|
5 | 9.236 ± 1.010 | |
|
1 | 11.103 ± 0 |
Significant difference of YY1 and p53 gene concentrations to cancer operability are observed (p: < 0.0001). Inoperable patients have 7.917 ± 1.110 and 10.492 ± 1.050 for YY1 and p53 concentration respectively, while operable patients have 12.891 ± 1.509 and 7.735 ± 1.153. On the other hand, age and marriage do not contribute to the operability of cervical cancer patients (p: 0.0971 and 0.9550) with an 8.00 years difference of age and 0.1 years old gap of marriage age between groups.
The correlation of gene expression and cancer stage as well as between the gene itself produces significant results (p: 0.023, 0.035, and < 0.0001). Although, p53 expression has a proportional relationship with a coefficient of 0.473, YY1 expression has an inverse correlation with coefficient of -0.505. Likewise, the expression of YY1 against p53 shows a strong correlation, yet inversely proportional (r: -0.905). Viral load measurements were done and showed negative results on all respondents.
Table 5: Cancer Characteristics with Operability and Cancer Stage
| Variable | Inoperable | Operable | r | p – value |
| YY1 expression (fc) | 7.917 ± 1.110 | 12.891 ± 1.509 | < 0.0001 | |
| P53 expression (fc) | 10.492 ± 1.050 | 7.735 ± 1.153 | < 0.0001 | |
| Age | 53.9 ± 13.287 | 45.9 ± 5.68 | 0.0971 | |
| Marriage Age | 18.5 ± 4.766 | 18.6 ± 2.790 | 0.9550 | |
| YY1 expression vs. cancer stage | – 0.505 | 0.023 | ||
| P53 expression vs. cancer stage | 0.473 | 0.035 | ||
| YY1 vs. P53 expression | -0.905 | < 0.0001 | ||
Figure 1 demonstrates the correlation of YY1 gene expression on the cervical cancer stage showed that each increase of the cancer stage is followed by a decrease in YY1 gene expression. Although mildly related, this trend signifies the action of YY1 as an inhibitor of cell growth as well as YY1 incapability to keep up with the cell growth as cancer progresses.
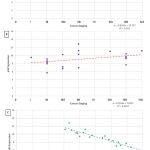 |
Figure 1: (A) YY1 Expression and Cancer Staging, (B) p53 Expression and Cancer Staging |
Nevertheless, the correlation coefficient between p53 and cervical cancer stage display that each increase in staging is followed by an increase in p53 expression due to the gene mutations or dysregulation. YY1 and p53 gene expression relationship has the largest statistical correlation where p53 increase was followed by a significant YY1 decrease (p < 0.0001).
The study limitations include small quantities of respondents as compared to similar studies, study design, and study timing; in which cervical cancer can develop until 10-20 years after HPV infection.
Conclusion
The study describes the correlation between YY1 and p53 gene expressions in cervical cancer stages. It was found that the downregulated YY1 expression in cervical cancer as the stage progress is directly opposed to the p53 gene trend. The higher YY1 and lower p53 gene expressions dictate the likelihood of surgery. Therefore, YY1 and p53 can be utilized as a cervical cancer progression indicator and surgery indicator.
Further Prospects
The study serves as a basis for further investigation to analyze YY1 as well as p53 gene inhibitor and dysregulation nature respectively in neoplasm.
Acknowledgement
The authors would like to thank Mr. Romi Usman, Mus Helminus, and Marwani for their excellent technical laboratory work in Molecular Biology and Immunology Laboratory for Infectious Diseases, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia. Furthermore, the authors would like to appreciate Mr. Rendy Reynaldi and Ms. Erica Widodo for their help throughout the concoction of the documents.
Conflict Of Interest
All authors declare no conflict of interest.
Funding Source
The study was funded through authors’ personal accounts without any external sponsors.
References
- Catarino R. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World Journal of Clinical Oncology. 2015;6(6):281.
CrossRef - Khalissa D, Abdelhalim K, Xie X, Li Y, Soraya O, Abbes M. Immunohistochemical Expression of p53 and Bcl-2 in Algerian Cervical Carcinoma. Biomedical and Pharmacology Journal. 2018;11(1):67–75.
CrossRef - Liu Z-C, Liu W-D, Liu Y-H, Ye X-H, Chen S-D. Multiple Sexual Partners as a Potential Independent Risk Factor for Cervical Cancer: a Meta-analysis of Epidemiological Studies. Asian Pacific Journal of Cancer Prevention. 2015;16(9):3893–900.
CrossRef - Yeo-Teh N, Ito Y, Jha S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. International Journal of Molecular Sciences. 2018;19(6):1706.
CrossRef - Martinez-Zapien D, Ruiz FX, Poirson J, Mitschler A, Ramirez J, Forster A, et al. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature. 2016;529(7587):541–5.
CrossRef - Wai DC, Shihab M, Low JK, Mackay JP. The zinc fingers of YY1 bind single-stranded RNA with low sequence specificity. Nucleic Acids Research. 2016;
CrossRef - Inoue K, Fry EA, Frazier DP. Transcription factors that interact with p53 and Mdm2. International Journal of Cancer. 2015;138(7):1577–85.
CrossRef - Liu D, Zhang J, Wu Y, Shi G, Yuan H, Lu Z, et al. YY1 suppresses proliferation and migration of pancreatic ductal adenocarcinoma by regulating the CDKN3/MdM2/P53/P21 signaling pathway. International Journal of Cancer. 2017;142(7):1392–404.
CrossRef - Meliala ITS, Hosea R, Kasim V, Wu S. The biological implications of Yin Yang 1 in the hallmarks of cancer. Theranostics. 2020;10(9):4183–200.
CrossRef - Wang W, Yue Z, Tian Z, Xie Y, Zhang J, She Y, et al. (2018) Expression of Yin Yang 1 in cervical cancer and its correlation with E-cadherin expression and HPV16 E6. PLoS ONE 13(2): e0193340. https://doi.org/10.1371/journal. pone.0193340
CrossRef - Munger K. Expert Views on HPV Infection. McBride A, editor. Switzerland: MDPI AG – Multidisciplinary Digital Publishing Institute; 2018.
- Ribeiro A, Caodaglio A, Sichero L. Regulation of HPV transcription. Clinics. 2018;73(Suppl 1).
CrossRef - Vieler M, Sanyal S. p53 Isoforms and Their Implications in Cancer. Cancers. 2018;10(9):288.
CrossRef - Radhika Mucharla, Pingle Prathyusha, Geeta Voolapalli, T. Ravinder, Vemula Sreenivas. Study of p53 Expression in Carcinoma Cervix and Normal Cervical Epithelium. IAIM, 2019; 6(7): 37-48
- Reynolds JP, Miller-Delaney SFC, Jimenez-Mateos EM, Sano T, Mckiernan RC, Simon RP, et al. Transcriptional Response of Polycomb Group Genes to Status Epilepticus in Mice is Modified by Prior Exposure to Epileptic Preconditioning. Frontiers in Neurology. 2015;6.
CrossRef - Hatta M, Surachmanto EE, Islam AA, Wahid S. Expression of mRNA IL-17F and sIL-17F in atopic asthma patients. BMC Res Notes. 2017;10(1):202. Published 2017 Jun 12. doi:10.1186/s13104-017-2517-9
CrossRef - Sirait RH, Hatta M, Ramli M, Islam AA, Arief SK. Systemic lidocaine inhibits high-mobility group box 1 messenger ribonucleic acid expression and protein in BALB/c mice after closed fracture musculoskeletal injury. Saudi J Anaesth. 2018;12:395‑8. DOI: 10.4103/sja.SJA_685_17
CrossRef - Tambaip T, Karo BR, Hatta M, Dwiyanti R, Natzir R, Massi MN, Islam AA, Djawad K, Immunomodulatory effect of orally red fruit (Pandanus conoideus) extract on the expression of CC chemokine receptor 5 mRNA in HIV patients with antiretroviral therapy. 2018. Res. J. Immunol., 11: 15-21. DOI: 10.3923/rji.2018.15.21.
CrossRef - Tohidi FZ, Toosi MH, Azimian H, Khademi S, Fardid R, Sarab G. The gene expression level of p53 and p21 in mouse brain exposed to radiofrequency field. International Journal of Radiation Research. 2015Sep;13.
- Qiao, K., Ning, S., Wan, L. et al. LINC00673 is activated by YY1 and promotes the proliferation of breast cancer cells via the miR-515-5p/MARK4/Hippo signaling pathway.J Exp Clin Cancer Res 38, 418 (2019). DOI: 10.1186/s13046-019-1421-7
CrossRef - Meliala ITS, Hosea R, Kasim V, Wu S. The biological implications of Yin Yang 1 in the hallmarks of cancer. Theranostics. 2020;10(9):4183–200.
CrossRef - Sarvagalla S, Kolapalli SP, Vallabhapurapu S. The Two Sides of YY1 in Cancer: A Friend and a Foe. Frontiers in Oncology. 2019;9.
CrossRef - Perri F, Pisconti S, Scarpati GDV. P53 mutations and cancer: a tight linkage. Annals of Translational Medicine. 2016;4(24):522.
CrossRef - Mo J, Lin M, He B, Tan K, Jin C, Jiang H, et al. Recombinant human adenovirus‑p53 improves the outcome of mid‑late stage pancreatic cancer via arterial infusion. Oncology Letters. 2017;
CrossRef - Wang L-P, Jia Z-B, Liu Y, Gao Q, Cheng S-J, Jin D, et al. Inhibitory effect of wild-type P53 gene transfer on graft coronary artery disease. Transplant Immunology. 2018;48:1–9.
CrossRef - Garima, Pandey S, Pandey LK, Saxena AK, Patel N. The Role of p53 Gene in Cervical Carcinogenesis. The Journal of Obstetrics and Gynecology of India. 2015Apr;66(S1):383–8.
CrossRef








