Medical Laboratory Department, Turabah University College, Taif University, Saudi Arabia.
Corresponding Author E-mail: adelglhk@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/1996
Abstract
Amethopterin (methotrexate) is a well-known chemotherapy agent that has been used for the treatment of different types of cancers, such as leukemia and breast cancer. It is also used for the treatment of rheumatoid arthritis as an immunosuppressor; however, its numerous adverse effects limit its use in clinical settings. The aim of this study was to determine the protective effect of Moringa oleifera leaves extract on the intestinal toxicity caused by amethopterin exposure. Forty adult male mice were divided into four groups (10 mice each): the control group, a group treated orally by moringa extract (300 mg/kg) daily, a group treated by a single intraperitoneal injection dose of amethopterin at (20mg/kg ) on the seventh experimental day, and a group given orally moringa extract (300 mg/kg) orally daily and a single intraperitoneal injection dose of amethopterin (20mg/kg) on the seventh experimental day. A serum analysis of TNF-α and CRP showed a significant elevation of both parameters in amethopterin-treated mice, while the co-treatment of moringa extract protected the intestines and restored both to normal control values. The immunohistochemistry and histopathology images indicated inflammation in the intestinal lamina as a response to the amethopterin toxicity supported by the downregulation of GLP2 and TGFβ2 genes in the laminal tissues. These structural alterations were prevented by moringa extract co-treatment in the fourth group. In conclusion, Moringa oleifera leaves extract exerts an anti-inflammatory and anti-stress effect on intestinal tissues caused by amethopterin toxicity exposure in experimental mice.
Keywords
Amethopterin; GLP2; Intestinal injury; Methotrexate, Moringa; TGFβ2
Download this article as:| Copy the following to cite this article: Adel Q. A. The Protective Effect of Moringa Oleifera Leaves Extract on Intestinal Injury Caused by Amethopterin Administration in Mice. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Adel Q. A. The Protective Effect of Moringa Oleifera Leaves Extract on Intestinal Injury Caused by Amethopterin Administration in Mice. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/3iwjZm2 |
Introduction
Amethopterin, or methotrexate as it is commercially known, is a synthetic organic compound, and its chemical name is 2,4-diamino-N10-methyl propyl glutamic acid.1 It was introduced in 1950 as a treatment for acute lymphocytic leukemia.1 Amethopterin has been widely used as a chemotherapy agent and for rheumatoid arthritis treatment as an immunosuppression;2-4 however, it has some side effects that vary in severity, such as nausea, fatigue, recurrent infections, liver injury, and renal failure.5-8 In addition, J A Taminiau et al. have reported that amethopterin caused an intestinal injury in rats that were injected with just a single dose, which in turn caused malnutrition in experimented animals.9 Another study revealed that amethopterin caused significant damage in the small intestinal epithelium in experimental mice.10 A study has also shown that amethopterin causes intestinal mucositis that delays gastric emptying in treated rats.11 Therefore, doctors are highly cautious when prescribing this medication, and they always prescribe vitamins as well, such as folic acid, vitamin A, or vitamin D analogues to avoid these side effects.12-14
In another context, some traditional medicinal plants can be used in place of these supplements to provide more protection with fewer side effects. Moringa oleifera Lam is a fast-growing tree belonging to the family Moringaceae.15 The leaves of this plant possess several beneficial organic compounds. They are rich in vitamins, minerals, flavonoids, and polyphenols that consumers benefit from as nutrients and antioxidants.16, 17 This plant has long been used for medical purposes in some ancient nations.15 It has been reported that Moringa oleifera Lam has some medicinal attributes, such as antimicrobial activity and an antiulcer effect as well as antispasmodic, anti-inflammatory, and anti-tumor growth effects.18-21 Therefore, the objective of this study was to investigate the efficiency of Moringa oleifera Lam leaves extract as an anti-inflammatory and an antioxidant caused by amethopterin administration to mice and to what extent it can protect the intestinal lining of these animals after two weeks of co-treatment.
Materials and Methods
Materials
Ethanol 99.8% hplc grade, dNTPs, Oligo (dT), Reverse transcriptase, and Dimethyl ether were purchased from Sigma Aldrich St. Louis, Missouri, United States. The hematoxylin, eosin, anti-Bcl2 antibody, Rabbit Anti-Mouse IgG H&L (HRP), C-reactive protein Elisa kit, and TNF-alpha Elisa kit were purchased from Abcam, Discovery Drive, Cambridge, UK. Amethopterin (Methotrexate) was purchased from Biovectra, Charlottetown, Canada. Primers were purchased from Macrgen, Beotkkot-ro, Geumcheon-gu, Seoul, South Korea. The real-time PCR kit and biozol reagent were purchased from Qiagen, Skelton house, Manchester, UK.
Moringa ethanolic extraction
Fresh Moringa Oleifera leaves were collected from the local markets in Taif, Saudi Arabia. The plant was identified by a botanist colleague from the College of Science, Taif University, Saudi Arabia. The leaves were washed with distilled water and dried in the dark and then powdered using a high-speed electrical grinder. 25 grams of the powder were mixed with 70% of ethanol and stored in the dark for 48 hours at room temperature. The mixture was then filtered using Whatman No1 filter paper, and the filtrate was dried and concentrated at a reduced pressure at a controlled temperature.
Experimental design
All animal model experimental protocols were approved by the Scientific Research and Ethical Committee (SREC) of the University Collage of Tarabah, Taif University. Forty adult male mice were purchased from the animal house of the King Fahad Research Center, Jeddah, Saudi Arabia. The mice were accommodated for two weeks prior to beginning the experiment and were divided into four groups. Group 1 was the negative control (10 mice had free access to food and water). Group 2 had 10 mice exposed to a single intraperitoneal injection dose of Amethopterin (Methotrexate) of 20 mg/kg on the 7th day. Group 3 had 10 mice that were orally administered Moringa Oleifera leaves extract (MOLE) at 300mg/kg daily for the 12 experimental days. Group 4 had 10 mice that were orally administered Moringa Oleifera leaves extract (MOLE) at 300mg/kg daily for the 12 experimental days with exposure to a single intraperitoneal injection dose of Amethopterin (Methotrexate) at 20 mg/kg on the 7th day. On the 13th day, the mice inhaled dimethyl ether and were slaughtered for serum and intestinal tissue collection [22, 23].
Serum analysis
The serums were collected via centrifugation of the whole blood samples at 4000rpm. The samples then were treated using sandwich ELISA technology commercial kits. At the end of the procedure according to the manufacturer instructions, the reactions were read using a Bio-Rad xMark™ Microplate Absorbance Spectrophotometer at 450nm.
Gene expression analysis
The intestinal tissues were collected and immersed in 1ml of biozol reagent and frozen immediately at -20°C. On the day of total RNA extraction, the samples were homogenized and centrifuged at 4°C in a chloroform solution. In equal volumes, Isopropanol was added to each supernatant. The samples then were centrifuged at a high speed, and the supernatants were removed. The precipitates (total RNAs) were rinsed with 70% ethanol and dissolved in DEPC water. For cDNA forming, the extracted total RNAs were mixed with oligo dT and incubated for 5 min at 70°C in a thermal cycler. The denatured RNAs were then mixed with 10mM of dNTPs, 100U of M-MulV, the reverse transcriptase, and 2uL of 10X RT-buffer for each sample. The mixtures were incubated at 37°C for one hour in the thermal cycler followed by 10 min at 90°C to stop enzyme activity. The primers of the genes investigated listed in table 1 were used for the qRT-PCR analysis. The PCR reactions were conducted by following the optimized protocol provided with the kit. The reactions were analyzed using a Bio-Rad CFX Real-Time PCR Detection System.
Table 1: Real time PCR primers used in this study
| Gene | Primer sequence | Product size | Annealing temp. (°C) | |
| GLP-2R | GLP-2-F | GGCGGCCTGAAGAGGACTTG | 128 | 60 |
| GLP-2-R | CCTTCTGGCGCCCTTTCACA | |||
| TGF-b2 | TGFb-2-F | GCGGAGGGTGAATGGCTCTC | 122 | 60 |
| TGFb-2-R | GACGGCACGAAGGTACAGCA | |||
| b-actin | b-actin-F | CCAGCCTTCCTTCTTGGGTA | 143 | 60 |
| b-actin-R | CAATGCCTGGGTACATGGTG |
Histopathological investigation
Intestinal tissues were collected from the mice of different groups and immediately fixed in 10% of formalin. The specimens were then washed under running tap water, dehydrated by alcohol, and then cleared in xylene. In paraffin, the samples were embedded, casted, and sectioned into five micron sections. Finally, routine staining was performed using the H&E stain for the microscopic investigation.
Immunohistochemical examination of Bcl2
The intestinal tissue slides were washed twice with xylene for three min each followed by serial washes in gradient ethanol concentrations for deparaffinization. The slides were then washed under cold tap water to remove the remaining ethanol. The slides were treated with 3% H2O2 for 10 min for peroxidases suppression and then heated in a 10-mM citrate buffer at 121 °C for 30 min for antigen retrieval. The next step was blocking in a 5% normal serum for 15 min. The slides were incubated with a rabbit polyclonal anti-Bcl2 antibody (1:100; sc- EPR17509; Abcam, Discovery Drive, Cambridge, CB2 0AX, UK) in phosphate-buffered saline (PBS) overnight at 4 °C. The slides were then washed three times with PBS and incubated with a Rabbit polyclonal Secondary Antibody to Mouse IgG – H&L (HRP) for 15 min at room temperature. Samples then underwent further incubation with diamino-benzidine (DAB) and were counterstained with hematoxylin for 10 s at room temperature.
Statistical analysis
Data are presented as means ± standard error of means for 10 animals of each group. A two-way ANOVA was performed using SPSS data analysis software. P values < 0.05 were considered significant.
Results and Discussion
Methotrexate (amethopterin) is an anti-cellular growth agent used for treating different types of cancers, such as breast cancer, leukemia, and melanoma.24 It is also used for treating rheumatoid arthritis and psoriasis.25, 26 To some extent, exposure to methotrexate can cause severe toxicity that injures the liver, kidneys, and nervous system.22 In addition, it harms the intestinal lamina and the intestinal mucosa absorptive area.27, 28 In a study published in 2013, scientists reported that exposure to methotrexate for just four days can lead to intestinal mucositis, which is indicated by TNF-α and other cytokines.29 In fact, the challenge is determining how to manage the beneficial effects of methotrexate while avoiding its serious side effects, such as intestinal injury. Interestingly, reviewing several reports on the antioxidant and health benefits of Moringa oleifera Lam Leaves extract (MOLE) encouraged our lab to examine the positive effects of this extract on methotrexate toxicity. The health benefits of MOLE could be due to some of its valuable organic components, such as vitamins, short chain fatty acids, flavonoids, and polyphenols.30, 31
Moringa oleifera leaves extract (MOLE) reduces serum levels of both CRP and TNF-α that are elevated by methotrexate (MTX) exposure
In the current study, the serum levels of both CRP and TNF-α were estimated for all experimental mice as inflammatory condition markers. CRP is a protein produced by hepatocytes, clinically known as an acute-phase inflammatory marker when its concentration is increased in the plasma. In addition, TNF-α is a small protein released mainly by macrophages, natural killer cells, and T-lymphocytes and is known as a proinflammatory cytokine.32, 33 Figure 1 clearly shows a significant increase in both markers. CRP and TNF-α in mice exposed to a single dose of MTX at 20 mg/kg on the seventh experimental day both had P values of ≤ 0.05 corresponding to normal control mice; however, figure 1 shows there was almost no difference between the control group and mice administered Moringa Oleifera leaves extract (MOLE) at 300mg/kg daily over the 12 experimental days, which might mean there is no possible toxicity at that dose for that period. Interestingly, the serum levels of both the CRP and the TNF-α of mice treated with Moringa Oleifera leave extract (MOLE) at 300mg/kg daily over the 12 experimental days with exposure to a single dose of amethopterin (Methotrexate) at 20 mg/kg on the 7th day were significantly reduced to the normal levels, and the P values were ≤ 0.05 corresponding to mice treated with MTX alone. These findings may indicate that the inflammatory response caused by MTX toxicity can be prevented by either pre- or co-treatment of Moringa Oleifera leaves extract (MOLE).
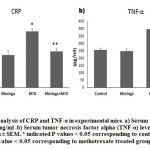 |
Figure 1: Serum analysis of CRP and TNF-α in experimental mice. |
Co-treatment of moringa oleifera leaves extract (MOLE) prevents the downregulation of both GLP2 and TGB2 genes caused by methotrexate (MTX) treatment
Two important genes have been investigated: the Glucagon-like peptide-2 (GLP2) gene and the transforming growth factor beta 2 (TGB2) gene. Glucagon-like peptide-2 (GLP2) is a polypeptide produced by intestinal cells to enhance the absorption process and to protect the gut epithelium.34, 35 Transforming growth factor beta 2 (TGB2) is an anti-inflammatory cytokine released by different cell types to exert a sort of control on the immune response and the bowel anti-inflammatory cytokine.36 Several studies have reported that both proteins are endogenous intestinal lamina protectors against any oxidative stress caused by different types of medications.37-40 B van’t Land and colleagues found that TGB2 exerts some protection in the small intestine of rats when co-treated with methotrexate.41 In another study, GLP2 was reported to be a useful treatment for enteritis caused by chemotherapy.42 Herein, a single dose of methotrexate significantly downregulated both GLP2 and TGB2 gene expression in the intestinal tissues P values of ≤ 0.05 corresponding to normal control mice, while MOLE significantly kept the expression levels at almost normal whereas the P values were ≤ 0.05 corresponding to mice treated with MTX alone, as clearly shown in figure 2.
 |
Figure 2: Real Time PCR analysis of GLP2 and TGFβ2 genes. |
Histopathological investigation
Figures 3A and 3B show a normal histological depiction of the intestinal samples of the control group and the moringa-administered group, which strikingly show normal submucosa, normal lamina epithelials, and normal lamina propria. In the 3C picture of figure 3, methotrexate is shown to have caused a massive infiltration of round cells in the lamina propria accompanied with goblet cell metaplasia and the desquamation of lamina epithelials. The abnormal depiction caused by methotrexate was entirely prevented in the intestinal tissues of mice that were co-treated with moringa leaves extract, as can be observed in figure 3D.
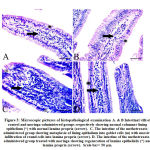 |
Figure 3: Microscopic pictures of histopathological examination |
Immunohistochemical investigation of Bcl2
In the current study, the intracellular Bcl2 levels were examined to investigate the extent of MTX toxicity for cellular apoptosis. Bcl2, or B-cell lymphoma 2, is a family member of proteins that regulate the apoptosis process. Some previous studies have shown that MTX treatment downregulates the expression of the Bcl2 gene.43, 44 Figure 4C demonstrates a significant decrease in cellular Bcl2 in the intestinal tissues of mice as a response to MTX toxicity, while MOLE prevented the reaction, as shown in figure 4D. These findings indicate a fairly apoptotic process in these tissues, which is consistent with previous reports as well as with both serum CRP and TNF-α data and the histological depictions presented in figures 1 and 2. These results add further technical validation to the data that demonstrated both the toxicity effect of methotrexate on the intestinal lamina and the strong prevention of moringa leaves extract against the toxicity.
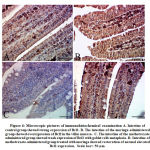 |
Figure 4: Microscopic pictures of immunohistochemical examination |
Conclusion
In conclusion, this study clarified that a single dose of amethopterin (Methotrexate) at 20 mg/kg can cause moderate to severe intestinal injury and Moringa leaves Extract has powerful content that can protect the gut from such toxicity.
Acknowledgment
The authors greatly thank and acknowledge Taif University Saudi Arabia, for its support for facilities.
Grant Support & Financial Disclosures
None
Conflict of Interest
The author declares that there is no conflict of interest.
References
- L.M. Meyer, F.R. Miller, M.J. Rowen, G. Bock, J. Rutzky, Treatment of acute leukemia with amethopterin (4-amino, 10-methyl pteroyl glutamic acid), Acta haematologica 4(3) (1950) 157-167.
CrossRef - J.R. Lurain, E.P. Elfstrand, Single-agent methotrexate chemotherapy for the treatment of nonmetastatic gestational trophoblastic tumors, American journal of obstetrics and gynecology 172(2) (1995) 574-579.
CrossRef - G. Rustin, E. Newlands, J. Lutz, L. Holden, K. Bagshawe, J. Hiscox, M. Foskett, S. Fuller, D. Short, Combination but not single-agent methotrexate chemotherapy for gestational trophoblastic tumors increases the incidence of second tumors, Journal of clinical oncology 14(10) (1996) 2769-2773.
CrossRef - J.M. Kremer, G.S. Alarcón, R.W. Lightfoot Jr, R.F. Willkens, D.E. Furst, H.J. Williams, P.B. Dent, M.E. Weinblatt, Methotrexate for rheumatoid arthritis, Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 37(3) (1994) 316-328.
CrossRef - T.A. Knox, M.M. Kaplan, J.A. Gelfand, S.M. Wolff, Methotrexate treatment of idiopathic granulomatous hepatitis, Annals of internal medicine 122(8) (1995) 592-595.
CrossRef - B. Hernandez-Cruz, M. Cardiel, A. Villa, J. Alcocer-Varela, Development, recurrence, and severity of infections in Mexican patients with rheumatoid arthritis. A nested case-control study, The Journal of rheumatology 25(10) (1998) 1900-1907.
- M. Berends, J. Snoek, E. De Jong, P. Van De Kerkhof, M. Van Oijen, J. Van Krieken, J. Drenth, Liver injury in long‐term methotrexate treatment in psoriasis is relatively infrequent, Alimentary pharmacology & therapeutics 24(5) (2006) 805-811.
CrossRef - S. Ahmad, F.-h. Shen, W.A. Bleyer, Methotrexate-induced renal failure and ineffectiveness of peritoneal dialysis, Archives of internal medicine 138(7) (1978) 1146-1147.
CrossRef - J. Taminiau, D. Gall, J. Hamilton, Response of the rat small-intestine epithelium to methotrexate, Gut 21(6) (1980) 486-492.
CrossRef - M. Nakamaru, Y. Masubuchi, S. Narimatsu, S. Awazu, T. Horie, Evaluation of damaged small intestine of mouse following methotrexate administration, Cancer Chemother Pharmacol 41(2) (1998) 98-102.
CrossRef - P.M.G. Soares, L.O. Lopes, J.M.S.C. Mota, J.N. Belarmino-Filho, R.A. Ribeiro, M.H.L.P.d. Souza, Methotrexate-induced intestinal mucositis delays gastric emptying and gastrointestinal transit of liquids in awake rats, Arquivos de Gastroenterologia 48 (2011) 80-85.
CrossRef - B. Shea, M.V. Swinden, E. Tanjong Ghogomu, Z. Ortiz, W. Katchamart, T. Rader, C. Bombardier, G.A. Wells, P. Tugwell, Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis, Cochrane Database of Systematic Reviews (5) (2013).
CrossRef - H. Zachariae, Methotrexate side‐effects, British journal of Dermatology 122 (1990) 127-133.
CrossRef - S. Lamba, M. Lebwohl, Combination therapy with vitamin D analogues, British Journal of Dermatology 144 (2001) 27-32.
CrossRef - F. Anwar, S. Latif, M. Ashraf, A.H. Gilani, Moringa oleifera: a food plant with multiple medicinal uses, Phytotherapy Research 21(1) (2007) 17-25.
CrossRef - P. Siddhuraju, K. Becker, Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves, Journal of agricultural and food chemistry 51(8) (2003) 2144-2155.
CrossRef - B. Moyo, P.J. Masika, A. Hugo, V. Muchenje, Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves, African Journal of Biotechnology 10(60) (2011) 12925-12933.
CrossRef - A. Caceres, O. Cabrera, O. Morales, P. Mollinedo, P. Mendia, Pharmacological properties of Moringa oleifera. 1: Preliminary screening for antimicrobial activity, Journal of ethnopharmacology 33(3) (1991) 213-216.
CrossRef - S.K. Pal, P.K. Mukherjee, B. Saha, Studies on the antiulcer activity of Moringa oleifera leaf extract on gastric ulcer models in rats, Phytotherapy research 9(6) (1995) 463-465.
CrossRef - A. Cáceres, A. Saravia, S. Rizzo, L. Zabala, E. De Leon, F. Nave, Pharmacologie properties of Moringa oleifera. 2: Screening for antispasmodic, antiinflammatory and diuretic activity, Journal of ethnopharmacology 36(3) (1992) 233-237.
CrossRef - M.M. Khalafalla, E. Abdellatef, H.M. Dafalla, A.A. Nassrallah, K.M. Aboul-Enein, D.A. Lightfoot, F.E. El-Deeb, H.A. El-Shemy, Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma, African Journal of Biotechnology 9(49) (2010) 8467-8471.
- M. Ouédraogo, A. Lamien-Sanou, N. Ramdé, A.S. Ouédraogo, M. Ouédraogo, S.P. Zongo, O. Goumbri, P. Duez, P.I. Guissou, Protective effect of Moringa oleifera leaves against gentamicin-induced nephrotoxicity in rabbits, Experimental and Toxicologic Pathology 65(3) (2013) 335-339.
CrossRef - M.M. Abdel-Daim, H.A. Khalifa, A.I. Abushouk, M.A. Dkhil, S.A. Al-Quraishy, Diosmin attenuates methotrexate-induced hepatic, renal, and cardiac injury: a biochemical and histopathological study in mice, Oxidative medicine and cellular longevity 2017 (2017).
CrossRef - A.S. Knoop, H. Knudsen, E. Balslev, B.B. Rasmussen, J. Overgaard, K.V. Nielsen, A. Schonau, K. Gunnarsdóttir, K.E. Olsen, H. Mouridsen, Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group, Journal of Clinical Oncology 23(30) (2005) 7483-7490.
CrossRef - S. Prey, C. Paul, Effect of folic or folinic acid supplementation on methotrexate‐associated safety and efficacy in inflammatory disease: a systematic review, British Journal of Dermatology 160(3) (2009) 622-628.
CrossRef - J.M. Kremer, G.S. Alarcón, R.W. Lightfoot Jr., R.F. Willkens, D.E. Furst, H.J. Williams, P.B. Dent, M.E. Weinblatt, Methotrexate for Rheumatoid Arthritis, Arthritis & Rheumatism 37(3) (1994) 316-328.
CrossRef - B. Carneiro-Filho, I. Lima, D. Araujo, M. Cavalcante, G. Carvalho, G. Brito, V. Lima, S. Monteiro, F. Santos, R. Ribeiro, Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis, Digestive diseases and sciences 49(1) (2004) 65-72.
CrossRef - J.S. Trier, Morphologic Alterations Induced by Methotrexate in the Mucosa of Human Proximal Intestine: I. Serial Observations by Light Microscopy, Gastroenterology 42(3) (1962) 295-305.
CrossRef - K. Hamada, N. Kakigawa, S. Sekine, Y. Shitara, T. Horie, Disruption of ZO-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis, Cancer Chemotherapy and Pharmacology 72(4) (2013) 757-765.
CrossRef - M. Vergara-Jimenez, M.M. Almatrafi, M.L. Fernandez, Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease, Antioxidants (Basel) 6(4) (2017) 91.
CrossRef - M.A. Valdez-Solana, V.Y. Mejía-García, A. Téllez-Valencia, G. García-Arenas, J. Salas-Pacheco, J.J. Alba-Romero, E. Sierra-Campos, Nutritional Content and Elemental and Phytochemical Analyses of <i>Moringa oleifera</i> Grown in Mexico, Journal of Chemistry 2015 (2015) 860381.
CrossRef - N.R. Sproston, J.J. Ashworth, Role of C-Reactive Protein at Sites of Inflammation and Infection, Front Immunol 9 (2018) 754-754.
CrossRef - W.-M. Chu, Tumor necrosis factor, Cancer Lett 328(2) (2013) 222-225.
CrossRef - D.J. Drucker, Glucagon-like peptide 2, J Clin Endocrinol Metab 86(4) (2001) 1759-64.
CrossRef - G.W. Moran, C. O’Neill, J.T. McLaughlin, GLP-2 enhances barrier formation and attenuates TNFα-induced changes in a Caco-2 cell model of the intestinal barrier, Regulatory Peptides 178(1) (2012) 95-101.
CrossRef - H.S. Oz, M. Ray, T.S. Chen, C.J. McClain, Efficacy of a transforming growth factor beta 2 containing nutritional support formula in a murine model of inflammatory bowel disease, J Am Coll Nutr 23(3) (2004) 220-6.
CrossRef - Q. Lei, J. Bi, X. Wang, T. Jiang, C. Wu, F. Tian, X. Gao, X. Wan, H. Zheng, GLP-2 prevents intestinal mucosal atrophy and improves tissue antioxidant capacity in a mouse model of total parenteral nutrition, Nutrients 8(1) (2016) 33.
CrossRef - P.D. Cani, S. Possemiers, T. Van de Wiele, Y. Guiot, A. Everard, O. Rottier, L. Geurts, D. Naslain, A. Neyrinck, D.M. Lambert, Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability, Gut 58(8) (2009) 1091-1103.
CrossRef - B. Dass, P. Udayakumar, J. Malaiyan, B. Krishnaswamy, P. Krishnan, A. PGIBMS, Temperature and oxidative stress as triggers for virulence gene expression in pathogenic Leptospira spp, (2016).
- B.A. de Koning, B. Philipsen‐Geerling, M. Hoijer, K. Hählen, H.A. Büller, R. Pieters, Protection against chemotherapy induced mucositis by TGF‐β2 in childhood cancer patients: Results from a randomized cross‐over study, Pediatric blood & cancer 48(5) (2007) 532-539.
CrossRef - B. Van’t Land, H. Meijer, J. Frerichs, M. Koetsier, D. Jager, R. Smeets, L. M’Rabet, M. Hoijer, Transforming Growth Factor-β2 protects the small intestine during methotrexate treatment in rats possibly by reducing stem cell cycling, British journal of cancer 87(1) (2002) 113-118.
CrossRef - A. Tavakkolizadeh, R. Shen, P. Abraham, N. Kormi, P. Seifert, E. Edelman, D. Jacobs, M. Zinner, S. Ashley, E. Whang, Glucagon-like peptide 2: a new treatment for chemotherapy-induced enteritis, Journal of Surgical Research 91(1) (2000) 77-82.
CrossRef - L. Ding, X.-M. Hu, H. Wu, G.-X. Liu, Y.-J. Gao, D.-M. He, Y. Zhang, Combined transfection of Bcl-2 siRNA and miR-15a oligonucleotides enhanced methotrexate-induced apoptosis in Raji cells, Cancer biology & medicine 10(1) (2013) 16.
- K.V. Floros, M. Talieri, A. Scorilas, Topotecan and methotrexate alter expression of the apoptosis-related genes BCL2, FAS and BCL2L12 in leukemic HL-60 cells, Biological chemistry 387(12) (2006) 1629-33.
CrossRef








