Syeda Ayesha Siddiqua1 , Md. Zubair Ahmed2*
, Md. Zubair Ahmed2* and Abdullah Ansari3
and Abdullah Ansari3
1Department of Pharmacology, Shadan Institute of Medical Sciences, Teaching Hospital and Research Centre, Hyderabad – 500008, Telangana, India.
2Department of Pharmacology, Mamata Academy of Medical Sciences, Bachupally, Hyderabad – 500090, Telangana, India.
3Department of Pharmacology, Ayaan Institute of Medical Sciences, Teaching Hospital and Research Centre, Hyderabad- 501504, Telangana, India.
Corresponding Author E-mail : drzubair8880@yahoo.co.in
DOI : https://dx.doi.org/10.13005/bpj/2007
Abstract
Background: Urticaria has a wide spectrum of clinical presentations and causes. Patients with urticaria make up a large proportion of referrals to allergy clinics. Urticaria is characterized by wheals with swelling which can appear on any body part. This study is attempted to match the efficiency and safety of olopatadine with rupatadine in the treatment of chronic urticaria of idiopathic origin. Methods: Based on the inclusion and exclusion criteria, sixty patients of urticaria were divided into two treatment groups, olopatadine, and rupatadine groups. The groups received olopatadine and rupatadine, orally with doses of 10mg/day respectively, for 8weeks. Clinical improvement was evaluated through parameters in terms of change in Absolute Eosinophil Count (AEC), serum IgE level, Total Symptom Score (TSS), and safety was considered on the adverse events reported. Results: AEC was considerably depressed by both drugs but the olopatadine group was superior in its action. Within the olopatadine group, a greater decline in the serum IgE level is observed than that of the rupatadine group. Olopatadine was found to be better in the reduction of TSS. The prevalence of adverse effects (gastric irritation, drowsiness, headache, and dryness of mouth) was less in the olopatadine group when compared to the rupatadine group. Conclusions: From the analysis of results, the study shows that olopatadine is a better choice in the treatment of chronic urticaria of idiopathic origin in comparison to rupatadine as a result of its better efficiency and safety profile.
Keywords
Absolute Eosinophil Count; Chronic Urticaria of Idiopathic Origin; Olopatadine; Rupatadine; Serum IgE; Total Symptom Score
Download this article as:| Copy the following to cite this article: Siddiqua S. A, Ahmed Z, Ansari A. The Efficiency and Safety of Olopatadine against Rupatadine in Chronic Urticaria of Idiopathic Origin: A Comparative Study. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Siddiqua S. A, Ahmed Z, Ansari A. The Efficiency and Safety of Olopatadine against Rupatadine in Chronic Urticaria of Idiopathic Origin: A Comparative Study. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/2QalBqc |
Introduction
Urticaria is a cutaneous disorder present with angioedema1 (swelling that arises below the skin). Urticaria is a worldwide disease and may occur at any age. The lifetime occurrence of urticaria in the general population ranges from 1% to 5%. 2, 3 Chronic urticaria repeatedly follows a relapsing course and at night it is worst.4 Chronic urticaria affects mostly adult patients. A higher frequency of chronic urticaria was observed in women (0.45%) than men (0.24%) in the adult population.5 The lesions vary in size from millimeters to centimeters in diameter, and are short-lived, disappearing within 24 hours without damaging the skin, however, some lesions may last up to 48 hours.6 Urticaria is generally classified as acute urticaria/angioedema (<6 weeks) and chronic/autoimmune urticaria (>6 weeks). Acute urticaria or chronic urticaria, in which no cause can be identified, is found in most patients and is called idiopathic urticaria.7
Immunologic mechanisms are involved more often in urticaria. The most common mechanism is hypersensitivity due to triggered mast cells.8 Mast cells are well known for their role in allergic and anaphylactic reactions, 9 their degranulation leads to the rapid release of newly synthesized inflammatory mediators (histamine, leukotrienes, prostaglandins, and cytokines). These all cause vasodilation, leakage of plasma within the skin, and inflammatory responses leading to lesions. Some studies have demonstrated a defective histamine release in patients which may result from a qualitative abnormality of basophil bound IgE.10
Antihistamines are widely used for treating chronic urticaria of idiopathic origin. Antihistamines like olopatadine and rupatadine have been indicated in treating chronic urticaria of idiopathic origin, due to its better efficiency and safety. Olopatadine is a selective H1-receptor antagonist and inhibitor of histamine released from the mast cells. Rupatadine is a selective histamine receptor (H1-receptor) and platelet-activating factor antagonist. This study focuses on the efficiency and safety of olopatadine versus rupatadine in chronic urticaria of idiopathic origin.
Methods
Sixty patients of chronic urticaria of idiopathic origin attending the Department of Dermatology, at Prathima Institute of Medical Sciences, Nagunur, Karimnagar were enrolled for the study. The study comprised of patients of either gender, aged between 18-70 years if they had urticarial episodes, 3 times every week for a period of 4 weeks, without a detectable cause.
ICMR’s Ethical guidelines, 11 were implemented
Group Allocation
60 patients were divided into two groups.
Olopatadine group: 30 patients received olopatadine 10mg/day orally, for 4 weeks.
Rupatadine group: 30 patients received rupatadine 10mg/day orally, for 4 weeks.
Efficiency Measures
Patients were assessed for the degree of urticarial pruritus, size of the urticarial wheals, number of the urticarial wheals, and number of isolated urticarial episodes. Assessments were completed for each patient.
The efficiency was recorded according to the following measures: 12
Number of urticarial wheals: 0 – none, 1 (1-10 wheals), 2 (11-20 wheals) and
3 (> 20 wheals)
Size of urticarial wheals (mean diameter): 0 – no lesion, 1 (< 1.27 cm), 2 (1.27-2.54 cm) and 3 (> 2.54 cm)
Severity of urticarial pruritus: 0 – none, 1 (mild), 2 (moderate) and 3 (severe)
Number of separate urticarial episodes: 0 – no episode, 1 (1 episode), 2 (2-3 episodes) and
3 (> 3 episodes)
Total Symptoms Score (TSS) was 12.
Follow Up
In a month follow up, 5 patients in olopatadine and 4 patients in the rupatadine group were lost. Finally, 51 patients, 25 patients within the olopatadine group, and 26 patients within the rupatadine group completed this study. Detached resume of clinical states was made including investigations and therapy of following parameters,
Absolute Eosinophil Count (AEC)
Serum IgE levels
Total Symptom Score (TSS) and
The assessment of safety was done based on adverse events.
Informed Consent
All the subjects were instructed to fill informed consent before enrolment in the study.
Statistically Analyzed Data
Data is stated as Mean ± SD, and in percentage (%), p value <0.05 was considered statistically significant. Data analysis was done by statistical tools,
a) Paired t test, b) Unpaired t test and c) Fisher’s exact test
Results
60 patients participated, out of which 51 patients, 25 patients within the olopatadine group, and 26 patients within the rupatadine group completed the study.
The parameters Absolute Eosinophil Count (AEC), serum IgE, and Total Symptom Score (TSS) were analyzed statistically and the p value is found to be statistically significant in all of them, data is interpreted in Table 1. Based on the occurrence of adverse events (gastric irritation, drowsiness, headache, and dryness of mouth) assessment of safety was analyzed.
Table 1: Comparison of Absolute Eosinophil Count, Serum IgE level and Total Symptom Score at 1st and 2nd visit in Olopatadine and Rupatadine groups
| Variables | Olopatadine group | Rupatadine group | Mean
Difference between the groups ∆O vs. ∆R |
||||||
| 1st visit | 2nd visit | ∆O | p value | 1st visit | 2nd visit | ∆R | p
value |
||
| Absolute Eosinophil Count (AEC) | 683.92 ± 206.93 | 363.96 ± 115.69 | 320 | 0.831 | 672.70± 172.83 | 484.85± 153.58 | 188 | 0.003 | 0.001 |
| Serum IgE level | 347.96
± 66.73 |
243.0
± 33.09 |
104 | 0.746 | 341.92
± 65.53 |
284.53
± 72.06 |
57 | <0.001 | 0.003 |
| Total Symptom Score (TSS) | 7.58
± 2.1 |
4.48
± 0.653 |
3 | 0.924 | 7.63
± 1.925 |
5.12
± 1.071 |
2 | 0.014 | 0.001 |
Data are in Mean ± SD, ∆ Mean difference
Change in Absolute Eosinophil Count (AEC) is calculated in both study groups. Absolute Eosinophil Count in the olopatadine group at 1st visit is 683.92 and after treatment at 2nd visit is 363.96 and in the rupatadine group at 1st visit is 672.7 and after treatment at 2nd visit is 484.85.
A mean decrease of 320 in AEC in the olopatadine group and 188 in the rupatadine group was found when tested by the Paired t test. When the Mean difference within the two groups was compared by Unpaired t test, the difference was established to be statistically significant (p=0.001)
Serum IgE levels were measured at 1st and 2nd visits, respectively. Serum IgE in the olopatadine group at 1st visit is 347.96 and after treatment at 2nd visit is 243 and in the rupatadine group at 1st visit is 341.72 and after treatment at 2nd visit is 284.53. In the olopatadine group, there was a Mean decrease of 104 in serum IgE in comparison to 57 in the rupatadine group when compared by the Paired t test. The comparative analysis of the Mean difference in distinct groups by Unpaired t test was shown to be statistically significant (p=0.003)
Total Symptom Score (TSS) was assessed at both visits. TSS in the olopatadine group at 1st visit is 7.58 and after treatment at 2nd visit is 4.48 and in the rupatadine group at 1st visit is 7.63 and after treatment at 2nd visit is 5.12.
There was a Mean decrease of 3 in Total Symptom Score (TSS) in the olopatadine group whereas it was 2 in the rupatadine group when related by using the Paired t test. The comparison of the Mean difference was also found to be significant statistically (p=0.001), which was compared by the Unpaired t test.
Absolute Eosinophil Count has a major relationship (coefficient of correlation = 0.482, p<0.001) with TSS, and the above finding has been also presented in the following scatter diagram where the line shows a rise in Absolute Eosinophil Count with a rise in Total Symptom Score. (Figure 1)
 |
Figure 1: Correlation between Absolute Eosinophil Count and Total Symptom Score |
Coefficient of correlation = 0.482, p<0.001
Assessment of Safety
Both the drugs were well tolerated with none new side effects. In the olopatadine group, out of 5 patients, 2 had gastric irritation, 1 of them had drowsiness, 1 had a headache, and 1 patient showed dryness of the mouth.
In the rupatadine group from 6 patients, 1 had gastric irritation, 2 suffered from drowsiness, 2 complained headache, and 1 of the patient had dryness of mouth, comparison of adverse effects in both study groups is given in Table 2.
Table 2: Comparison of adverse effects in olopatadine and rupatadine groups
| Adverse effects | Olopatadine | Rupatadine | Total | p value |
| Gastric irritation | 2 | 1 | 3 | 0.5 |
| Drowsiness | 1 | 2 | 3 | 0.5 |
| Headache | 1 | 2 | 3 | 0.5 |
| Dryness of mouth | 1 | 1 | 2 | 0.75 |
| Total | 5/25 (20%) | 6/26(23.07%) | 11 |
An incidence of adverse effects was 20 % in olopatadine and 23.07 % in the rupatadine group. By performing Fischer’s exact test the p values were obtained, gastric irritation (p=0.5), drowsiness (p=0.5), headache (p=0.5), and dryness of the mouth (p=0.75) respectively were not found to be statistically significant.
A comparison of adverse effects in both olopatadine and rupatadine groups has been depicted in the graphical form (Figure 2). The occurrence of adverse effects (gastric irritation, drowsiness, headache, and dryness of mouth) dropped in the olopatadine group, indicating olopatadine is a safer Antihistaminic.
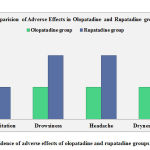 |
Figure 2: Incidence of adverse effects of olopatadine and rupatadine groups respectively |
Discussion
Urticaria has an intense impact on the day-to-day activities of the life of patients. Treating urticaria and ensuring the standard of life is difficult. In previous years, the efficiency and safety of the new generation antihistaminic in treating chronic urticaria of idiopathic origin cannot be denied.
In chronic urticaria of idiopathic origin, Absolute Eosinophil Count is tested regularly because it is a superior parameter. As eosinophil is the most important blood corpuscle contributing to any allergic response, its control is of paramount importance. Olopatadine is an antiallergic drug with selective and potent histamine H1 receptor antagonist activity, 13 it controls AEC levels. In this study, olopatadine was found to be superior in controlling AEC than rupatadine. There was a mean decrease of 320 in Absolute Eosinophil Count (AEC) in the olopatadine group and 188 in the rupatadine group. The mean difference in both olopatadine and rupatadine groups was statistically significant when it was tested by unpaired t-test.
Allergic reaction identification can be done in most of the patient cases by assessing serum IgE levels. Chronic urticaria is a serum IgE mediated immunological response, treatment strategies depend on the modulation of the immune response to interfere with the function of IgE antibodies. Olopatadine has an immunomodulatory action that helps in improving the chronic urticaria condition.14 Serum IgE levels in the olopatadine group were reduced to 104 and in the rupatadine group, 57 reading was found. The mean difference in both the study groups was statistically significant. It is found that olopatadine is superior in comparison to rupatadine in reducing serum IgE levels.
Patients were evaluated for the degree of urticarial pruritus, size of the urticarial wheals, number of the urticarial wheals, and the number of urticarial episodes in both 1st and 2nd visits, respectively. The Total Symptoms Score (TSS) calculation was 12. There was a mean reduction of 3 in Total Symptom Score in the olopatadine group while it was 2 in the rupatadine group. The mean difference in both olopatadine and rupatadine study groups was found to be statistically significant when tested by unpaired t test, the change in Total Symptom Score showed statistical significance with olopatadine group. Total Symptom Score is a dependable means to evaluate the efficiency of a drug and a higher reduction15 in it shows an improvement of the disorder.
The frequency of adverse effects (gastric irritation, drowsiness, headache, and dryness of mouth) was of the smaller amount in the olopatadine group, indicating olopatadine is a safer Antihistaminic. Olopatadine therapy was well tolerated without major side effects by most patients in chronic urticaria studies.16
From the analysis of results, the study reveals that olopatadine is a better choice in the treatment of chronic urticaria of idiopathic origin as compared to rupatadine due to its better efficiency and safety profile.
Conclusion
The analysis of the present comparative clinical study between olopatadine and rupatadine reveals that Absolute Eosinophil Count was significantly lowered by olopatadine and it was found to be better than rupatadine.
Olopatadine group showed a significant reduction of serum IgE, and it was found to be better than that of rupatadine.
Olopatadine was found to be better in the reduction of Total Symptom Score than rupatadine.
The frequency of adverse effects was less in the olopatadine group than in the rupatadine group.
It can be concluded that olopatadine is a superior choice in chronic urticaria of idiopathic origin.
Ethical Approval
The study was approved by the institutional ethics committee.
Acknowledgments
We express our immense gratitude to our Lab technician in the Department of Pharmacology (Neeli Naresh), Prathima Institute of Medical Sciences and Dr. Sameer Valsangkar for his assistance during statistical analysis
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding source
References
- Zuberbier T, Maurer M. Urticaria: Current opinions about etiology, diagnosis and therapy. Acta Derm Venereol. 2007;87(3):196–205.
- Schäfer T, Ring J. Epidemiology of urticaria. Monogr Allergy. 1993;31.
- Paul E, Greilich KD, Dominante G. Epidemiology of urticaria. Monogr Allergy. 1987;21.
- Grattan CEH, Francis DM, Greaves MW. A histamine releasing factor in serum of chronic urticaria with anti-IgE autoantibody-like properties. Br J Dermatol. 1990;123(s37):45–6.
CrossRef - Parisi CA, Ritchie C, Petriz N, Torres CM, Gimenez-Arnau A. Chronic urticaria in a health maintenance organization of buenos aires, Argentina – New data that increase global knowledge of this disease. Anais Brasileiros de Dermatologia. 2018;93(1).
CrossRef - Kanani A, Betschel SD, Warrington R. Urticaria and angioedema. Allergy, Asthma Clin Immunol. 2018;14.
CrossRef - Schwartz RA. The 13th East Zonal Indian Association of Dermatology, Venereology and Leprology (IADVL) Conference, 19th Annual IADVL West Bengal Chapter Conference and the Indian Journal of Dermatology Diamond Jubilee Celebration, Kolkata, 2015: A Triple Educational Tr. Indian J Dermatol. 2016;61(2):231–3.
CrossRef - Monroe EW, Jones HE. Urticaria: An Updated Review. Arch Dermatol. 1977;113(1):80–90.
CrossRef - Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, et al. Mast cells and inflammation. Biochim Biophys Acta – Mol Basis Dis. 2012;1822(1).
CrossRef - Kern F, Lichtenstein LM. Defective histamine release in chronic urticaria. J Clin Invest. 1976;57(5):1369–77.
CrossRef - Ananthakrishnan N, Shanthi AK. ICMR’s ethical guidelines for biomedical research on human participants: need for clarification. Indian J Med Ethics. 2012;9(3):207–9.
CrossRef - Maiti R, Jaida J, Raghavendra BN, Goud P, Ahmed I, Palani A. Rupatadine and levocetirizine in chronic idiopathic urticaria: A comparative study of efficacy and safety. Journal of Drugs in Dermatology. 2011;10(12).
CrossRef - Miyake K, Ohmori K, Ishii A, Karasawa A. Inhibitory effect of olopatadine hydrochloride (KW-4679), a novel antiallergic drug, on peptide leukotriene release from human eosinophils. Allergology International. 2001;50(1):113–6.
CrossRef - Mahawar D, Mathur S, Sankhla S, Aseri M, Dass B, Gaur S. A prospective study of comparison of efficacy and safety between levocetirizine and olopatadine in chronic idiopathic urticaria. Indian Journal of Allergy, Asthma and Immunology. 2014;28(2).
CrossRef - Dakhale GN, Wankhede SS, Mahatme MS, Hiware SK, Mishra DB, Dudhgaonkar SS. Comparison of efficacy, safety and cost-effectiveness of rupatadine and olopatadine in patients of chronic spontaneous urticaria: A randomized, double-blind, comparative, parallel group trial. Indian Journal of Dermatology. 2016;61(1).
CrossRef - Godse K. Olopatadine in chronic idiopathic urticaria. Indian Journal of Dermatology. 2009;54(2):191–2.
CrossRef








