R. V. Rohit Vishwanath1, S. Saradha2* , A. Ruckmani2, A. Sindhana1, R. Vijayashree2, T. Sobita Devi3
, A. Ruckmani2, A. Sindhana1, R. Vijayashree2, T. Sobita Devi3
1Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India.
2Department of Pharmacology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India
3Department of Pathology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakam, Chennai-603103, Tamilnadu, India.
3Central Animal House, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Chennai-603103, Tamil Nadu, India.
Corresponding Author E-mail : saradhachoks@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1997
Abstract
Objective: To evaluate the effect of Resveratrol on testicular functions in rapid eye movement (REM) sleep deprived Wistar rats. Method: Eighteen male Wistar albino rats were divided into three groups of 6 rats each. Group1,(Control) had rats with normal sleep/wake cycle. Group 2 and 3 rats were subjected to seven days of REM sleep deprivation. Group 3 in addition received 20 mg/kg/day of Resveratrol. REM sleep deprivation was done using the inverted flower pot technique. Sperm count and motility, testosterone level, and histo pathology of testes was assessed among the three groups. One way ANOVA was used to assess the difference among the groups. p value<0.05 was considered statistically significant. Results: Sperm count and motility as well as testosterone level were within normal range in group 1. Group 2 had significant reduction in sperm count, motility and testosterone level (p<0.05). Group 3, treated with resveratrol in addition to REM sleep deprivation, showed improved sperm count, motility and testosterone and these levels were equivalent to group 1.The difference between group 2 and group 3 was found to be statistically significant(p <0.05). Histopathological analysis of the testes revealed normal architecture in group 1 and mild spermatogenic disarray with distorted architecture in group 2. However in group 3 no significant change was observed. Conclusion: Resveratrol was found to protect the testis from the damage due to REM sleep deprivation in rats in terms of sperm count, motility, testosterone levels and testicular morphology.
Keywords
Antioxidants; Infertility; REM Sleep Deprivation; Resveratrol; Sperm; Testes
Download this article as:| Copy the following to cite this article: Vishwanath R. V. R, Saradha S, Ruckmani A, Sindhana A, Vijayashree R, Devi T. S. Protective Effect of Resveratrol on REM Sleep Deprivation Induced Changes in Testicular Functions in Wistar Rats. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Vishwanath R. V. R, Saradha S, Ruckmani A, Sindhana A, Vijayashree R, Devi T. S. Protective Effect of Resveratrol on REM Sleep Deprivation Induced Changes in Testicular Functions in Wistar Rats. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/3fVfMIp |
Introduction
Infertility is a significant clinical problem today and it affects 8–12% of couples worldwide.1 Nearly fifty percent of infertility results from male factors and two percent of all men exhibit sub optimal sperm parameters1. Sleep is essential for mind and body. Lack of sleep and increased stress cause the production of ROS (reactive oxygen species) in the body leading to oxidative stress induced damage.2 Along with other organs, testis is also affected by the reactive oxygen species.2 This is evident by the presence of free radicals in the semen samples of infertile men.3 Sleep deprivation can cause reduction in sperm viability and motility in rats4. Resveratrol (trans3,4’,5-Trihydroxystilbene) is a phenolic compound that is extracted from the skin of red grapes5,Vitis vinifera.
Resveratrol is found to have anti-inflammatory, anti-aging, antimicrobial, anticancer and antioxidant properties. It also prevents cognitive deterioration and improves insulin sensitivity. The health benefits of resveratrol have also been studied in obesity and cardiovascular disease.6,7 Currently, Resveratrol is available as a nutritional supplement with a wide range of pharmacological effects, including cellular defense against oxidative stress.7 This research was done to assess the effect of resveratrol on testicular functions in REM sleep deprived adult Wistar albino rats.
Materials and Methods
Animals
Eighteen male Wistar albino rats,14 to 18 weeks old, weighing 150-200g, were used in this study. The animals were procured from the Central Animal House of the Institution and after 1 week of a quarantine and acclimatization. The animals were housed in large poly propylene cages in a room maintained at a temperature of 24°C±3°C and a relative humidity of 50%±10% and had 12+/-1 hour light dark cycle to maintain day and night rhythm throughout the experimental period. The animals received a balanced commercially available pelleted rat feed and clean water. The experimental procedures were done in accordance with CPCSEA guidelines. The study was conducted following the approval of the Institutional animal ethics committee (IAEC1/Proposal:19/A.Lr:03/Dt.01.03.18).
Drug
Resveratrol was obtained from Sharrets Nutritions® as 100 percent standardized 250mg capsules.
Study Details
The animals were divided into three groups of six animals each and treated as given in Table.1.
Table 1: Study design
| Group | Intervention | |
| Group 1 (n=6) | Control
REM sleep deprived REM sleep deprivation + Resveratrol |
|
| Group 2 (n=6) | ||
| Group 3 (n=6) | ||
Group 1 served as control group and had a normal sleep wake cycle. Groups 2 and 3 were subjected to REM sleep deprivation for a period of 7 days using the inverted flower pot technique8.Resveratrol was administered at a dose of 20mg/kg body weight/day orally.9The body weights of all the rats were recorded on day 0 and day 8. The general behavior, physical activity, food and water intake were observed throughout the study period. On day eight, 0.5 ml of blood was drawn for estimation of testosterone. All the animals were then sacrificed. Testicular and cauda sperm motility, testicular and cauda sperm count and histopathological examination of testis were carried out.
Sleep Deprivation
The duration of REM sleep deprivation was 7 days in groups 2 and 3. It was achieved using the inverted flower pot technique8. To produce sleep deprivation, the rats were placed on a circular platform of diameter 6.5cm (Figure 1) in the center of a small water tub in which water was filled till 1cm beneath the surface of the platform. This set up prevents REM sleep because reduction in muscle tone tends to make the rat fall in water which wakes it up. The inverted flower pot technique is the most widely used method for REM sleep deprivation in rats. The control group animals were placed on similar setup with diameter of the platform being 15 cm (Figure 1), large enough to permit the animal to go into REM sleep without falling into the water. The apparatus was designed with provisions for food pellets and clean water for the rats.
 |
Figure 1: REM Sleep deprivation setup and Control setup. |
Sperm Motility Assessment
Sperm motility assessment was done for all the groups on day eight. Once the rats were euthanized with halothane, removal of both testes and cauda epididymis was done and placed in 10% formalin. To detect motility, sperm wash media was kept in water bath at 370C. 5mL of the media was placed in a petri dish. To suspend the sperm, the distal part of the left cauda epididymis was dissected with a 23 gauge needle and sperm was exuded by applying gentle pressure in the proximal part. The motility of the sperms were assessed using a light microscope and was expressed in percentage. The same procedure was repeated to obtain motility % of sperms in the testis.
Sperm Count
Sperm count was done for all the groups on the eighth day. The left testis after dissection using sterile bard parker surgical blade no.22 was teased and was homogenized with sperm wash medium. The sperm suspension was placed in the Makler’s chamber, waited for stabilization of the sperm heads and the number of heads counted using a light microscope and the testicular sperm count obtained.
To measure the cauda epididymis sperm count, the left cauda epididymis was minced and placed in a 15mL test tube with 5 mL sperm wash medium. The suspension was homogenized for one minute. Cauda sperm counts performed similar to the testis using Makler chamber.
Serum Testosterone Estimation
On day eight, the rats were anaesthetized with halothane, 0.5ml of blood was collected from the periorbital plexus. Testosterone level was estimated by Enzyme Linked Immunosorbent Assay method.
Histopathological Examination
Histopathological examination of the testes was done on the eighth day. The right testis was embedded in paraffin wax. After deparaffinization, the tissue blocks were cut in 5μmthickness, stained with hematoxylin and eosin and examined under a students’ light microscope.
Statistics
Statistical analysis was performed using SPSS software. One-way ANOVA was used to assess the difference in sperm motility, cauda and testis sperm count and testosterone levels among the three study groups. p value of <0.05 was considered statistically significant.
Results
All the rats were alive throughout the study. The general physical activity, food and water intake was similar in group 1 and 3. The group 2 animals (sleep deprived) had shown reduced food and water intake. The body weight of animals before and after the study is shown in Table 2.
Table 2: Body weight of animals
| Weight (gm) – Day 0
Mean ± SD |
Weight (gm) – Day 8
Mean ± SD |
|
| Group 1 | 236.85±18.18 | 238.51±18.26 |
| Group 2 | 235.42±7.3 | 226.8±2.9* |
| Group 3 | 235.52±10.4 | 239±10.25 |
*p Value< 0.005 with statistical significance
There was a mean reduction in body weight of animals in group 2 following REM sleep deprivation, whereas there was no reduction in body weight in group 1 and 3.
The testicular and cauda sperm count and motility with serum testosterone levels are given in Table.3
A statistically significant difference was obtained among the three study groups with respect to testicular sperm count, cauda sperm count, motility of testicular sperms and testosterone levels.
The difference in testicular sperm count was significant when groups 1 and 3 were compared with group 2 as shown in (Figure 2, Table 3).
Table 3: Comparison of sperm count, motility and testosterone level on day 8
| Sperm Count (Millions/mL) | Sperm Motility (%) | Serum Testosterone level(ng/mL) | |||
| Testis | Cauda | Testis | Cauda | ||
| Group I | 65.17±7.2 | 102.83±13.77 | 17.33±1.37 | 37.17±5.53 | 1.3±0.2 |
| Group II | 37.83±6.61* | 66.33±6.37* | 15.33±1.86* | 34.67±3.88* | 0.56±0.39* |
| Group III | 67±8.46* | 108.83±26.33* | 29.5±4.03* | 43.5±9.52* | 1.96±0.85* |
*p Value < .005 and statistically significant
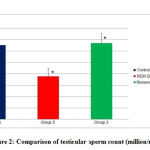 |
Figure 2: Comparison of testicular sperm count (million/mL) |
Comparison of cauda sperm count among the three groups (Figure 3, Table 3) showed statistical significance among groups 1 and 3 in comparison with group 2. REM sleep deprivation has reduced the cauda sperm count. However following treatment with Resveratrol the sperm count has improved and comparable to that of group 1.
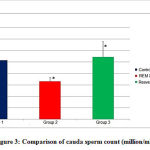 |
Figure 3: Comparison of cauda sperm count (million/mL) |
Comparison of testosterone levels among the groups (Figure 4, Table 3) showed a significant change (p<0.05) between groups 1 and 3 when compared with group 2.
 |
Figure 4: Comparison of testosterone levels (ng/mL) |
The testicular sperm motility was significantly reduced in REM sleep deprived rats. (Figure 5, Table 3). Co administration of resveratrol had significantly improved the motility better than that of normal rats.
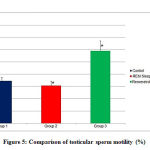 |
Figure 5: Comparison of testicular sperm motility (%) |
Comparison of cauda sperm motility (Figure 6,Table 3) showed a statistically significant difference (p<0.05)in groups 1 and 3 when compared with group 2.
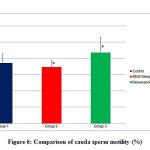 |
Figure 6: Comparison of cauda sperm motility (%) |
The results have shown that resveratrol improved the sperm count, motility as well as increased serum testosterone level.
Histopathological analysis of the testes revealed normal architecture in control (Figure 7).
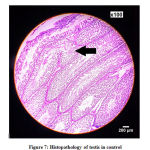 |
Figure 7: Histopathology of testis in control |
Histo pathological image of testicular tissue in group 2 rats showed mild spermatogenic disarray with distorted architecture (Figure 8).
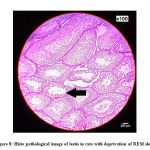 |
Figure 8: Histo pathological image of testis in rats with deprivation of REM sleep |
Histopathology of testis in Resveratrol treated group shows normal architecture (Figure 9).
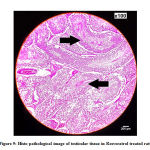 |
Figure 9: Histo pathological image of testicular tissue in Resveratrol treated rats |
Discussion
Sleep is a physiologic process that is needed for life and health. Sleep has an important part in the functioning of central nervous system and other systems. It also has an influence on immune function. Sleep is considered to be normal and healthy if it is of good quality, appropriate duration and regular in nature.10 Sleep can be staged in to two, “rapid eye movement (REM) sleep” and “non-rapid eye movement (NREM) sleep”. A cycle between stages of NREM and REM sleep occurs throughout the night, with REM sleep constituting about 20-25%. REM sleep has a desynchronized brain wave activity, reduced muscle tone and rapid eye movements. There are many physiological differences between NREM and REM sleep. During NREM sleep heart rate, blood pressure, brain activity, flow of blood to the brain, muscle tone and the process of respiration are reduced in comparison with wakefulness, whereas during REM sleep, these parameters are increased.
Sleep is very important for most of the biological processes and deprivation can cause adverse consequences to health.11
Sleep deprivation can lead to increase in stress level, depression, anxiety, memory disturbances as well as poor performance in normal individuals. Chronic sleep deprivation may cause increase in blood pressure, abnormalities in lipid profile, cardiac disorders, gain in body weight, increase in gastric acid secretion and impaired glucose tolerance.11
In mice, it was found that five hours of sleep deprivation decreased memory by affecting neuronal connectivity in the Hippocampus.12
REM sleep deprivation in humans enhances emotional reactivity both at behavioural and neural levels.11
REM sleep deprivation is a potent oxidative stressor that could cause, behaviour and performance alteration in both experimental animals as well as humans.8 Oxidative stress response occurs when there is an increased formation of ROS and decreased neutralization. This can precipitate a reduction in sperm concentration and motility and changes in the testicular morphology, leading to infertility. “Spermatozoa are particularly vulnerable to the harmful effects of ROS.” Oxidative stress can affect the activity of sperms, cause damage to DNA and hasten the apoptosis leading to decreased sperm count, motility and function.13
Choi et.al., demonstrated that REM sleep deprivation in Wistar rats caused significant reduction in sperm motility and testosterone levels. However they did not observe a significant difference in testis and cauda sperm counts.14 In our study significant improvement was seen in testicular and cauda sperm counts in resveratrol treated group.
Alvarenga et al have demonstrated that rats exposed to 96 hours of paradoxic sleep deprivation showed a reduction in testosterone level and sperm count by affecting testicular nitric oxide pathway. They have observed that “sleep deprivation has increased expression of genes encoding inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS).” These enzymes synthesize nitic oxide which may mediate free radical injury to testis.4In our study a significant reduction in testosterone levels and sperm count was seen in the sleep deprived group when compared with Resveratrol treated group and control, though the molecular basis of free radical injury was not studied. This could have been mediated by NO.
Ebtihal et al observed that decrease in testosterone level in sleep deprived male rats was due to oxidative stress which was evident by an increase in malondialdehyde and glutathione in testicular tissue15 and the observations on testosterone level were similar to our study.
Many studies conducted both in vitro and in vivo demonstrated the beneficial effects of antioxidants on fertility and recommend their use for the treatment of male infertility.16
Several plant products like ellagic acid in Pomegranate, ascorbic acid in Gooseberry, Lycopene in tomato are well known antioxidants and Resveratrol, a phyto chemical derived from the skin of red grapes is one among them.
In a meta analysis done by Armand Zini et al, it was observed that Randomized controlled trials on antioxidant therapy for male infertility showed a beneficial effect on sperm parameters. The oral antioxidants that demonstrated positive effects on sperm parameters in terms of sperm count, motility and morphology were vitamin C, vitamin E, selenium, zinc, glutathione, L-carnitine and N-acetyl cysteine.17 In our study we have observed similar effects on sperm count, motility and morphology in Resveratrol treated group.
Florin Muselin et al have observed the role of Resveratrol in prevention of trace element imbalance in older rats, caused by aluminium induced oxidative stress.18 In our study we have used sleep deprivation to induce oxidative stress in rats.
“Resveratrol has been found to act as an antioxidant by augmenting the action of enzymes catalase, superoxide dismutase and glutathione reductase and enhancing the expression of the transcription factor nuclear factor erythroid factor 2 (Nrf2).19”
Sleep deprivation in men is associated with reduced testosterone level and sperm quality.20 In this study it was observed that the sperm count, sperm motility as well as the testosterone levels showed an improvement in Resveratrol treated sleep deprived rats. As Resveratrol is commercially available it may be used as an adjunctive therapy in male infertility if there is an associated sleep deprivation.
The limitation of our study is that molecular mechanisms relating to the functioning of Resveratrol within the testis has not been properly understood and leaves room for further research.
Conclusion
Sleep deprivation is an important factor that can cause male infertility by reducing testosterone levels, sperm count and sperm motility. Our study has shown that Resveratrol has a protective effect on the testicular damage caused by sleep deprivation. Hence Resveratrol may be used as an adjuvant therapy in male infertility associated with sleep deprivation. However future human studies are needed to establish its effects.
Acknowledgements
All the authors are thankful to Chettinad Hospital and Research Institute for providing the facility to conduct this research. The authors would like to acknowledge the contribution made by Dr.Ranjani, Department of IVF, Chettinad Hospital and Research Institute for her support in the conduct of the study.
Funding Source
This research did not receive any grant from any funding agencies.
Conflict of Interest
All the authors have declared nil conflict of interest.
References
- Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. Journal of Human Reproductive Sciences. 2015;8(4):191-196.
CrossRef - Ashok Agarwal, DamayanathiDurairajanayagam, Jacques Halabi, Jason Peng, Monica Vazquez-Levin. Proteomics, oxidative stress and male infertility. Reproductive BioMedicine Online (2014) 29, 32– 58
CrossRef - Yumura Y, Takeshima T, Kawahara T, et al. Reactive oxygen species measured in the unprocessed semen samples of 715 infertile patients. Reproductive Medicine and Biology. 2017;16(4):354-363.
CrossRef - Alvarenga, Tathiana A. et al. Impairment of male reproductive function after sleep deprivation. Fertility and Sterility, Volume 103, Issue 5, 1355 – 1362.
CrossRef - Singh CK, Liu X, Ahmad N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann N Y Acad Sci. 2015;1348(1):150–160.
CrossRef - Ramírez-Garza SL, Laveriano-Santos EP, Marhuenda-Muñoz M, Storniolo CE, Tresserra-Rimbau A, Vallverdú-Queralt A, Lamuela-Raventós RM. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients. 2018 Dec 3;10(12):1892.
CrossRef - Salehi B, Mishra AP, Nigam M, et al. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines. 2018;6(3):91. Published 2018 Sep 9.
CrossRef - Mathangi DC, Shyamala R, Subhashini AS. Effect of REM sleep deprivation on the antioxidant status in the brain of Wistar rats.Annals of Neurosciences. 2012;19(4):161-164.
CrossRef - Juan, Vinardell, Planas. The daily oral administration of high doses of resveratrol to rats for 28 days is not harmful. The Journal of Nutrition. 2002.
CrossRef - Yusuf Patrick, Alice Lee, OishikRaha, Kavya Pillai, Shubham Gupta, SonikaSethi et.al. Effects of sleep deprivation on cognitive and physical performance in university students. Sleep Biol. Rhythms (2017) 15:217–225.
CrossRef - Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption.Nat Sci Sleep. 2017;9:151-161.
CrossRef - Havekes R, Park AJ, Tudor JC, et al. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife. 2016;5:e13424. Published 2016 Aug 23. doi:10.7554/eLife.13424
CrossRef - Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66(1):60–67.
CrossRef - Choi JH, Lee SH, Bae JH, et al. Effect of Sleep Deprivation on the Male Reproductive System in Rats. Journal of Korean Medical Science. 2016;31(10):1624-1630.
CrossRef - Ebtihal A. Abd El-Aziz, Dalia G. Mostafa. Impact of sleep deprivation and sleep recovery on reproductive hormones and testicular oxidative stress in adult male rats. AAMJ,2012:10(3).
- Gambini J, Inglés M, Olaso G, et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid Med Cell Longev. 2015;2015:837042.
CrossRef - Zini A, Al-Hathal N. Antioxidant therapy in male infertility: fact or fiction?.Asian J Androl. 2011;13(3):374–381.
CrossRef - Homeostatic changes of some trace elements in geriatric rats in the condition of oxidative stress induced by aluminum and the beneficial role of resveratrol. J.Trace Elem.Med.Biol, 2019; 55:136-142.
CrossRef - Malhotra A, Bath S, Elbarbry F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol.Oxid Med Cell Longev. 2015;2015:803971.
CrossRef - Wise LA, Rothman KJ, Wesselink AK, et al. Male sleep duration and fecundability in a North American preconception cohort study. FertilSteril. 2018;109(3):453‐
CrossRef








