Manuscript accepted on :17-07-2020
Published online on: 23-07-2020
Plagiarism Check: Yes
Reviewed by: Vikrant Rai
Second Review by: Abhijit Nirwane
Final Approval by: Dr. Javad Sharifi-Rad
Jaganathan Murugasan Kuppuswamy and Barathi Seetharaman
and Barathi Seetharaman
Department of Biotechnology, School of Bioengineering, Faculty of Engineering and Technology, SRM Institute of Science and Technology, Tamil Nadu – 603203
Corresponding Author E-mail : barathi_micro@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1998
Abstract
Monocrotophos is one of the most widely used pesticides in India. The agricultural workers were exposed to high concentrations of these pesticides due to unsafe work practices, and also the individual exposed to these pesticides shows alterations in their neurobehavioral. The present study aimed to evaluate the toxic effects of Monocrotophos on the locomotor activity, antioxidant activity, lipid peroxidation, and expression of genes related to apoptosis using adult zebrafish (Danio rerio) as a model. The neurotoxic effects of sub-lethal concentration of the Monocrotophos on fish were evaluated. The locomotor activity of the fishes was determined, followed by an antioxidant activity, and lipid peroxidation assays were performed. The expression of genes related to apoptosis in the brain was also determined by qPCR. The Lethal Concentration for 96 hrs. was estimated to be 36.5 mg/L. When exposed to sub-lethal concentration, marked changes were observed in locomotor activity (distance traveled, swimming speed and meandering), acetylcholinesterase activity, oxidative stress markers (lipid peroxidation) and antioxidant defenses in the brain. Expression of apoptotic genes determined by qPCR revealed an increase in Bax, caspase-3, and caspase-9 expression and a decrease in Bcl-2 expressions. Data from this study suggest that Monocrotophos based pesticides induce cellular toxicity, and has the potential to alter the behavior and health.
Keywords
Behavior Response; Lipid Peroxidation; Monocrotophos; Oxidative Stress; Zebrafish
Download this article as:| Copy the following to cite this article: Kuppuswamy J. M, Seetharaman B. Monocrotophos Based Pesticide Alters the Behavior Response Associated with Oxidative Indices and Transcription of Genes Related to Apoptosis in Adult Zebrafish (Danio rerio) Brain. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Kuppuswamy J. M, Seetharaman B. Monocrotophos Based Pesticide Alters the Behavior Response Associated with Oxidative Indices and Transcription of Genes Related to Apoptosis in Adult Zebrafish (Danio rerio) Brain. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/2WLAmmI |
Introduction
Organophosphate (OP) compounds are a group of chemicals agents used for protecting crops, livestock, and human health.1 Monocrotophos (MCP) (C7H14NO5P) is an organophosphate insecticide that is used to protect various crops such as cotton, coconut, coffee, maize, sugarcane, paddy, and pulses from various pests.2 In India, Monocrotophos is banned for use on vegetables and is under the “restricted use” category.3 It inhibits acetylcholinesterase (AchE) activity and prevents the breakdown of the neurotransmitter acetylcholine (Ach), results in the accumulation of acetylcholine in the synapses inducing neurotoxicity.4–7 Monocrotophos is particularly predominant in India, where more than 22% of the Indian market is dependent on cotton pesticides (Government of India, 2019), and it is one of the primary agents used in the current epidemic of farmer suicides in India.3 Due to its easy availability, they have been used extensively, and this pesticide becomes the most common household item in rural areas of developing countries resulting in enhanced utilization for homicidal and suicidal poisoning cases .9,10 As per the World Health Organization, OP kills around 200,000 people each year, mainly in the Asia-Pacific region ( World Health Organization, 2001).
In India, agricultural workers were exposed to high concentrations of pesticides due to unsafe work practices, like mixing of pesticides with bare hands, spraying of pesticides without a mask, and leakages from the container and tanks of pesticide during the spraying operation.3 Moreover, follow-up studies in the individuals exposed to high levels of organophosphorus compounds have shown specific neurobehavioral changes such as anxiety, mood swings, emotional liability, depression, fatigue, irritability, drowsiness, and confusion.12 These neurobehavioral changes have been termed together as chronic organophosphate induced neuropsychiatric disorder (COPIND).12,13 Previous reports have suggested that exposure to a higher concentration of agricultural pesticides, especially to organophosphates, produces anxiety and depression, which is a risk factor for suicide.14 Recent data from the National crime bureau of India show in 2018, 23331 people committed suicide by consumption of pesticides, which account for 17.3 % of all cases of suicidal poisoning.15 Organophosphate use in agriculture was the leading method of suicide in both men and women, corresponding to about 92000 deaths nationally at ages 15 years and older,16 and also reported that suicide attempts might be an effect of exposure to pesticides.17,18
Many studies established the link between pathological anxiety and oxidative stress and also a possible relationship between cellular oxidative stress and emotional stress.19,20 OP pesticides can induce oxidative stress, by increasing the production of free radicals and its accumulation in the cell thereby altering the antioxidant defense mechanisms, including detoxification and scavenging enzymes including catalasei(CAT),iglutathioneiS-transferasei(GST),iand superoxide dismutase (SOD), or by increasing lipid peroxidation.21–23 Free radicals produced by these pesticides attack glial cells and neurons, ending in oxidative stress leading to programmed cell death, i.e., apoptosis.24 Oxidative stress and free radical generation are the main culprits in neuropsychiatric diseases and neurodegenerative diseases.19,25,26 OP also shows various adverse effects on the replication and differentiation of the brain cells and even alters the synaptic development and function, and ultimately alteration in behavioral performance.27
MCP is one of the most widely used pesticide in India, and there is not much data related to the mechanisms of toxicity other than AchE inhibition. Therefore, in this study, MCP induced changes in the behavior, oxidative stress biomarkers, and expression of apoptotic pathway-related genes in the brain were assessed using adult zebrafish (Danio rerio) on exposure to sub-lethal concentration of MCP. The genome of zebrafish was well characterized, and this allows us to use more sophisticated molecular methods to investigate the toxicity mechanisms.28–31 Our experimental approach was to expose zebrafish to sub-lethal concentration of MCP near to the lethal concentration and to assess the effect of exposures on behavior, oxidative stress biomarker (CAT, SOD, GSH, lipid peroxidation), and expression of the gene related to apoptosis in the brain tissue. This information would provide new understanding of the toxicological function of MCP other than the acetylcholinesterase inhibition.
Materials and Methods
Experimental Fish
Six-month-old, Zebrafish (Danio rerio) (0.87± 0.06 g wet-mass and 4.5 ± 0. 7 cm length) wild-type of both sexes were procured from a local aquarium. Before the exposure, the fishes have been acclimated in aerated tanks for two weeks. Twice a day zebrafish were fed with commercial fish food and kept at ambient temperaturei(28 ± 1 ͦ C) with a photoperiod consisting of 12 h: 12 h light-dark cycle, pH 7.0 ± 0.25, DO 8.5 ± 1.0 and conductivity 700–900 μS. All the experimental protocols were conducted as per the Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA) guidelines, Government of India.
Fish Exposure and Sample Collection
The experiment was carried out by using the technical grade of monocrotophos (36 % Soluble Liquid) manufactured by Insecticides (India) Ltd., having 36% W/W of MCP as an active ingredient (a.i), since this grade is commonly used in the agricultural field practices. The desired concentrations of the test solutions were prepared using 1 mg/mL stock solution. Pilot studies were conducted to determine the LC50 of MCP on adult zebrafish in the laboratory as per the OECD test guidelines.32 Based on the pilot study, the nominal concentrations (10, 20, 40, 50, and 60 mg a.i L-1) were chosen to determine the median lethal concentration (LC50). The mortality percent of the fish was recorded for every 24 h, and dead fishes were removed in each concentration of the pesticide. Theidataiwereiuseditoiestimate median lethal concentrations (LC50) using probit analysis 33 for 24, 48, 72, and 96h.
For assessment of behavior activity, oxidative stress, and apoptosis in the zebrafish brain, Ten fishes were reared in 10 L glass aquarium, and fishes were exposed to three nominal concentrations of MCP (9, 18, and 27 mg a.i L-1) in dechlorinated water .These concentrations were selected because, due to improper handling of pesticides in India agricultural workers were exposed to high concentrations of pesticides. Concentrations of test compound were maintained semi-statically, and for each treatment triplicate exposures were performed, and water without toxicant was used as control.
Locomotor Activity Assay
The experiment was conducted as described previously 34. Briefly, the tests were performed in a circular plastic container with a diameter of 28 cm and filled with water to a depth of 7 cm. For each analysis, 5 fishes were transferred to the experimental arena and acclimated for 30 mins. The video was recorded for 5 min using a Logitech C270 HD Webcam, which is mounted above the water surface. Immediately after behavior recording, the fish were anesthetized on ice, and the brain from each treatment group was excised and snap-frozen, resulting in five pooled samples for mRNA transcription analysis and ten pooled samples for the Antioxidant Enzyme Assays. These samples were stored at -80 ͦ C until they were analyzed.
Image Acquisition and Video Analysis
The video was captured using the open-source program VirtualDub 1.9.10 in uncompressed AVI format at 30 frames per second and imported to the open-source program Id Tracker to measure the fish trajectories and give an output that results in a readable form.35 Data were subsequently imported to Microsoft Excel, and per frame measurements for each fish was obtained after converting pixel into the absolute distance, and locomotor behavior (distance traveled in mm and swimming speed in mm/sec) was determined for each of the individual targets.36
Acetylcholinesterase Activity
The brain of zebrafishes was homogenized in 0.1M phosphate buffer (pHi7.4)and were centrifuged at 5000g for 10 min, and then the supernatant was used for estimation of AchE, antioxidant enzymes, and lipid peroxidation. Reagents and chemicals used in the assays were of analytical grade (Himedia, India), and the sample preparations were carried out at 4°C. The total protein concentration in the sample was estimated by the Bradford method.37
The AchE activity was measured as described by Ellman et al .38 Briefly, the homogenate of the brain from different groups was added to the mixture containing 0.34 mM DTNB, 1mM acetylthiocholine and 0.05 M Tris–HCl buffer (pH 8.0) made upto a final volume of 3.0ml. The thiocholine–DTNB complex formed at room temperature (25°C) was measured spectrophotometrically at 412 nm. The activity of the AchE was expressed as mmole/min/mg protein.
Antioxidant Enzyme Assays
Superoxide dismutase: The activity of superoxide dismutase (SOD) was measured in terms of percentage inhibition of epinephrine auto-oxidation, as described previously.39 In brief, the homogenate of the brain from different groups was added to 0.1 M carbonate buffer (pH 10). To this equal volume of 1.3 mM, epinephrine in carbonate buffer and 0.6 mM EDTA were added. Using Multiskan Microplate Spectrophotometer (ThermoFisher, USA, USA) the absorbance was measured at 480 nm and was expressed as Unit per mg of protein. One unit was defined as the amount of protein required to inhibit oxidation reaction by 50%.
Catalase Activity
The total Catalase (CAT) activity was measured as described previously.40 In brief, the homogenate was diluted with 60 mM sodium phosphate buffer (pH 7.4) and then 65 µM hydrogen peroxide solution is added and incubated for 4 minutes. By adding 32.4 mM ammonium molybdate, the enzymatic reaction was stopped and the absorbance of the yellow complex was measured at 405 nm using Multiskan Microplate Spectrophotometer (ThermoFisher, USA). The activity of the catalase was expressed in µmole per mg of protein.
Glutathione S-Transferase (GST) Activity
The glutathione-S-transferase (GST) activity was determined as described previously.41 In brief, the sample was diluted with 0.5 M phosphate buffer (pH 6.5), and then 25 mM 1-chloro 2, 4-dinitrobenzene (CDNB) in 95% ethanol is added. The absorbance was measured immediately at 340 nm after adding 20 mM reduced glutathione to the reaction mixture at 30-second intervals for 1.5 min using Multiskan Microplate Spectrophotometer (ThermoFisher, USA). was expressed as Unit per mg of protein. One unit was defined as the amount of protein required to produce one μmole of GS-DNB conjugate/min.
Lipid Peroxidation (LPO)
The lipid peroxidation was measured initerms of the formation of adducts with thiobarbituric acid 42. The protein in the samples were precipitated by incubated for 15 min with ice-cold 10% trichloroacetic acid. To the supernatants an equal volume of 0.67% TBA is added and incubated for 10 mins in the water bath at 100⁰ C. The samples were cooled, and the absorbance was measured at 532 nm using Multiskan Microplate Spectrophotometer (ThermoFisher, USA). The results were expressed in terms of MDA equivalents in nmol per mg of protein.
Gene Expression Analysis
Total RNA from the zebrafish brain was extracted and converted into cDNA using TriSoln reagent (Merk-Genei, India) and M-MLV reverse transcriptase kit (NEB, BioLab, England) respectively. A 1µL of the RT product was used directly for real-time polymerase chain reaction (PCR). Quantitative real-time PCR amplifications were performed on a CFX96 Touch Real-Time PCR Detection System (Biorad, CA, USA) using the KAPA SYBR FAST qPCR Kits (Kapa, USA). The sample was analyzed according to the protocol mentioned previously (Jin et al., 2008, 2010b).
The mRNA levels of the apoptotic pathway genes, including B-cell lymphoma/leukemia-2 gene (Bcl-2 ), Bcl-2 -associated X protein (Bax), caspase-3 (Cas3) and caspase-9 (Cas9) were analyzed to evaluate the effect of MCP on the expression of the apoptosis-related gene. The primers for the genes mentioned were indicated in previous reports.43 The housekeeping gene beta-actin was used as an internal control. Quantification of the transcripts was performed using the 2 -ΔΔCt method.
Statistical Analysis
The Data normality was checked using the Kolmogorov-Smirnov test, and the data were analysed using one way ANOVA followed by Dunnet’s multiple comparisons test to compare means from experimental groups against a control group mean. The results are expressed as a mean ± standard deviation of the mean (SD). SPSS 13.0, is used for all statistical analysis and the significance level was set to p < 0.05. All statistical analyses were conducted using SPSS 13.0, and the level of significance was set at p < 0.05.
Results
Acute Toxicity Test
All group survival rates were combined to determine LC50 values and related confidence intervals of 95 percent of MCP by probit analysis (Table 1 and Fig 1). In zebrafish, the acute toxicity parameters for MCP were 65.69 mg / L (LC50-24 h), 50.13 mg / L (LC50-48 h), 43.46 mg / L (LC50-72 h), and 36.55 mg / L (LC50-96 h).
Table 1: LC50 Values for monocrotophos in adult zebrafish.
| Time point (h) | LC50 (mg/L) | Confidence interval (mg/L) |
| 24 | 65.69 | 53.23 – 93.86 |
| 48 | 50.13 | 37.38 – 68.88 |
| 62 | 43.46 | 32.71 – 55. 94 |
| 96 | 36.55 | 27.70 – 45.37 |
 |
Figure 1: Dose-response curves of monocrotophos. |
Locomotor Activity Assay
As shown in Fig 2, the locomotor and behavior activity of the fish exposed to the MCP. The distance travel (Fig 2a) and swimming speed (Fig 2b) were significantly decreased when the fishes are exposed to sublethal concentration of MCP. The time spent in rest (Fig 2c) is significantly increased as the concentration increase. The effect of MCP on the meandering (Fig 2d) shows it was significantly decreased at all concentrations when compared with the control group and observed a maximum decrease in meandering at the higher concentration of 27mg/L. At low concentration 9mg/L, there was an increase in the average inter-fish distance, but there was no significant at concentration 18mg/L and 27mg/L; there was a significant decrease in the average inter-fish distance (Fig 2e). Time spent in the Thigmotaxis zone (Fig 2f) shows that there was a significant increase in time spent in the thigmotaxis zone (260.8±21 s at 9 mg/L, 265.2±18s at 18 mg/L, , 278.0±19 s at 27 mg/L) as the concentration increase than the control (237.5± 21s).
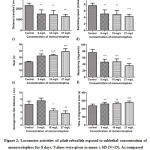 |
Figure 2: Locomotor activities of adult zebrafish exposed to sublethal concentration of monocrotophos for 8 days. |
Acetylcholinesterase Activity
Fig 3. Represent the results of the AchE. When compared to control, there is a significant decrease in the activity of AchE in zebrafish at 18mg/L and 27 mg/L concentration (0.40 ± 0.07 mmole/min/mg and 0.38±0.06 mmole/min/mg respectively), whereas there is no significant change observed at 9mg/L concentration . It is observed that the activity of the AchE is significantly decreased as the concentration of the MCP increase. The maximum activity was observed in the 27 mg/L MCP exposed zebrafish.
 |
Figure 3: Activities of acetylcholinesterase in the brain of adult zebrafish exposed to sublethal concentration of monocrotophos for 8 days. |
Antioxidant Enzyme Assays
The results of the antioxidant enzymes are presented in Fig 4. Fig. 4a illustrated the effects of MCP on the CAT activity. In comparison with the control and 9 mg/L, CAT activity was significantly higher in the brain of 18 mg/L and 27 mg/L MCP exposed zebrafish. The maximum CAT activity in the fish brain was observed in 27 mg/L MCP than that of the controls. On exposure to 9 mg/L MCP, there is no significant change in CAT activity than the control. Fig. 3b shows the SOD activity of the zebrafish brain exposed to MCP. At the concentration of 9 mg/L (1.32±0.014 U/mg Protein), there is no significant change in the activity, and then it starts to increase significantly with the concentration of pesticide (1.47±0.012 U/mg Protein at 18 mg/L, 1.49+0.022 U/mg Protein at 27 mg/L) than the control ( 1.31±0.012 U/mg Protein). The maximum SOD activity in the fish brain was observed at 27 mg/L of MCP that of the controls. Fig. 4c shows GST activity in the zebrafish brain exposed to MCP. At 9 mg/L, there was a significant increase in GST activity, but as the concentration of pesticide increases, there is a significant decrease in GST activity. The activity of GST decreased in tested groups exposed to 18 mg/L and 27 mg/L of MCP compared to the control group. The lowest and a significantly different value of GST activity was found in the concentration of 27 mg/L.
Similarly, Fig. 4d shows the effect of MCP on Lipid Peroxidation. The MDA concentration significantly increases with an increase in the concentration of the MCP. It concentrations that the 27 mg/L concentration of MCP showed a significant increase in MDA formation.
 |
Figure 4: Activities of Catalase (a), superoxide dismutase (b), glutathione S-transferase (c), and levels of a thiobarbituric acid reactive substance (d) in adult zebrafish brain |
Effect of MCP on Transcription of Gene Related to Apoptosis
Exposure to different concentrations of monocrotophos for 96 h, the transcriptional levels of genes involved in the apoptotic pathway in the adult zebrafish brain were altered (Fig.5). The expression of the gene encoding the anti-apoptotic protein Bcl-2 was not affected in a lower concentration of monocrotophos, but when the concentration increases, the expression of the gene decreases. Bcl-2 gene expression was a significant decrease when compared to control after exposure to 27 mg/L MCP (Fig. 5a). Moreover, the expression of the Bax, there was a significant increase in the gene expressions with respect to the concentration of the monocrotophos (Fig. 5b).
In addition to Bcl-2, and Bax, the genes in the caspase pathway,the mRNA level of genes such as Cas3 and Cas9, were also determined by RT-qPCR (Fig 5c&d). The results showed that there was no significant change in the level of expression for both cas3 and cas9 after the exposure with the low concentration of monocrotophos (9 mg/L), while there was a significant increase in the expression at higher exposure concentration group (27 mg/L) with increase in 5.43 and 3.04 fold of cas3 and cas9 respectively when compared to the control.
 |
Figure 5: mRNA expression level of Bax, Bcl2, caspase 3, and caspase 9 in adult zebrafish brain exposed to sublethal concentration of monocrotophos for 8 days. |
Discussion
MCP poisoning can result from occupational, accidental, or intentional exposure. The current treatment for OP poisoning is based on the mechanism, and its depends on the severity of the poisoning. First-line therapy involves the administration of atropine to counteract muscarinic over-stimulation, and pralidoxime (2-PAM) to reactivate acetylcholinesterase. In some supportive and intensive care therapy, benzodiazepines to control convulsions may be administrated.1 In some follow-up studies in the individuals exposed to high levels of organophosphorus compounds shown specific neurobehavioral changes12, but the available data is not much. The limitation of our current studies is that there may be some species sensitivity to pesticides may vary. However, the studies show that zebrafish behave like humans to the xenobiotics.44
Our results showed that zebrafish exposure to MCP resulted in a concentration and time-dependentidecreaseiinisurvivaliandishowedisymptomsiof lethargy, loss of coordination, and in consistent swimming behaviour. Moreover, also, the mucous secretion on the body increases as the concentration of pesticide increases. Similar results were reported by Qayoom et al., (2016a), who evaluated the effects of organophosphate pesticide dimethoate on juvenile Cyprinus carpio. The results also showed that different concentrations (9 – 27 mg/L) of MCP exposure significantly changes the locomotor, behavior, oxidative stress endpoints and also alter the transcriptional of genes related to the apoptosis pathway.
Behavior endpoints are becoming the most effective way to study both chronic and acute toxicity studies because of their high sensitivity and their usefulness in ecological risk assessment. It is also reported that zebrafish shows similar behavior responses with environmental toxicants like humans.46 MCP affected the erratic movements of zebrafish, and also significantly decrease the meandering at all concentrations when compared with the control group (Figure 1d). Previous studies showed that inhibition of the AchE enzyme by MCP was responsible for the reduction in a locomotor activity such as distance traveled, swimming speed, and meandering of fish.47 A dose-dependent reduction in the activity of AchE was observed relative to the activity of controls. Similarly, when zebrafish were exposed to organophosphate pesticide malathion, it significantly inhibits the acetylcholinesterase.48 Our results indicate that fish’s altered locomotive activity could be due to the accumulation of acetylcholine (Ach) that disrupted nervous and muscular junction’s synchronization. Previous studies have also proposed the use of brain AchE activity as an excellent diagnostic tool for OP poisoning.49
Thigmotaxis or wall-hugging behavior is one of the proxy tests for the measurement of anxiety in fish.50 Thigmotaxis is observed in fish and rodents and is linked to anxiety-related behaviors.51–53 Reported studies showed that fish under stress would remain closer to the edges of the experiment tank 52. It is also observed in our study that the time spent in thigmotaxis zone and resting time significantly increased as the concentration increase. Similar observations been documented that several compounds such as nicotine, buspirone, chlordiazepoxide and ethanol have anxiogenic and anxiolytic effects in zebrafish.54–56 At Lower concentration the Inter- fish distance is increased. It is known that teleost fish after emergence, the individual fishes will stay away from conspecifics to avoid competition while when searching for food in the new enviroment.57 At higher concentrations, there was a significant decrease in the average inter-fish distance. This shows that MCP induces anxiety-related behaviors in adult zebrafish. It is also previously reported that pesticides like organophosphates inhibit AchE, such as malathion and chlorpyrifos have opposite effects on neurobehavior that are not correlated to alteration in activity of AchE.58
Oxidative stress is characterized as aniimbalance between reactive oxygen speciesproduction and the body’s ability to counteract or detoxify it with an antioxidant defence system. Many pesticides, including organophosphate, induce the production of additional reactive oxygen species (ROS), which impairs the antioxidant defense system that lead to damage tissues and initiate neuronal death.59 In this study, we examined the activity of exogenous antioxidant proteins to determine if MCP induces oxidative stress in the brain of zebrafish. The brain of adult zebrafish exposed to MCP shows alteration in the antioxidant enzyme such as CAT, SOD, GST activities, as well as in the extent of lipid peroxidation. We found that MCP increased the activity ofiCAT andiSOD at different concentrations (Figures 3a and 3b). Similar to another organism, fish can combat the elevated levels of ROS with the help of the ROS-scavenging enzymes such as CAT and SOD.60 Induction of these two enzymes provides the first line of defense against the ROS generated by xenobiotics.61,62 SOD converts the radical superoxide anion to hydrogen, while CAT converts the peroxide to water and molecular oxygen.63 GST catalyzes the conjugation reaction between reduced glutathione and xenobiotic metabolite, thereby it accelerates their excretion of the xenobiotic and also GST in conjugation with reduced glutathione (GSH) act as a defense against ROS and protect against lipid peroxidation.64 In our study, we found that the activity of GST in the brain is increased in lower concentration, and as the concentration increases, the activity of the GST decreases.
At lower concentration, the activity of the GST is increased to eliminate the ROS induced by the MCP, but as the concentration increases the activity decrease significantly, this may be due to the overproduction of ROS which leads to enzyme inactivation or GSH depletion, inducing GST to lose its activity. Malondialdehyde (MDA) is a final oxidation product of, and increased, peroxidised polyunsaturated fatty acids; it serves as an important indicator of the level of lipid peroxidation. The results of the lipid peroxidation assay showed that the level of MDA in the brain of zebrafish exposed to sublethal concentration of MCP increased. Similar observations were reported when Gambusia affinis were exposed to MCP.65 Vadhva and Hasan66 found that exposure of dichlorvos shows an increase in the MDA levels of Heteropneustes fossilis brain on the seventh day, which concurs with the present results The reason for the increase in MDA level may be due to ROS induction, which increases the oxidation of polyunsaturated fatty acids and contributes to lipid peroxidation. The brain has a large membrane surface due to axon extensions and neuronal dendrites that are rich in PUFAs and high intakes of oxygen, making them particularly vulnerable to ROS attack.67 Our results indicate that MCP exposure causes oxidative stress in fishes and results in LPO and may lead to the destruction of membrane lipids.
The generation of additional ROS triggered by oxidative stress is related to apoptotic cell death via the mitochondrial apoptosis pathway.68 Bcl-2, which plays an important role in promoting cell survival and inhibiting pro-apoptotic protein actions, and regulatesiitheiantioxidant pathway atiselected ROS generation sites to prevent apoptosisiiand cellular damage.69 Oxidative stress has been reported to inhibit expression of Bcl-2 mRNA in zebrafish.70,71 In this study, the transcript of Bcl-2 decreased after exposure to MCP, as well as the mRNA level of Bax is increased. The Bcl-2 and Bax work oppositely in term of cell death, Bax homodimer accelerates apoptosis and the Bax heterodimer, and Bcl-2 polypeptides inhibit or prolong cell death.72 Consequently, an increase in Bax homodimer resulting from Bax up-regulation and Bcl-2 down-regulation may induce the release of cytochrome C from the mitochondria, which triggers the activation of Cas9 and other downstream caspases. It is also observed that upregulation of Cas3 and Cas9 after the exposure of zebrafish with MCP. Previously it been reported that environmental contaminants and pesticides also showed caspase activation and their function in apoptosis, which supports our results.43,73–75
In conclusion, our study shows that exposure to sublethal concentration of MCP alters the locomotor behavior and induce anxiety-like behavior accompanied by oxidative stress and alteration in the transcription of genes related to apoptosis. So, future studies aimed at correlating oxidative stress with changes in neurotransmitter signalling pathways that will bring new opportunities to understand the role of MCP in vertebrate psychiatric disorders.
Acknowledgments
The authors are thankful to the Faculty of Engineering and Technology, SRM Institute of Science and Technology, Kattankulathur, Tamilnadu.
Ethical Statement
The authors declare no ethical issues.
Conflict of Interests
All authors declare that there is no conflict of interest associated with this article.
Funding Source
There is no funding source.
References
- Katz AKD, Editor C, Tarabar A. Organophosphate Toxicity. 2016:10-12.
- Pamanji R, Bethu MS, Yashwanth B. Developmental toxic effects of monocrotophos , an organophosphorous pesticide , on zebrafish ( Danio rerio ) embryos. Environ Sci Pollut Res. 2015;22(10):7744-7753. doi:10.1007/s11356-015-4120-8
CrossRef - World Health Organization. Health Implications from Monocrotophos Use : A Review of the Evidence in India.; 2009.
- Casida JE, Quistad GB. Organophosphate Toxicology: Safety Aspects of Nonacetylcholinesterase Secondary Targets. Chem Res Toxicol. 2004;17(8):983-996.
CrossRef - Jindal R, Kaur M. Acetylcholinesterase inhibition and assessment of its recovery response in some organs of Ctenopharyngodon idellus induced by chlorpyrifos. Int J Sci Environ Technol. 2014;3(2):473-480.
- Ansari S, Ansari BA. Embryo and Fingerling Embryo and Fingerling Toxicity of Dimethoate and Effect on Fecundity, Viability, Hatchability and Survival of Zebrafish, Danio rerio (Cyprinidae). World J Fish Mar Sci. 2011;3(2):167-173.
- Maniyar RA, Ahmed RN, David M. Monocrotophos: Toxicity Evaluation and Respiratory Responses Of Cyprinus Carpio (Linnaeus). Recent Res Sci Technol. 2011;3(1):51-54.
- Goverment of India. Major Uses of Pesticides. Directorate of Plant Protection, Quarantine & Storage. http://ppqs.gov.in/divisions/cib-rc/major-uses-of-pesticides. Published 2019.
- Eddleston M. Self poisoning with pesticides. BMJ. 2004;328(7430):42-44. doi:10.1136/bmj.328.7430.42
CrossRef - Rao CHS, Venkateswarlu V, Surender T, Eddleston M, Buckley NA. Pesticide poisoning in south India : opportunities for prevention and improved medical management. 2005;10(6):581-588.
CrossRef - Nations U, Programme E. Guidelines to Classification 2000-2002. 2001.
- Ghimire SR, Parajuli S. Chronic organophosphate-induced neuropsychiatric disorder: A case report. Neuropsychiatr Dis Treat. 2016;12:275-277. doi:10.2147/NDT.S91673
CrossRef - Singh S, Sharma N. Neurological Syndromes Following Organophosphate Poisoning. Neurol India. 2000;48:308-313.
- Parrón T, Hernández AF, Villanueva E. Increased risk of suicide with exposure to pesticides in an intensive agricultural area. A 12-year retrospective study. Forensic Sci Int. 1996;79(1):53-63. doi:10.1016/0379-0738(96)01895-6
CrossRef - National Crime Records Bureau. Accidental Deaths and Suicides In India. Natl Crime Rec Bur (Ministry Home Aff Gov India Natl. 2018;53(9):1-312. doi:10.1017/CBO9781107415324.004
CrossRef - Patel V, Ramasundarahettige C, Vijayakumar L, et al. Suicide mortality in India: A nationally representative survey. Lancet. 2012. doi:10.1016/S0140-6736(12)60606-0
CrossRef - London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am J Ind Med. 2005;47(4):308-321. doi:10.1002/ajim.20147
CrossRef - Beseler C, Stallones L, Hoppin JA, et al. Depression and pesticide exposures in female spouses of licensed pesticide applicators in the Agricultural Health Study cohort. J Occup Environ Med. 2006;48(10):1005-1013. doi:10.1097/01.jom.0000235938.70212.dd
CrossRef - Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev. 2009;2(2):63-67. doi:10.4161/oxim.2.2.7944
CrossRef - Hovatta I, Tennant RS, Helton R, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438(7068):662-666. doi:10.1038/nature04250
CrossRef - Possamai FP, Fortunato JJ, Feier G, et al. Oxidative stress after acute and sub-chronic malathion intoxication in Wistar rats. Environ Toxicol Pharmacol. 2007;23(2):198-204. doi:http://dx.doi.org/10.1016/j.etap.2006.09.003
CrossRef - Zhang X, Xie P, Li D, Tang R, Lei H, Zhao Y. Time-dependent oxidative stress responses of crucian carp (carassius auratus) to intraperitoneal injection of extracted microcystins. Bull Environ Contam Toxicol. 2009;82(5):574-578. doi:10.1007/s00128-009-9671-2
CrossRef - Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf. 2006;64:178-189. doi:10.1016/j.ecoenv.2005.03.013
CrossRef - Zhan CD, Sindhu RK, Pang J, Ehdaie A, Vaziri ND. Superoxide dismutase, catalase and glutathione peroxidase in the spontaneously hypertensive rat kidney: effect of antioxidant-rich diet. J Hypertens. 2004;22(10):2025-2033. doi:00004872-200410000-00027 [pii] CrossRef
- Uttara B, Singh A V, Zamboni P, Mahajan RT. Oxidative Stress and Neurodegenerative Diseases : A Review of Upstream and Downstream Antioxidant Therapeutic Options. 2009:65-74.
CrossRef - Xu S, Zhang R, Niu J, Cui D, Xie B, Zhang B. Oxidative Stress Mediated-Alterations of the MicroRNA Expression Profile in Mouse Hippocampal Neurons. Int J Mol Sci. 2012;13:16945-16960. doi:10.3390/ijms131216945
CrossRef - Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: Nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198(2):132-151. doi:10.1016/j.taap.2003.06.001
CrossRef - Nikonov a a, Caprio J. Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J Neurophysiol. 2001;86(4):1869-1876.
CrossRef - Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: Effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25(1):51-57. doi:10.1016/S0892-0362(02)00322-7
CrossRef - Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol Teratol. 2004;26(6 SPEC. ISS.):719-723. doi:10.1016/j.ntt.2004.06.013
CrossRef - Crosby EB, Bailey JM, Oliveri AN, Levin ED. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebra fi sh. Neurotoxicol Teratol. 2015;49:81-90.
CrossRef - OECD. OECD guideline for testing of chemicals. 1992;(July):1-9.
CrossRef - Finney DJ. Probit Analysis: A Statistical Treatment of the Sigmoid.; 1971.
- Barry MJ. Application of a novel open-source program for measuring the effects of toxicants on the swimming behavior of large groups of unmarked fish. Chemosphere. 2012;86(9):938-944. doi:10.1016/j.chemosphere.2011.11.011
CrossRef - Pérez-Escudero A, Vicente-Page J, Hinz RC, Arganda S, De Polavieja GG. IdTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat Methods. 2014;11(7):743-748. doi:10.1038/nmeth.2994
CrossRef - Audira G, Sampurna B, Juniardi S, Liang S-T, Lai Y-H, Hsiao C-D. A Simple Setup to Perform 3D Locomotion Tracking in Zebrafish by Using a Single Camera. Inventions. 2018;3(1):11-20. doi:10.3390/inventions3010011
CrossRef - Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-254. doi:10.1016/0003-2697(76)90527-3
CrossRef - Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88-95. doi:10.1016/0006-2952(61)90145-9
CrossRef - Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 1978;90(1):81-89. doi:10.1016/0003-2697(78)90010-6
CrossRef - Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196(2-3):143-151. doi:10.1016/0009-8981(91)90067-M
CrossRef - Habig WH, Jakoby WB. Assays for differentiation of glutathione S-Transferases. Methods Enzymol. 1981;77:398-405. doi:10.1016/S0076-6879(81)77053-8
CrossRef - Yagi K. Simple Procedure for Specific Assay of Lipid Hydroperoxides in Serum or Plasma. Armstrong D, ed. Free Radic Antioxid Protoc. 1998;108:107-110. doi:10.1385/0-89603-472-0:107
CrossRef - Jiang J, Wu S, Wu C, An X, Cai L, Zhao X. Embryonic exposure to carbendazim induces the transcription of genes related to apoptosis, immunotoxicity and endocrine disruption in zebrafish (Danio rerio). Fish Shellfish Immunol. 2014;41(2):493-500. doi:10.1016/j.fsi.2014.09.037
CrossRef - de Souza Anselmo C, Sardela VF, de Sousa VP, Pereira HMG. Zebrafish (Danio rerio): A valuable tool for predicting the metabolism of xenobiotics in humans? Comp Biochem Physiol Part – C Toxicol Pharmacol. 2018:34-46. doi:10.1016/j.cbpc.2018.06.005
CrossRef - Qayoom I, Shah FA, Mukhtar M, Balkhi MH, Bhat FA, Bhat BA. Dimethoate Induced Behavioural Changes in Juveniles of Cyprinus carpio var. communis under Temperate Conditions of Kashmir, India. Sci World J. 2016. doi:10.1155/2016/4726126
CrossRef - Baggio S, Mussulini BH, de Oliveira DL, Gerlai R, Rico EP. Embryonic alcohol exposure leading to social avoidance and altered anxiety responses in adult zebrafish. Behav Brain Res. 2018;352(8):62-69. doi:10.1016/j.bbr.2017.08.039
CrossRef - Venkateswara Rao J, Begum G, Jakka NM, Srikanth K, Nageswara Rao R. Sublethal effects of profenofos on locomotor behavior and gill architecture of the mosquito fish, Gambusia affinis. Drug Chem Toxicol. 2006;29(3):255-267. doi:10.1080/01480540600651543
CrossRef - Ansari BA, Kumar K. Malathion toxicity: In vivo inhibition of acetylcholinesterase in the fish Brachydanio rerio (Cyprinidae). Toxicol Lett. 1984;20(3):283-287.
CrossRef - Üner N, Oruç EÖ, Sevgiler Y, Şahin N, Durmaz H, Usta D. Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol. 2006;21(3):241-245. doi:10.1016/j.etap.2005.08.007
CrossRef - Sackerman J, Donegan JJ, Cunningham CS, et al. Zebrafish Behavior in Novel Environments: Effects of Acute Exposure to Anxiolytic Compounds and Choice of Danio rerio Line. Int J Comp Psychol. 2010;23(1):43-61.
CrossRef - Seibenhener ML, Wooten MC. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J Vis Exp. 2015;96(e52434). doi:https://doi.org/10.3791/52434
CrossRef - Richendrfer H, Pelkowski SD, Colwill RM, Creton R. On the edge: Pharmacological evidence for anxiety-related behavior in zebrafish larvae. Behav Brain Res. 2012;228(1):99-106. doi:10.1016/j.bbr.2011.11.041
CrossRef - Barry MJ. Effects of fluoxetine on the swimming and behavioural responses of the Arabian killifish. Ecotoxicology. 2013;22:425-432. doi:10.1007/s10646-012-1036-7
CrossRef - Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90(1):54-58. doi:10.1016/j.physbeh.2006.08.026
CrossRef - Mathur P, Berberoglu MA, Guo S. Preference for ethanol in zebrafish following a single exposure. Behav Brain Res. 2011;217(1):128-133. doi:10.1016/j.bbr.2010.10.015
CrossRef - Baggio S, Mussulini BH, de Oliveira DL, Gerlai R, Rico EP. Embryonic alcohol exposure leading to social avoidance and altered anxiety responses in adult zebrafish. Behav Brain Res. 2018;352:62-69. doi:https://doi.org/10.1016/j.bbr.2017.08.039
CrossRef - Pitcher TJ, Parrish JK. Functions of shoaling behaviour in teleosts. In: Behaviour of Teleost Fishes. ; 1993:363 –439. doi:10.1007/978-94-011-1578-0_12
CrossRef - Richendrfer H, Creton R, Biology C. Chlorpyrifos and Malathion have opposite effects on behaviors and brain size that are not correlated to changes in AChE activity. Neurotoxicology. 2015;49:50-58. doi:10.1016/j.neuro.2015.05.002.Chlorpyrifos
CrossRef - Pearson JN, Patel M. The role of oxidative stress in organophosphate and nerve agent toxicity. Ann N Y Acad Sci. 2016;1378(1):17-24. doi:10.1111/nyas.13115
CrossRef - Jin Y, Zhang X, Shu L, et al. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere. 2010;78(7):846-852. doi:10.1016/j.chemosphere.2009.11.044
CrossRef - Pandey S, Ahmad I, Parvez S, Bin-Hafeez B, Haque R, Raisuddin S. Effect of endosulfan on antioxidants of freshwater fish Channa punctatus bloch: 1. Protection against lipid peroxidation in liver by copper preexposure. Arch Environ Contam Toxicol. 2001;41(3):345-352. doi:10.1007/s002440010258
CrossRef - Pandit S, Mundhe A. Assessment of toxicity of monocrotophos in freshwater bivalve, Lamellidens marginalis, using different markers. Toxicol Int. 2014;21(1):52. doi:10.4103/0971-6580.128793
CrossRef - Shao B, Zhu L, Dong M, et al. DNA damage and oxidative stress induced by endosulfan exposure in zebrafish (Danio rerio). Ecotoxicology. 2012;21(5):1533-1540. doi:10.1007/s10646-012-0907-2
CrossRef - Yang Y, Cheng JZ, Singhal SS, et al. Role of glutathione S-transferases in protection against lipid peroxidation: Overexpression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J Biol Chem. 2001;276(22):19220-19230. doi:10.1074/jbc.M100551200
CrossRef - Kavitha P, Venkateswara Rao J. Oxidative stress and locomotor behaviour response as biomarkers for assessing recovery status of mosquito fish, Gambusia affinis after lethal effect of an organophosphate pesticide, monocrotophos. Pestic Biochem Physiol. 2007;87(2):182-188. doi:10.1016/j.pestbp.2006.07.008
CrossRef - Vadhva P, Hasan M. Organophosphate dichlorvos induced dose-related differential alterations in lipid levels and lipid peroxidation in various regions of the fish brain and spinal cord. J Environ Sci Heal Part B. 1986;21(5):413-424. doi:10.1080/03601238609372534
CrossRef - Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi:10.3389/fnagi.2010.00012
CrossRef - Livingstone DR. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull. 2001;42(8):656-666. doi:10.1016/S0025-326X(01)00060-1
CrossRef - Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75(2):241–251. doi:10.1016/0092-8674(93)80066-N
CrossRef - Deng J, Yu L, Liu C, et al. Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat Toxicol. 2009;93(1):29-36. doi:10.1016/j.aquatox.2009.03.001
CrossRef - Sun Y, Li H, Liu Y, Mattson MP, Rao MS, Zhan M. Evolutionarily conserved transcriptional co-expression guiding embryonic stem cell differentiation. PLoS One. 2008;3(10). doi:10.1371/journal.pone.0003406
CrossRef - Zhang Z, Lapolla SM, Annis MG, et al. Bcl-2 homodimerization involves two distinct binding surfaces, a topographic arrangement that provides an effective mechanism for Bcl-2 to capture activated bax. J Biol Chem. 2004;279(42):43920-43928. doi:10.1074/jbc.M406412200
CrossRef - Jin Y, Zheng S, Pu Y, et al. Cypermethrin has the potential to induce hepatic oxidative stress , DNA damage and apoptosis in adult zebrafish (Danio rerio). Chemosphere. 2011;82(3):398-404. doi:10.1016/j.chemosphere.2010.09.072
CrossRef - Saquib Q, Attia SM, Siddiqui MA, et al. Phorate-induced oxidative stress , DNA damage and transcriptional activation of p53 and caspase genes in male Wistar rats. Toxicol Appl Pharmacol. 2012;259(1):54-65. doi:10.1016/j.taap.2011.12.006
CrossRef - Ahmad MI, Zafeer MF, Javed M, Ahmad M. Pendimethalin-induced oxidative stress, DNA damage and activation of anti-inflammatory and apoptotic markers in male rats. Sci Rep. 2018;8(1):17139. doi:10.1038/s41598-018-35484-3
CrossRef







