Kusum Kumari1, Manju Gari1 , Priyanki2* and Pramveer Kumar1
1Department of Pharmacology and Therapeutics, Rajendra Institute of Medical Sciences, Ranchi, Jharkhand, 834009
2MGM Medical College , Jamshedpur, India
Corresponding Author E-mail : priyanki.mishra@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2010
Abstract
Pain is a major burden on limited health care resources for all the nations. In the research model called “pain-induced functional impairment in the rat” (PIFIR model), both nociception and antinociception happen similar to that experienced in arthritic -type pain situations. This experiment can be used to determine efficacy, potency and the duration of antinociceptive effect caused by a drug that is either administered alone or in combination with some other drug. In this study, the drugs that have been used are Ketorolac, paracetamol and Tramadolfor evaluating and comparing their efficacy. Healthy male albino rats have been used for the study. Pain was induced with an intra articular injection of uric acid (32% ) in the right side knee joint of the animal. Electrodes were attached to each of the hind paw between the plantar pads. The animal was then placed on an aluminium cylinder . The cylinder was rotated which forced the rat to walk. The time of contact between each of the hind paw with the rotating cylinder was recorded. When the conducting pad placed under the animal’s paw contacted the cylinder floor, a circuit was closed and the time for which the circuit conducted electricity was recorded. 2.5 hrs. after uric acid injection , analgesic agents under study were administered. This time was recorded as time zero (t0) for measurement of effects of the analgesic drugs. After the Drugs were adminstered at time zero, the rats were made to walk on rotating cylinder for 2 minutes every one hour for next 4 houts and the time of contact of both the paws was measured. The ratio of time of contact of both the legs was expressed in terms of Functional Index (FI) and the time required to reach this response (t) was recorded .The maximum efficacy is seen with Tramadolfollowed by paracetamol closely followed by Ketorolac. Paracetamol has a fast onset while Tramadolis slower in onset.
Keywords
PIFIR; Antinociceptive Effect; Ketorolac; Paracetamol; Tramadol
Download this article as:| Copy the following to cite this article: Kumari K, Gari M, Priyanki P, Kumar P. Evaluation of Antinociceptive Effect of Paracetamol, Ketorolac and Tramadolhydrochloride in Arthritic Rat by Pain Induced Functional Impairment Model. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Kumari K, Gari M, Priyanki P, Kumar P. Evaluation of Antinociceptive Effect of Paracetamol, Ketorolac and Tramadolhydrochloride in Arthritic Rat by Pain Induced Functional Impairment Model. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/2QT22CM |
Introduction
Pain is a major social, economic and clinical problem. It is witnessed across all ages. The estimates of the monthly prevalence of pain ranges from 1.0% to around 60.0%.1 Cancer, Arthritis (osteo and rheumatoid), Surgery and injuries, and spinal problems are the four top most causes of pain. The etiology of pain is a complex and transdisciplinary affair.2 Pain can be charecterised as a disease in itself, with major cognitive and psychological correlates.3 The prevalence of chronic pain in adults ranges from 2 to around 40% and a median of 15%,with a higher percentage of women being affected with it.4 It poses a major economic concern for both patients and health service providers across all societies. Differences in orientation and study methodology in different countries make the comparison difficult, but it is evident that pain poses a major burden on the health care resources of all the nations.5
In the animal model research methodolgy named “pain-induced functional impairment in the rat” (PIFIR), nociception and antinociception are dealt with in a similar way to that in arthritic-type pain . This experimental technique is used to ascertain the efficacy, potency and duration of the antinociceptive effect of the drug (or combination of drugs) under study. The PIFIR model is an good method to analyze the pharmacokinetic and pharmacodynamic effects in preclinical reserach,6 It has been used for different works, time to time, mentioned below.
PIFIR model helped ascertain that a combination of caffeine and ketoprofen had a more preferable mechanism of action rather than the use of a single drug alone. Similar studies using morphine, metamizole and their synergy have been performed in uric acid induced arthritic rats to study their antinociceptive efficacy with different levels of inflammatory pain.7 The profile of activity of drugs like acetyl salicylic acid, morphine and rofecoxib have also been mapped using this model.8.9 The analgesic activity of natural plant products like Rosemarinus officinalis, Tilia americana and Auguste mexicana have been studied extensively by using this preclinical model.1 0,11,12
In this study, the drugs that have been used are Ketorolac, paracetamol and Tramadolfor evaluating and comparing their efficacy.
In order to exhasutively evaluate the potential efficacy of an analgesic, one must not only analyze its efficacy at a fixed time, but also quantitively evaluated the total duration of the analgesic activity. This could be called the kinetics of the drug concerned.2 4 Hence, this model was chosen for the aforementioned study. This model has not yet been used in India.
So, in the present study, we attempt to evaluate and compare the analgesic efficacy of paracetamol, Ketorolac and Tramadolusing a model of arthritic pain in rats to assess the anti nociceptive efficacy of paracetamol, Ketorolac and Tramadoland to compare the functional index at different durations of the study due to the above drugs.
Materials and Method
This study has been conducted at Department of Pharmacology and Therapeutics of Rajendra Institute of Medical Sciences, Ranchi. The study was duly approved to be undertaken from Institutional Animal Ethics Committee(IAEC), RIMS.
Healthy adult male albino rats(n=24),of an average weight of 150-250 gms were chosen for the experiment. We made four groups each group pertaining to a single drug under study . Six rats were assigned to each group . Rats of each group was placed in separate cages. The room temperature was maintained at 25+ – 2degree centigrade. Light and dark cycle of 12:12 hour was also maintained. Animals were fed on standard laboratory diet which included bread, crushed maize and soyabeans with water ad libitum. Regular cleaning of animal house and disposal of excreta was taken care of. Before starting the experiment, they were left for ten days of acclimatization in the new environment.
The Drugs and Doses Were as Follows
Uric acid-5 ml(8mg/dl) from Uric Acid Kit, Coral Clinical Systems, Phase III-b, Verna Industrial Estate, Salcete, Goa, India. Paracetamol -Inj. Neomol,2 ml(150mg/ml) from Neon Laboratories Limited, Dhamji Shamji – Industrial Complex, Mahakali Caves Road, Andheri(E), Mumbai, Maharashtra, India. Ketorolac tromethamine-Inj. Ketorol,1ml(30 mg/ml),from Dr. Reddy’s, Hyderabad, Telangana, India. Tramadol Hydrochloride –Inj.Tramazac,2 ml(100 mg/2 ml),from Zydus Cadilla, Ahmedabad, Gujarat, India. All the drugs were procured from the local market.
Animals were divided into four groups, C(Control), P(Paracetamol), K(Ketorolac) and T(Tramadol). Each group contained six animals. They were kept in six cages, each cage containing one animal from each group, to avoid overcrowding in cages. The cages were labelled from A to F. The tails of the rats of different groups were coloured with permanent markers of different colours for their recognition.
Group P rats were given 32% uric acid inj. 0.05ml intraarticular, then inj. paracetamol 0.8 ml was given after 2.5 hours. Group K rats were given 32% uric acid inj. 0.05 ml intraarticular, then inj. Ketorolac 0.01 ml was given after 2.5 hours. Group T rats were given 32% uric acid inj. 0.05 ml intraarticular, then inj. Tramadol0.2 ml was given after 2.5 hours. Group C rats were given 32% uric acid inj. 0.05 ml intraarticular, then inj. of 0.05 ml of normal saline was given after 2.5 hours. The experiment was performed and responses were observed hourly for 4 hours.
Uric acid was given intrarticular with the help of 26 G tuberculin syringe.
All drugs were given intraperitoneally with the help of 26 G tuberculin syringe.
All the rats (150 -250 gms) that were healthy and were active enough, to move and play were taken. Diseased and inactive rat were excluded from the study.
After properly holding the animal, pain was induced with an intra articular injection of 32% uric acid in the right knee joint of the animal. An conducting metal pad was attached to each of the hind paws. The animal was then placed on an aluminium cylinder, 15 cm wide and 30cm in diameter. The cylinder was rotated at 5 rpm, forcing the rat to walk. A training period of 2 mins. before the injection was found appropriate as the rat was able to perform the act well after that. The time of contact between each of the rat’s hind paw and the conducting aluminium cylinder was measured. When the conducting pad placed under the animal’s paw came in contact with the aluminium cylinder, the circuit would complete and a very low voltage current would transmit to the measuring device . The time of that current flow was recorded. The recordings were done while the cylinder rotated for 2 mins at the end of each hour. The analgesis drug under study were administered 2.5 hrs. after the initial injection of the uric acid. The time if injection of the aalgesic aganet was recorded as time zero(t0) for subsequent measurements of it’s analgesic effect. After time zero, the time of contact was measured for 2 mins. After lapse of one hour each for the next 4 hours. All experiments and recordings were done between 10 A.M. and 5 p.m.
The rotary walking surface on which the animals walked was made of hollow aluminium cylinder 15 cm in width and 30 cm in diameter. The aluminium cylinder was covered with a mesh to provide a non-slippery surface to the animals, t. Using this arrangement, one animal was studied at a time. An electrical synchronous AC 220/240 V AC motor was used to roate the cylinder at a fixed RPM of 5.
The time of contact of each conducting pad in each hind paw with the rotaitng cylinder was recorded. Each conducting pad consisted of a soldering wire that was coiled between the fingers of the hind paw to prevent it from coming out. The other end of the electrode was connected to an Arduino. This code is loaded into the Arduino which allows Arduino to record contact timing of left and right leg with the cylinder for two minutes at a time. Arduino emits the data on serial port at a baud rate of 9600.The data is read on a Window PC over serial port using a program called Putty. This data is saved as CSV file for further analysis.
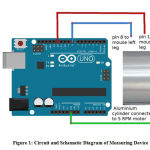 |
Figure 1: Circuit and Schematic Diagram of Measuring Device |
Data Analysis
The main output of experiment was stated as functionality index (FI). Functionality Index can be defined as the ratio of the total time of contact of the cylinder and the injected leg to the total time of contact of cylinder with the left leg (control), expressed as percentage.
The maximum observed effect was stated in terms of FI and the time required to reach this response (t).Thus, with the help of these parameters, we analyze and reflect upon the effectiveness and the rate of onset of the different drugs’ action by the intraperitoneal route that has been used here . Furthermore, by following the total duration of the effect, the cumulative analgesic effect during the entire observation period was measured by calculating the area under the curve(AUC).
AUC represents the total analgesic effect during the study duration, including both the maximum response and the total time of activity of the analgesic. So, this parameter was also used to draw the dose response curve. The AUC was calculated by the trapezoidal rule. The maximum AUC that can be attained is 375 units i.e. 100% by this method. So , we also compared the AUCs of different drugs.
The Emax and Tmax were directly obtained from analyzing the FI as against the time curve by doses that produced the maximum AUC of that drug.
Observations
FI was approximately 100% at the start of the experiments, since the cumulative time of contact (msec) of both hind legs with the rotary device while ambulation was nearly same. Intra-articular injection of uric acid suspension resulted in a dysfunction of the right leg that was apparent as a gradual reduction of FI. The maximum decrease was observed around 2.5 hours in all the animals (reaching a FI of almost zero). Then, the drugs were given, and readings of time of contact of each paw of the animal was obtained at 1, 2, 3 and 4 hours respectively. This data was used to calculate the functional index of each rat at each hour. Then, the median for each FI and the AUC for each drug and control group was calculated. Data analysis was done by Wilcoxon signed rank test for comparison of FI at different time interval for each group while Mann Whitney U test was used for inter-group comparisons. This was followed by Bon Ferroni – post hoc comparison
Table 1: P value of Functional Index in t2,t3,t4 in comparison to t1;t3 and t4 in comparison to t2 and t4 in comparison to t3 in paracetamol. (where n in tn is variable 2 to 4 according to the comparisons made)
| P value(t1,tn) | 0 | .0319 | 0 | .0002 | 0 | .0001 |
| (t1,tn) | 0 | .027708 | 0 | .027708 | 0 | .027708 |
| (t2,tn) | 0 | .027708 | 0 | .027708 | ||
| (t3,tn) | 0 | .027708 |
Table 2: P value of Functional Index in t2,t3,t4 in comparison to t1;t3 and t4 in comparison to t2 and t4 in comparison to t3 in Ketorolac (where n in tn is variable 2 to 4 according to the comparisons made)
| P value(t1,tn) | 0 | .0271 | 0 | .0001 | 0 | .0001 |
| (t1,tn) | 0 | .027708 | 0 | .027708 | 0 | .027708 |
| (t2,tn) | 0 | .027708 | 0 | .027708 | ||
| (t3,tn) | 0 | .027708 |
Table 3: P value of Functional Index in t2,t3,t4 in comparison to t1;t3 and t4 in comparison to t2 and t4 in comparison to t3 in Tramadol
| P value(t1,tn) | 0.0001 | 0.0001 | 0.0001 |
| (t1,tn) | 0.027708 | 0.027708 | 0.027708 |
| (t2,tn) | 0.027708 | 0.027708 | |
| (t3,tn) | 0.027708 |
Table 4: P value after comparison of Functional Index of different drugs in t4
| P-K | 0.002537 |
| P-T | 0.002537 |
| P-C | 0.002537 |
| K-T | 0.002537 |
| K-C | 0.002537 |
| T-C | 0.002537 |
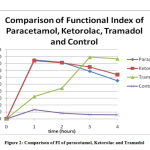 |
Figure 2: Comparison of FI of paracetamol, Ketorolac and Tramadol |
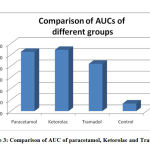 |
Figure 3: Comparison of AUC of paracetamol, Ketorolac and Tramadol |
Results
Table. 1 shows that the p value is significant for all comparisons between FI of t1 with t2 ,t3 and t4 and t2 with t3 , t4 and t3 with t4.
Table.2 shows that the p value is significant for all comparisons between FI of t1 with t2 ,t3 and t4 and t2 with t3 , t4 and t3 with t4. The Table.3 shows that the p value is highly significant for all comparisons between FI of t1 with t2 ,t3 and t4 and t2 with t3 , t4 and t3 with t4.
Results of ‘p’ value in Table. 4 shows the comparison of the result of FI at t4 of different drugs and control also show highly significant values.
Figure. 1 is the schematic circuit diagram of the device that was used to record the time of contact between both the rat’s hind paw and the rotating cylinder.
Figure. 2 shows the comparison of the FI of all the four groups.
Figure. 3 shows the comparison of the AUC of all the four groups.
The maximum efficacy is seen with Tramadolfollowed by paracetamol closely followed by Ketorolac.
The results obtained for different drugs confirm the pattern of drug action. The change of Functional Index with time are in accordance with the pharmacological properties which are characteristic for that class of drug. For e.g. Paracetamol has a fast onset while Tramadol is slower acting.
Discussion
This study was performed in the Department of Pharmacology and Therapeutics, Rajendra Institute of Medical Sciences, Ranchi. Permission was duly obtained the Institutional Animal Ethics Committee(IAEC) at the Rajendra Institute of Medical Sciences, Ranchi(Jharkhand).
The PIFIR method seems to be more suitable for the assay of analgesic drugs. It is however not so suitable to characterize agents that have a delayed onset or agents that have a longer duration of action. The estimation of the time course of study would be required to accommodate such compounds.
Intra-articular injection of Uric acid induces a more acute inflammatory response compared to sodium urate crystals. Hypothesis also suggests that MSU crystals based micro-particles and chondrocytes based proteoglycans can induce subclinical , low-grade inflammation. This can aggravate degradation of the cartilage and can also cause knee OA progression .13Uric acid injection on the other hand produced a consistent dysfunction of the injured leg due to acute inflammation which persisted for at least for 4 hours. Therefore, the employed method can be considered as a suitable model to realistically simulate temporal clinical pain.
Uric acid injection causes a motor dysfunction in the injected leg. The rats avoided the use of the injured leg when they were compelled to walk on the cylinder. The dysfunction onset happened gradually with a maximal (almost 100%) effect in 2.5 h. This is taken as a pain measure and has been shown as paw elevation in urate induced arthritis in rats as shown by Neugebauer et al.13 The main advantage of this type of gait analysis is that the quantitation is independent of the observer.
Paracetamol showed an efficacy which was considerably lower than Tramadolbut roughly equal to that of Ketorolac in our experiment. The maximum functional index is seen at t1which corresponds to the peak plasma concentration of the drug. This is comparable to a research done by Amran et al , where oral paracetamol attains peak plasma concentrations within 30–60 minutes.14The FI declines to almost 55% at t4 in our study. In a study done by Dominguez-Ramirez et al, the FI at t4 done by the PIFIR method, for an oral dose of paracetamol is 28% of the maximum.15 Thus we find that the nociceptive effect of paracetamol considerably declines within 4 hours in both the studies. The AUC for paracetamol is 220.4 for 4 hours after administration in our study which corresponds to 55% bioavailability. In the study by Amran et al, the bioavailability was found to be 70% which is higher than that in our study. 15This difference may be due to difference in the route of administration or difference in species used in the two experiments. It is almost equal to the study by Dominguez-Ramirez et al who obtained AUC of 239.1 at the end of 4 hours.16
For a long time, it has been thought that paracetamol reveals analgesic and antipyretic properties by acting centrally and its inhibitory effect on COX1 and COX-2 activity, i.e., prostaglandin synthesis is low.17
Ketorolac shows maximum efficacy at t1 which is slightly less than paracetamol in our experiment . A low dose of i.m. Ketorolac has shown a significant and immediate analgesic effect on jaw muscle pain in human beings in an experiment by Bendixen et al.18 On the contrary, Ketorolac i.p. was not seen to reduce pain in mice by a study done by Rouf et al.19 The onset of action is 30 minutes with peak effect at 45-60 minutes as suggested by Mallinson TE which corresponds to the time of onset and maximum efficacy in our experiment.20 FI of 64% at t4 for Ketorolac in our experiment shows that the duration of action of Ketorolac is approximately the same as above. The AUC of ketorolc is 230.0 which roughly corresponds to 57% bioavailability in our experiments. In a similar earlier study with with dose-dependent maximal concentration, a similar result was observed in around 20 minutes. The half-life was observed at about 6 hours. The relationship of AUC and the dose was observed to be linearly increasing. The difference in AUC/dose between doses was not found to be statistically significant. This suggests that pharmacokinetics of Ketorolac has a linear structure for a variety of doses under study.21
In several studies, Ketorolac was found to be equally effective analgecic as meperidine or morphine post some specific types of surgery. However, many subsequent studies reported unwanted side effects of Ketorolac. This include side effects such as nephrotoxicity, gastrointestinal issues, coagulopathy and others. As a result use of other classes of non-opioid analgesics is being increasing observed22.
In our experiments we observed that the opioid drug, Tramadol exhibited highest efficacy. But, the onset of the effect was slow and the duration of action seemed to be prolonged; i.e. the FI was observed for a shorter period than required to observe the total time course and activity of action of Tramadol. These results are in conformity with those of Gholami et al. Their study found the duration of analgesic effect of Tramadol100 mg ( single dose, oral) was about 6 hours. The peak analgesic effect of Tramadol recorded in their studies was 3.7 hours in wistar rats.23 Hoenemoff et al showed that the i.p. administration of Tramadol (12.5 mg each kilogram of body weight) exhibited protracted delay in hot plate and tail flick tests’ outcome to acute thermic pain without causing skin lesions.24 These results are comparable to the analgesic effect of i.p. Tramadol in our experiment. Following i.p. administration, the bioavailability of Tramadolwas found to be 67% according to Vaz et al.25 In our experiment, we found the AUC which corresponds to the bioavailability to be 48% of the maximum.
Berrosco et al suggested that 5-HT1Aautoreceptor modulation from the raphe nuclei and spinal cord seem to be participating in the antinociceptive effect of Tramadol.26
The FI and AUC remains very low in the control group ,showing that antinociception is achieved with the drugs only and uric acid induced arthritis further deteriorates in this group in 4 hours. The outcomes of these experiment agree with the outcome of both catwalk analysis in freely moving animals and gait analysis on treadmills. This procedure can thus be considered as a suitable model of tonic temporal pain and thus may be used to realistically simulate clinical pain in temporal settings.27 The onset of activity of Tramadol is very slow with maximum efficacy at t3.The AUC of Tramadolin 4 hours is much lower than both the above drugs. This warrants further studies with a longer time duration to study the pharmacokinetic action of Tramadol.
However since the quick pace of joint disruption in our studies does not accurately model the slow onset and growth of osteoarthritis pathology, there may be difference in results of the treatment modalities.
Conclusion
Current study shows that the antinociceptive efficacy of Tramadolis better than paracetamol and Ketorolac. Paracetamol and Ketorolac show almost equal antinociceptive efficacy.
Paracetamol and Keterolac have an early onset of action whereas Tramadol acts for a long time. So in order to achieve an immediate and sustained antinociception, as in rheumatoid arthritis, a combination of either paracetamol or keterolac along with Tramadolcan be very good antidote.
Further research on these drugs using a longer time duration, different doses and different routes of administration, serum plasma concentration measurements and in combinations may add to the existing knowledge on the antinociceptive efficacy of these drugs in arthritis.
Acknowledgements
We are thankful to Bhaskar Chaudhary for making the apparatus.
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding source.
References
- Henschke N, Camper SJ, Maher CG, The epidemiology and economic consequences of pain: Mayo. Clin. Proc., January 2015;90(1):139-147
CrossRef - Mcgee SJ, Goldberg DS, Pain as a global health priority, BMC Public Health 2011,11:770
CrossRef - Gereau IV KV, Sluka KA, Maixner W, Savage SR, Price TJ, Murinson BB et al , The Journal of Pain; Volume 15(12),December,2014,1203-14
CrossRef - Olesen AE, Andresen T, Staahl C, Drewes AM, Human Experimental Pain Models for Assessing the Therapeutic Efficacy of Analgesic Drugs; Pharmacol Rev 64:722–779, 2012
CrossRef - Phillips CJ, The cost and burden of economic pain; Reviews in pain; Volume 3(1),June 2009, 2-5
CrossRef - Kumar KH, Elvarasi P, Definition of pain and classification of pain disorders; Journal of Advanced Clinical & Research Insights (2016), 3, 87–90
CrossRef - Dubin AE, Patapoutian A, Nociceptors: the sensors of the pain pathway; : J Clin Invest. 2010;120(11):3760–3772. doi:10.1172/JCI42843.
CrossRef - Visser EJ, Stephanie D, What is Pain? I: Terms, Definitions, Classification and Basic Concepts ;Australian Anaesthesia,2009,29-339.
- Marttinez RV et al;Involvement of cycloxygenase1 and cycloxygenase 2 in peripheral pain(abstract), J Pharm Pharmacol.2002 Mar;54(3):405-12.
- Martinez LA, Truzano-Gunzalez ME, Aguierre-Hernandez E, Moreno J, Antinociceptive activity of Tilia americana var. mexicana inflorescences and quercetin in the formalin test and in an arthritic pain model in rat, Neuropharmacology56(2):564-71 · December 2008 DOI: 10.1016/j.neuropharm.2008.10.010 ·
CrossRef - Gonzalez-Truzano ME, Pena EI, Martinez AL, Moreno J, Guevara-Fefer P,Deciga-Campose M et al,Evaluation of the antinociceptive effect of Rosmarinus officinalisL. using three different experimental models in rodents; https://doi.org/10.1016/j.jep.2006.12.011
CrossRef - Gonzalez-Ramirez A, Eva GT M , Fransisco P, Lopez-Munoz FJ, Anti-nociceptive and anti-inflammatory activities of the Agastache mexicana extracts by using several experimental models in rodents; Journal of ethnopharmacology142(3):700-5 · June 2012 DOI: 10.1016/j.jep. 2012.05.044
CrossRef - Ma CA, Leung YY; Exploring the link between uric acid and arthritis, Frontiers in medicine, Front. Med., 13 December 2017 | Volume 4 https://doi.org/10.3389/fmed.2017.00225
CrossRef - Neugbauer V, Han J S , Adwanikar H, Fu Y, Ji G; Techniques for assessing knee joint pain in arthritis, Molecular pain 2007,3:8
CrossRef - Amran S et al; The Pharmacokinetic Study of Aspirin, Paracetamol and Naproxen with Magnesium Sulfate , Amran et al., Pharm Anal Acta 2015, 6:5
- Ramirez A M D et al; HPLC-PDA method for the quantification of paracetamol in plasma: application to pk/pd studies with arthritic rats , International Journal of Pharmacy and Pharmaceutical Sciences, Vol 9, Issue 5, 2017
CrossRef - Bebenista M J, Nowak Z J; paracetamol: mechanism of action, applications and safety concern, Acta Poloniae Pharmaceutica ñ Drug Research, Vol. 71 No. 1 pp. 11ñ23, 2014
- Christophe Mallet, Alain Eschalier and Laurence Daulhac; Paracetamol: Update on its Analgesic Mechanism of Action; http://dx.doi.org/10.5772/66649
CrossRef - Bendixen KH, Baad-Hansen L, Cairns BE, Svensson P; Effects of low-dose intramuscular Ketorolac on experimental pain in the masseter muscle of healthy women , J Orofac Pain. 2010 Fall;24(4):398-407.
- Granados-Soto V1, Flores-Murrieta FJ. Pharmacokinetics of oral Ketorolac in the rat. Methods Find Exp Clin Pharmacol. 1995 Oct;17(8):535-8.
- Cepeda S M, Daniel B. Carr, Nelcy Miranda, R.N., Adriana Diaz, Claudia Silva, Olga Morales ; Comparison of Morphine, Ketorolac, and Their Combination for Postoperative Pain,Results from a Large, Randomized, Double-blind Trial , Anesthesiology 2005; 103:1225–32
CrossRef - Mishra H, Khan F A; A double-blind, placebo-controlled randomized comparison of pre and postoperative administration of Ketorolac and Tramadolfor dental extraction pain, Journal of Anaesthesiology Clinical Pharmacology , April-June 2012 , Vol 28 , Issue 2
CrossRef - Heo B H, Park J H, Choi J I, Kim W M, Lee H G, Cho S Y, Yoon M; A Comparative Efficacy of Propacetamol and Ketorolac in Postoperative Patient Controlled Analgesia, Korean J Pain 2015 July; Vol. 28, No. 3: 203-209
CrossRef - Gholamia M, Sabooryb E,l Mehrabana S, Niakania A, Banihabiba N, Azadc M , Fereidonid J; Time Dependent Antinociceptive Effects of Morphine and Tramadol in the Hot Plate Test: Using Different Methods of Drug Administration in Female Rats, Gholami M et al. / IJPR (2015), 14 (1): 303-311
- Evaluation of dosages and routes of administration of Tramadolanalgesia in rats using hot-plate and tail-flick test, Volume 39, No. 11 | NOVEMBER 2010;344-50
CrossRef - Esther Berrocoso,,M. , Rojas-Corrales O, . Mico J A ;Differential role of 5-HT1Aand 5-HT1B receptors on the antinociceptive and antidepressant effect of Tramadolin mice, . Psychopharmacology September 2006, Volume 188, Issue 1, pp 111–118
CrossRef - Berge O G , Predictive validity of behavioural animal models for chronic pain. 1206 British Journal of Pharmacology (2011) 164 11
CrossRef








