Lavanya Prathap1* and Prathap Suganthirababu2
and Prathap Suganthirababu2
1Department of Anatomy, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai- 600077, Tamilnadu, India
2Saveetha College of Physiotherapy, Saveetha Institute of Medical and technical sciences.
Corresponding Author E-mail: lavanya.anatomist@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2001
Abstract
Background: The life time exposure to estrogen hormone and the family history describes the risk factors involved in hormone based life events in breast cancer population. Focusing the preventive strategies of breast cancer on genetic and epigenetic basis is pivotal. Aim: To explore the association of genetic base of reproductive factors and positive family history with genetic variants in breast cancer DNA repair genes. Methods: We conducted the study among 150 females in three groups, each comprising of 50 based on the selection criteria. The reproductive and hereditary risk factors are used as an outcome measure, which includes age >50 years, increased menstrual age, post-menopausal women, first full term pregnancy (FFTP)>30yrs, nulliparity, obesity, family history of breast cancer. The Genetic analysis is carried for four single nucleotide polymorphisms namely XRCC1 Arg 194 Trp, XRCC3 Thr 242 Met, ERCC4 Arg 415 Gln,, ERCC5 Asp 1104 His and the results are associated with outcome variables. Result and Conclusion: To encapsulate 34% of females with positive family history are associated with XRCC1 R/W, 8% of females with obesity are associated with ERCC4 R/Q, 11% of females with FFTP>30yrs of age are associated with XRCC3 T/M, 24% of nulliparous women, 26% of females with increased menstrual age, 22 % of postmenopausal women, and 23% of females with age >50 years are associated with ERCC5 D/H. The results suggested the association of the reproductive risk factors of breast cancer with the single nucleotide polymorphism status of DNA repair genes and recommends future researches to have a more precise outcome.
Keywords
Breast Neoplasm; DNA Repair; Estrogen Exposure; Oxidative stress: Single nucleotide polymorphism
Download this article as:| Copy the following to cite this article: Prathap L, Suganthirababu P. Estrogen Exposure and its Influence in DNA Repair Genetic Variants in Breast Cancer Population. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Prathap L, Suganthirababu P. Estrogen Exposure and its Influence in DNA Repair Genetic Variants in Breast Cancer Population. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/3iQFi2o |
Introduction
The life time exposure to estrogen hormone describes the factors involved in the hormone based life events in breast cancer population. Positive family history of breast cancer, on the other hand, helps to describe the genetic base of inherited breast cancer (5% to 10%). The Hormone Based Life Events can either directly inherits from parents or the epigenetic alteration of the gene of parents may be transferred to the daughter DNA pattern resulting in genomic instability. The crucial period of epigenetic programming occurs in-utero, during puberty and pregnancy period of a women’s life. This decisive period is highly influenced by environmental conditions like food, exercise, pollution, sleep, and stress. The fetal epigenetic programming and reprogramming during the maturation phase of women positively impact breast cancer risk. Epigenetic alteration is the term used to describe the life time factors that influence or regulates the gene without changing the sequence of DNA and regulate genes. Poor quality of life style induces oxidative stress, DNA methylation, and histone acetylation. As an effect of these changes at DNA level it can either produce negative effects resulting in polymorphism in which a base of nucleotide sequence is altered or affects the gene expression without altering the sequence of the gene resulting in poor quality of life with diseases and disorders.1-5 The risk factors associated with the hormone based life events are suggested to have genetic bases, thus the present study aims to explore the genetic base of the hormone based life events and positive family history by assessing its association with genetic variants in breast cancer population.
Materials and Methods
We conducted the study among 150 females in three groups, each comprising of 50 participants. The participants are age matched between 35-60 years. We commenced after getting approval from the Institutional Human Ethical Committee, Saveetha University -IHEC No-06/10/2012 Chennai, Tamilnadu. The participants are grouped based on selection criteria. Group-I includes females diagnosed histo-pathologically for breast cancer as their primary site of the carcinoma. Group-II includes females who are categorized as high risk for breast cancer based on detailed assessment emphasizing on their family history for breast cancer (mother, sister or daughter) or any two criteria based on their endogenous exposure to estrogen which includes Menstrual history (early menarche below 12 years, late menopause above 50 years), Parity status (first full term pregnancy (FFTP) above 30 years of age, Nulliparity), Personal history of fibro-adenoma, obesity, hormone replacement therapy (HRT). Group-III includes healthy females. We collected the data from the participants after providing them with detailed explanation about the procedure. Their cooperation and willingness is obtained with an informed consent prior to data collection. The Variables used as an outcome measure for Hormone Based Life Events and positive family history are collected as part of their subjective assessment (Table – I). The variables includes age > 50 years, increased menstrual age, post-menopausal women, first full term pregnancy (FFTP) >30yrs, nulliparity, obesity, family history of breast cancer (mother, sister, daughter). The Single nucleotide polymorphism of four DNA repair genes which are reported to have linked with breast cancer risk namely XRCC1 Arg 194 Trp, XRCC3 Thr 242 Met, ERCC4 Arg 415 Gln, and ERCC5 Asp 1104 His are used to associate with reproductive factors and family history. The Genetic analysis is carried as part of the study using Polymerase Chain Reaction and identification of single nucleotide polymorphism using Restriction Fragment Length Polymorphism and results are reported in our previous articles. (6, 7) The SNP results are associated with study outcome variables. The statistical procedure used to analyze the frequency of association between hormones based life events and variant allele of DNA repair gene is odds ratio and the level of significance was analyzed using p- value.
Table 1: Demographic Status of the Study Population
| S.N | Factors | Group I
Cases |
Group II
High risk cases |
Group III
Control |
| 1 | Age (SD± Mean) | 5.49± 49.4 | 5.69± 49.9 | 5.80± 49.68 |
| 2 | Age at menarche(SD ±Mean) | 1.73± 12.9 | 1.61± 12.7 | 1.20± 13.22 |
| 3 | Age at menopause (SD± Mean) | 1.76± 51.8 | 1.61± 52.5 | 1.86 ±49.84 |
| 4 | Age at First Full Term Pregnancy in Parous women (SD ±Mean) | 4.17 ±28.5 | 3.87± 29.5 | 3.57 ±24.77 |
| 5 | Nulliparity | 28 | 18 | 5 |
| 6 | Positive Family History For Breast Cancer | 26 | 21 | – |
| 7 | BMI(SD ±Mean) | 3.78± 26.6 | 3.70± 27.12 | 3.40± 25.36 |
| 8 | History of Fibro adenoma | 9 | 18 | – |
The distinct hormone based life events are those variables with increased exposure to estrogen hormone. Age >50 years, First Full Term Pregnancy (FFTP) >30 years are associated significantly at p<0.05 with XRCC1 Arg194Trp, XRCC3 Thr241 Met, ERCC4 Arg 415 Gln, ERCC5 Asp1104His in their homozygous wild and heterozygous mutant type. (Table -II, Figure I)
Results
Post-Menopausal women are associated with significant difference p<0.05 with XRCC1 Arg194Trp, ERCC4 Arg 415 Gln, ERCC5 Asp1104His in their homozygous wild and heterozygous mutant type and XRCC3 Thr241 Met showed significant difference with homozygous wild type alone. (Table -II, Figure I)
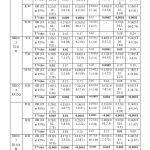 |
Table 2: Association between Hormone Based Life Events and Polymorphism Status. |
Increased menstrual age (early menarche and late menopause) is associated with significant difference p<0.05 with XRCC1 Arg194Trp, ERCC4 Arg 415 Gln, ERCC5 Asp1104His in their homozygous wild and heterozygous mutant type, XRCC3 Thr241 Met is not associated with increased menstrual age. (Table -II, Figure I)
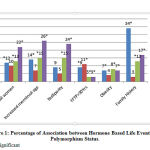 |
Figure 1: Percentage of Association between Hormone Based Life Events and Polymorphism Status. |
Nulliparity is associated with significant difference p<0.05 with ERCC4 Arg 415 Gln, and ERCC5 Asp1104His in their homozygous wild and heterozygous mutant type, XRCC1 Arg194Trp, XRCC3 Thr241 Met are not associated with Nulliparity. (Table -II, Figure I)
Obesity is associated significantly XRCC1 Arg194Trp in homozygous wild and heterozygous mutant type, XRCC3 Thr241 Met is associated in only homozygous wild type, ERCC4 Arg 415 Gln associated with all genotypes and ERCC5 Asp1104His is associated with homozygous wild and heterozygous mutant type. (Table -II, Figure I)
Family history of breast cancer in the first round relative mother sister and daughter is associated significantly with XRCC1 Arg194Trp, ERCC4 Arg 415 Gln, and ERCC5 Asp1104His in their homozygous wild and heterozygous mutant type, and ERCC5 Asp1104His is associated with homozygous mutant type also. XRCC3 Thr241 Met is not associated with Family history of breast cancer. (Table -II, Figure I)
Discussion
To encapsulate 34% of females with positive family history are associated with XRCC1 R/W, 8% of females with obesity are associated with ERCC4 R/Q, 11% of females with FFTP>30yrs of age are associated with XRCC3 T/M, 24% of nulliparous women, 26% of females with increased menstrual age, 22 % of postmenopausal women, and 23% of females with age >50 years are associated with ERCC5 D/H. The single nucleotide polymorphism XRCC1 rs 1799782 is associated significantly with increased age, FFTP >30 years, post-menopausal women, increased menstrual age, obesity and not associated with Nulliparity. XRCC3 rs 861539 is associated with increased age, FFTP>30years and not associated with other variables. ERCC4 rs1800067 and ERCC5 rs17655 are associated significantly with increased age, FFTP >30 years, post-menopausal women, increased menstrual age, Nulliparity and Obesity (both heterozygous and homozygous mutant type). The single nucleotide polymorphisms rs 1799782, rs1800067 and rs17655 are associated significantly with family history of breast cancer. ERCC5 rs 17655 is associated in homozygous genotype also. XRCC3 rs 861539 is not associated with family history of breast cancer.
One of the important pathway of carcinogenesis attributed to genetic alternation is oxidative stress which is exerted by the imbalance of production of free radicals and the antioxidant level. In the similar context the oxidative stress leads to the epigenetic alterations causing DNA hypo-methylation of promoter regions of tumor suppressor genes, DNA repair genes or apoptotic genes resulting in gene silencing and genomic instability. Epigenome-wide association study finds epigenetic markers associated with breast cancer risk. The findings of the study suggests 5% increase in breast neoplasm risk in one year longer estimated life time estrogen exposure (ELEE).8 A case control study evaluated the association between ERCC5 polymorphism and the breast neoplasm risk and their findings concluded that the variant ERCC5 rs2094258 may contribute to the risk of breast neoplasm.9
In a hospital based case control study, evaluated the synergistic effects of DNA repair single nucleotide polymorphism and endogenous estrogen in breast cancer population at risk. The findings of the study suggested the ERCC5 His 1104 Asp is associated significantly with odds ratio of 1.42 at confidence interval 1.08-1.97. This polymorphism status was found to be more linked in women with lengthy estrogen exposure. The synergistic influence of base excision repair and nucleotide excision repair genes on breast cancer risk was associated significantly in females with longer period of estrogen exposure, greater age at first full term pregnant >26 years , increased menstrual age and increased body mass index>22.10
A Case control study performed in northeastern region of India, assessed the DNA repair gene XRCC1 Arg194 Trp and XRCC3 (Thr241Met) polymorphism in breast cancer risk in premenopausal women. The findings suggests X-ray repair cross complementary 1A (Arg194Trp) associated significantly with the breast cancer risk and XRCC1 3(Thr241Met) not having any possible significant association with breast cancer risk.11
Hormone Based Life Events reflects the increased exposure to estrogen hormone which in turn reflects the exposure to oxidative stress. Epigenetics12 is a very useful and powerful tool which helps to regulate the genes. The nucleotide sequence of a gene cannot be changed normally by our daily life, if change occurs as a result of some inducing factors it is named as polymorphism or mutation. Epigenetic alteration is our nature’s gift to prevent and fight against cancer. Through Epigenetic alteration both positive and negative regulation can be attained, depending upon how an individual is operating the epigenetic mechanism. There are certain factors which positively regulates the gene through epigenetic mechanisms like physical activity, regular exercise, and balanced nutritious diet and ideal BMI. On the other hand there are certain factors which negatively regulate the gene through epigenetic mechanisms like lack of exercise, sedentary life style, obesity, unhealthy food habits and alcohol (modifiable risk factor). Increased estrogen exposure induces oxidative stress in breast cancer which in turn induces polymorphisms. The factors that result in increased estrogen exposure are named as Hormone Based life events, which is one of the important risk factor (non- modifiable risk factor). The positive regulation of epigenetic mechanism can in turn regulate the hormonal factor and maintain it in a balanced condition. The well balanced hormonal status can help to overcome the risks induced by the non-modifiable factors.13-16
On detailed observation of this concept it is evident that key to regulate our gene is in our hand. If the life is designed with factors that positively regulate the gene through epigenetics the individual can prevent, postpone the events of carcinogenesis to 60s or 80s of their life, can overcome the complications of breast cancer, and reduce the aggressiveness of cancer and many more beneficial effects which aids to lead a good quality of life.17- 19 This is the crucial time to take a wise decision to fight against breast cancer by just following simple life style modifications and awareness about the modifiable and non-modifiable risk factors. We can either prevent or delay the process of carcinogenesis and so the breast cancer. Breast cancer in 30s is more aggressive than 60s.20, 21 To investigate the hypothesis the hormone based life events and the positive family history are analyzed in the study with four DNA repair genes and our results have recommends that there is a possible association existing between these factors.
The limitation of the study is its sample size. We recommend to conduct numerous researches in this context with large sample size and more number of genetic variants to get precise results.
Conclusion
The findings suggests possible association between the duration of exposure to estrogen in a women’s life and the family history in initiating breast cancer risk through their synergistic influence in DNA repair gene polymorphism. Future researches should be conducted in large scale to come out with precise findings.
Acknowledgement
We would like to deliver sincere thanks to Saveetha Institute of Medical and Technical Sciences, Tamilnadu, India. and Dr. MGR Educational and Research Institute University, Chennai, India.
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding source.
References
- Christoph L and Gerd P.P, Epigenetic changes of DNA repair genes in cancer, J Mol Cell Biol.,2011: 3(1): 51-58.
- Egger G, Gangning L, Ana A, Peter A. J, Epigenetic human disease and prospects for epigenetic theory. Nature 2004: 429:457-463.
- Sarah M. Mense, Fabrizio R, Ahima B, Bhupendra Singh, Mahmoud E1- Tamer, Tom K.Hei and Hari K Bhat, Estrogen Induced Breast Cancer-Estrogen Induced Breast Cancer: Alterations in Breast Morphology andn Oxidative stress as a function of estrogen exposure. Toxicology and Applied Pharmacology, 2008: 232(1): 78-85.
- Lavanya Prathap, Vijayakumar Jagadeesan, Prathap Suganthirababu, Deepthi Ganesan, Association of Qualitative and Quantitative dermatoglyphic variable and DNA polymorphism in female breast cancer population, Online Journal of health and Allied sciences, 2017: Vol-16, Issue 2.
- Lavanya Prathap, Prathap Suganthirababu, Jagatheesan Alagesan, Digital and palmar dermal ridge patterns in population with breast carcinoma, Biomedicine, 2014: 34(3):315-321.
- Lavanya J, Kumar VJ, Sudhakar N, Prathap S. Analysis of DNA repair genetic polymorphism in breast cancer population. Int. J. Pharm. Bio. Sci. 2015; 6(3):966-73.
- Prathap L, Suganthirababu P, Ganesan D. Fluctuating Asymmetry of Dermatoglyphics and DNA Polymorphism in Breast Cancer Population. Indian Journal of Public Health Research & Development. 2019 Nov 1;10 (11).
- Johansson, A., Palli, D., Masala, G. et al.Epigenome-wide association study for lifetime estrogen exposure identifies an epigenetic signature associated with breast cancer risk. Clin Epigenet 2019: 11, 66 . https://doi.org/10.1186/s13148-019-0664-7)
- Na, N., Dun, E., Ren, L., & Li, G. Association between ERCC5 gene polymorphisms and breast cancer risk. International journal of clinical and experimental pathology,2015: 8(3), 3192–3197
- Ming-Shiean H, Yu JC, Wang HW, et al. Synergistic effects of polymorphisms in DNA repair genes and endogenous estrogen exposure on female breast cancer risk. Ann Surg Oncol. 2010; 17(3):760‐ doi:10.1245/s10434-009-0802-0
- Devi, K. R., Ahmed, J., Narain, K., Mukherjee, K., Majumdar, G., Chenkual, S., & Zonunmawia, J. C. DNA Repair Mechanism Gene, XRCC1A ( Arg194Trp) but not XRCC3 (Thr241Met) Polymorphism Increased the Risk of Breast Cancer in Premenopausal Females: A Case-Control Study in Northeastern Region of India. Technology in cancer research & treatment 2017: 16(6), 1150–1159. https://doi.org/10.1177/1533034617736162
- Kevin C J, Devin C K, Chao C, Brock C C,‘Age-related DNA methylation in normal breast tissue and its relationship with invasive breast tumor methylation’. Epigenetics, 2014: 9 (2):268-275.
- S, J. G. Tzanninis, A. Philippou, M. Koutsilieris, ‘Epigenetic regulation on gene expression induced by physical exercise’, J Musculoskelet Neuronal Interact, 2013 13(2): 133-146.
- David john Hunter, Lynsey James, Bethan Hussey, Alex J Wardley, Martin R Lindley, Sarabjit S Mastana, impact of aerobic exercise and fatty acid supplementation on global and gene specific DNA methylation,Epigenetics, 2019 14:3,294-309.
- S K, Stanczyk , Kusinska R , Kordek R, Majsterek I, Association of Arg 194 Trp and Arg 399 Gln Polymorphisms of XRCC1 gene woth risk of occurrence and the response to adjuvant therapy among polih women with breast cancer , Clin Breast Cancer 2013: 13(1): 61-68.
- Silva SN, Moita R, Azevedo AP, Gouveia , Manita I, Pina JE, Rueff J, Gaspar J, Menopasual age and XRCC1 gene polymorphisms: role in breast cancer risk, Cancer Detect Prev, 2007: 31(4): 303-309.
- Feng YZ, Liu YL, he XF, Wei W, Shen X L, Xie D L, Association between the XRCC1 Arg194Trp polymorphism and risk of cancer; evidence from 201 case control studies, TumourBiol, 2104: 35(11): 10677-10697.
- Hsu Ming S: Jyh-Cherng Y: Hsiao-Wei W, Shou- T C Liu MC, Synergistic effects of polymorphisms in DNA repair genes and endogenous estrogen exposure on female breast cancer risk, Ann SurgOncol, 2010: 17(3): 760-71.
- Crutis CD, Thorngren DL, Nardulli AM, Immunohistochemical analysis of Oxidative stress and DNA repair proteins in normal mammary and breast cancer tissues, BMC Cancer,2010: 10:9.
- Kanf DH, (2002), Oxidative stress, DNA damage and breast cancer, AACN Clin issues, 2002: 13(4): 540-549.
- Jorgensen TJ, HelzlsouerKJ,Clipp SC, Bolten JH, Crum RM, Visvanathan K., (2009), DNA Repair gene variants associated with Benign Breast Disease in High Cancer risk women, Cancer Epidemiol Biomarkers Prev, 2009:18(1): 346-50.








