Amin Mir1, Anuj Kumar2, Abhishek Goal2 and Priya Singh3
1Department of Mathematics and Natural Sciences PMU University Al Khobar Kingdom of Saudi Arabia
2College Kotdwar Pauri Garhwal
3IIIM Jammu
Corresponding Author E-mail : mohdaminmir@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1988
Abstract
Pteris vittata, Catharanthus roseus and Ganoderma lucidium plants were examined for the amount of Copper and cadmium by spectroscopically. The two metals so for analyzed have been found within a suitable range with no harmful effects. Copper and cadmium have a potential to act as co-enzymes in various metabolic cycles and also have preferential importance in other physiological pathways, so these elements are to be maintained under control within the biological systems. Since copper and Cadmium are both essential cofactors and can also act as toxic elements if not maintained, involving a complex network of metal trafficking pathways, so different strategies had come across, as to appropriately regulate homeostasis in plants. The strategies must be made in order to prevent accumulation of toxic metals in freely reactive form (metal detoxification pathways) and may ensure proper delivery of these elements to target metalloproteins in a controlled manner and at appropriate time. The plants and the fungi being the rich source of various types of essential minerals, so they should be consumed in any form as to meet the metal deficiency of the body.
Keywords
Catharanthus roseus; Copper; Cadmium; Ganoderma lucidium; Pteris vittata
Download this article as:| Copy the following to cite this article: Mir M. A, Kumar A, Goal A, Singh P. Estimation of Copper and Cadmium in Various Extracts of Pteris vittata, Catharanthus roseus and Ganoderma lucidum. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Mir M. A, Kumar A, Goal A, Singh P. Estimation of Copper and Cadmium in Various Extracts of Pteris vittata, Catharanthus roseus and Ganoderma lucidum. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/3eU3VsD |
Introduction
Catharanthus roseus is native to the Indian Ocean Island of Madagascar. In wild, it is found as an endangered plant which is mainly because of their decline is the habitat destruction by the slash and burn agriculture. However it is now common in various tropical and subtropical regions across the world, including the Southern United states. Catharanthus roseus (L) G. Don (formerly Vinca rosea L., Apocynaceae) is widely known as the Madagascar periwinkle. It is a perennial ever green herb, 30-100 cm tall originally (Lata B etal 2007). The plant is important as having its ability to synthesize a wide range of terpenoid indol alkaloids which possesses medicinal values. These compounds have application against leukemia in children, and lymphocytic leukemia, Wilkins’s tumor, neuroblastoma and reticulum cell sarcoma, Hodgkin’s disease besides lymphosarcoma, choriocarcinoma (Aslam J, Khan SH, et al 2010). Except alkaloids, other natural compounds in C. roseus have been less investigated (Karthikeyan B etal 2010). Catharanthus roseus, (K. Kabesh, P. Senthil Kumar 2015) mentioned this plant as an important plant with wide range of actions such as antimicrobial, antioxidant, anthelmintic, antifedant, antisterility, antidiarrheal, antidiabetic effect etc. Acetonic extract of leaves of Catharanthus roseus (Shahin Mardani-Nejada 2016) shows anti-bacterial action against three pathogenic micro-organisms, (Klebsiella pneumonia, Staphylococcus aureus, and Escherichia coli).
Nearly as per an estimate there are near about 1.5 million fungal species in world, among which approximately 82,000 have been noticed (Shahin Mardani-Nejada 2016)). Of these known species most of them belong to macro-fungi, of which about 5,000 are edible and 2,000 are safe for consumption (Kirk PM, Cannon PF 2001). Basidiomycota fungi are of great interest as they contain an enormous number of biologically active compounds (Hawksworth DL 2001). Ganoderma had been used traditionally from a long time ago and is still being used to reduce the ill effect of various diseases and virtually does bear the potential to cure all types’ diseases (Lorenzen K and Anke T, 1998 Kingston DG 2005 and Newman DJ Mizuno T 1995). Ling Zhi mentioned several Ganoderma species, which are widely used for various medicinal purposes e.g., Ganoderma lucidum, Ganoderma luteum steyaert, Ganoderma atrum Zhao, Xu and Zhang, Ganoderma applanatu (pers.:Wallr) pat.,Ganoderma australe (Fr.) pat., Ganoderma capense (Lloyd) Teng, Ganoderma tropicum (Jungh) Bres.,Ganoderma tenue Zhao, Xu and Zhang and Ganoderma sinense Zhao, Xu and Zhang. More over near about 250 Ganoderma species have been discovered till now (Moncalvo, J.M 1997, Wasser S.P, 1997), but for medicinal purposes and for literature citation, Ganoderma usually refers to the species of Ganoderma lucidum.
Pteris vittata L. (commonly known as Chinese brake fern) is a perennial evergreen fern which belongs to Pteridaceae family and is found in all tropical regions of Eurasia, Africa and America. (Ma LQ, Komar KM, 2001) and Chen et al. 2002) concurrently it had been discovered that this fern species do hyper accumulated carcinogenic metalloid arsenic (As). The concerned plant have a potential to accumulate up to 6,805 ppm of arsenic in just six weeks in contaminated soils; and up to 93% of the arsenic extracted from soil is concentrated in the fronds (Ma LQ, Komar KM, 2001, Chen T, Wei C, Huang Z, 2002, Tu C, Ma LQ 2002). The form of As in the leaves (with a concentration 25 times more than the roots and 142 times higher than soil) is inorganic As (III), and is mostly confined in the upper and lower epidermis (Tu C, Ma LQ, 2002, Lombi E, Zhao FJ, 2002, Zhang W, Cai Y, 2002).
Experimental
The “Ganoderma lucidum, Pteris vittata and Catharanthus roseus, was collected from the FRI, Dehradun (UK). The plant and the fungi was shade dried and powdered in to mixture.
Extraction
50 gms of each plant powder and fungi powder were segregated from the foreign matter and then dried and weighed separately and accurately and then extraction was carried out in a Soxhlet Apparatus using thimble to have the pure form of the extract. Various solvents with different polarity index were used as per their polarity index (Acetone, DMSO and water).
Extraction A
(Ganoderma lucidium) was extracted first with Acetone in a Soxhlet apparatus for the mentioned period followed by DMSO having higher polarity than acetone. After the Extraction with DMSO, the extract solution was subjected to filtration and the filtrate was then collected and evaporated to remove the volatile solvent to 1/4th its volume on water bath at a suitable temperature. The filtrate was then converted into powder form after being kept in an oven for a limited time. The residue from the extract was collected, and subjected to further extraction process.
Extraction B
The residue was then extracted with DMSO in the same manner as mentioned above, for extraction A.
Extraction C
The residue from extract B was subjected to water extraction by decoction technique, in which the extract was dissolved in 500 ml of water. The whole solution was heated over water bath to remove all the water from the extract and then additional 500 ml of water was added to the extract, and the extracted solution was finally evaporated as to remove 250ml of water from the whole solution. The solution was then subjected to filtration and the filtrate was collected and evaporated to remove nearly 1/4th of its volume. At last the water extract was dried in an oven within a temperature range 30-500C. The percentage yield of all the extracts were determined as w/w.
Estimation of Copper (Method by Amin et al, 2016)
All reagents were purchased from local market. The stock and standard solutions were prepared freshly throughout the experiment. Cu (II) metal was determined under required conditions in various water samples by UV-VIS Spectrophotometer. A pH meter (Elico) was used for the pH adjustments.
Procedure
Copper Sulphate Solution
0.1 g of copper sulphate solution was dissolved in 100 ml of deionized water.
Dimethylglyoxime Solution
1 g of Dimethylglyoxime was dissolved in 100 ml of ethanol.
The procedure followed by Amin et.al was modified in which 5 ml of each plant extract, 5 ml of Dimethylglyoxime, 2 ml of buffer and 8 ml of deionized water was taken in a test tube. The absorbance of the plant extracts was taken at 517 nm.
Estimation of cadmium
Reagents and Solutions
All the chemicals used were of analytical-reagent grade or the highest purity available. Doubly distilled and deionized water, ultraviolet treated water, was used throughout. Glass vessels were cleaned by soaking in acidified solutions of KMnO4 or K2Cr2O7 followed by washing with nitric acid (1+1) and rinsed several times with high-purity de-ionized water. More rigorous contamination control was used when the cadmium levels in the specimens were low.
Alizarin Red S Solution 139 x 10-3 M
Prepared by dissolving the requisite amount of alizarin red S. (1, 2 dihydroxyanthraqui-none-3-sulphonic acid, sodium salt) (BDH chemicals) in a known volume of deionized water. More dilute solutions of the reagent were prepared as required.
Cadmium Standard Solutions
A 100-mL amount of stock solution (1mg/1mL) of divalent cadmium was prepared by dissolving 0 2282mg of AR crystallized cadmium sulfate (3CdSO4, 8 H2O) (Merck) in doubly distilled de-ionized water. Aliquots of this solution were standardized by EDTA titration using xylenol orange as indicator. Extra more diluted standard solutions were prepared by appropriate dilution of aliquots from the stock solution with de-ionized water as and when required.
Procedure
1 ml of a neutral aqueous (pH 6) solution containing 5ml of each plant extract in a 10-mL calibrated flask was mixed with 1:5 molar excess of the alizarin red S. reagent solution (preferably 1.0 mL of 1.39 10 1M) followed by the addition of 1 ml of 0.05 M sulfuric acid (or pH 5.5, 6.1). The absorbance of the samples was measured at 422 nm against a corresponding reagent blank. The cadmium content in an unknown sample was determined using concurrently prepared calibration Figure.
Observations
The plant kingdom is being used as source of medicine for the treatment of various types of diseases of an unknown origin. The concerned plants and the fungi have also been identified to act against various ailments. The primary benefit of using medicinal plants is that they act against biological infections of unknown origin without having side effects associated with them. The study had been carried out upon the various extracts of the plant in reference to metal ions viz (Copper and Cadmium) concentration. The interesting results of the concerned study obtained through various analytical procedures are mentioned below.
Table 1: Showing the Concentration and Absorbance of Copper
| S. No. | Conc. of Copper g/l | Absorbance of copper |
| 1. | 6.6 | 0.099 |
| 2. | 13.2 | 0.142 |
| 3. | 19.8 | 0.187 |
| 4. | 26.4 | 0.235 |
| 5. | 33 | 0.275 |
| 6. | 39.6 | 0.315 |
| 7. | 46.2 | 0.353 |
| 8. | 52.8 | 0.389 |
| 9. | 59.4 | 0.426 |
| 10. | 66.0 | 0.461 |
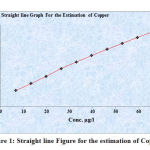 |
Figure 1: Straight line Figure for the estimation of Copper |
Table 2: Showing the concentration and Absorbance of Cadmium
| S. No. | Conc. of Cadmium µg/l | Absorbance of Cadmium |
| 1 | 1.66 | 0.200 |
| 2 | 3.32 | 0.277 |
| 3 | 4.98 | 0.350 |
| 4 | 6.64 | 0.422 |
| 5 | 8.3 | 0.499 |
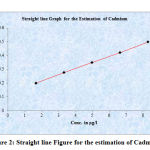 |
Figure 2: Straight line Figure for the estimation of Cadmium |
Table 3: Showing the concentration of copper in (Pteris vittata) plant extract
| S. No | Samples of plant extract | Absorb | Conc. of copper (µg/L) |
| 1 | Acetone plant extract | 0.372 | 32.34 |
| 2 | DMSO plant extract | 0.411 | 36.96 |
| 3 | Water plant extract | 0.313 | 25.09 |
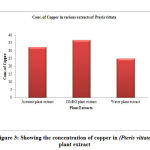 |
Figure 3: Showing the concentration of copper in (Pteris vittata) plant extract |
Table 4: Showing concentration of copper in (Catharanthus roseus) plant extract
| S. No. | Sample of plant extract | Absorb | Conc. of copper (µg/L) |
| 1 | Acetone plant extract | 0.445 | 40.92 |
| 2 | DMSO plant extract | 0.157 | 9.26 |
| 3 | Water plant extract | 0.196 | 13.2 |
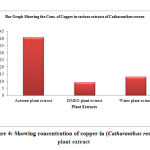 |
Figure 4: Showing concentration of copper in (Catharanthus roseus) plant extract |
Table 5: showing the concentration of copper in (Ganoderma lucidum) plant extract
| S. No. | Samples of plant extract | Absorb | Conc. of copper (µg/L) |
| 1 | Acetone plant extract | 0.316 | 25.74 |
| 2 | DMSO plant extract | 0.193 | 13.2 |
| 3 | Water plant extract | 0.160 | 10.56 |
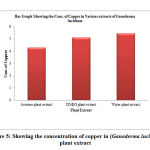 |
Figure 5: Showing the concentration of copper in (Ganoderma lucidum) plant extract |
Table 6: Showing the concentration of cadmium in (Pteris vittata) plant extract
| S. No. | Samples of plant extract | Absorb | Conc. of cadmium (µg/L) |
| 1 | Acetone plant extract | 0.388 | 4.648 |
| 2 | DMSO plant extract | 0.266 | 2.236 |
| 3 | Water plant extract | 0.351 | 4.15 |
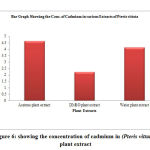 |
Figure 6: showing the concentration of cadmium in (Pteris vittata) plant extract |
Table 7: Showing the concentration of cadmium in (Catharanthus roseus) plant extract
| S. No. | Samples of plant extract | Absorb | Conc. of cadmium (µg/L) |
| 1 | Acetone plant extract | 0.304 | 3.154 |
| 2 | DMSO plant extract | 0.324 | 3.489 |
| 3 | Water plant extract | 0.369 | 4.478 |
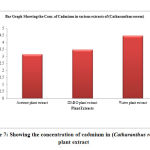 |
Figure 7: Showing the concentration of cadmium in (Catharanthus roseus) plant extract |
Table 8: Showing the concentration of cadmium in (Ganoderma lucidum) plant extract:
| S. No. | Samples of plant extract | Absorb | Conc. of cadmium (µg/L) |
| 1 | Acetone plant extract | 0.368 | 4.318 |
| 2 | DMSO plant extract | 0.407 | 5.146 |
| 3 | Water plant extract | 0.428 | 5.478 |
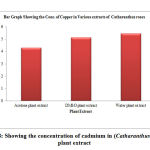 |
Figure 8: Showing the concentration of cadmium in (Catharanthus roseus) plant extract |
Results and Discussion
Heavy metals are significant environmental pollutants and their toxicity is an intolerable problem which is to be overcomed is the need of an hour. The term “heavy metals” refers to any metallic element that possesses relatively a high density. The major dangerous heavy metals in terms of their environmental pollution and hazardous health effects are lead, mercury, chromium, cadmium, copper and aluminium. Many heavy metals are considered to be essential for plant growth. Some of these heavy metals like Cu and Zn either serve as cofactor or as an activator of many biochemical reactions e.g. informing enzyme/substrate metal complex (Mildvan, A.S. 1970). Copper is an essential heavy metal for higher plants and algae particularly for photosynthesis (Chatterjee C, Dube BK, 20004) but enhanced industrial and mining activities have contributed to the increasing occurrence of Cu in ecosystems which makes it non supportive as the concentration is too high beyond the tolerance level. Exposure of plants to excess Cu generates oxidative stress and ROS. Oxidative stress causes disturbance of metabolic pathways and damage to macromolecules (Hegedus, A., Erdei,S, 2001). The concentration of copper in the concerned plant extracts have been found within the limits of its accessibility and being the important element for various metabolic process within the living being as mentioned by various authors.
Among the plant extracts different solvents behave differently as per the concentration of Copper and Cadmium. The concentration of copper in various extracts of Catharanthus roseus have been found as (4.92µg/L) in acetone extract and the DMSO extract shows (9.26µg/L), while water extract of shows (13.2µg/L) of copper. Similarly the acetone extract of Pteris vittata show (32.34µg/L) of copper, The DMSO extract shows (36.96µg/L), while water extract show (25.09µg/L). Also the acetone extract of Ganoderma lucidium show (25.74µg/L) of copper, the DMSO extract (13.2µg/L) of copper and the water extract show (10.56µg/L) of copper.
The cadmium ion concentration in the acetone extract of Catharanthus roseus show (3.154µg/L), the DMSO extract of shows (3.489µg/L) amount of cadmium, while the water extract show (4.478µg/L) amount of cadmium respectively. The acetone extract of Pteris vittata show (4.648µg/L) amount of cadmium the DMSO extract show (2.236µg/L) amount of cadmium, and the water extract of Pteris vittata show (4.15µg/L) amount of cadmium respectively. The Acetone extract of Ganoderma lucidium show (4.318µg/L) amount of cadmium. The DMSO extract of Ganoderma lucidium show (5.146µg/L) amount of cadmium respectively and the water extract of Ganoderma lucidium show (5.476µg/L) amount of cadmium.
Cadmium being a non-essential element affects negatively the plant growth and development. It enters into the environment via power stations, heating systems, metal-working industries or urban traffic. It is widely used in electroplating, pigments, plastic stabilizers and nickel-cadmium batteries (Sanita di Toppi L 1999). It is recognized as an extremely significant pollutant due to its high toxicity and large solubility in water (Pinto et al., 2004).
Wagner et.al 1993 estimated that non-polluted soil solutions contain Cd concentrations ranging from 0.04 to 0.32 mM as being found in the concerned plant extracts. Soil solutions containing Cd from 0.32 to about 1 mM can be regarded as polluted to a moderate level (Sanita di Toppi L 1999). Regarding its potential toxicity for soil organisms and soil microbial processes, Duxbury (1985) (Duxbury T 1985) classified Cd as an element of “intermediate” toxicity. Although the toxic effects of cadmium on biological systems have been reported by several authors (Bingham FT, 1976, Mukherjee A, 1984, Obata H, Umebayashi M, 1997, Das P, Samantaray S 1997), that the exact mechanisms of Cd toxicity is still unclear. Cadmium can alter the uptake of minerals by plants through its effects on the availability of minerals from the soil, or through a reduction in the population of soil microbes (Moral R, Palacios G, Gomez I 1994).
Cu concentrations in cells need to be maintained at low levels since this element is extremely toxic in view of its high redox potential. The average content of Cu in plant tissue is 10 µg/g dry weights (Baker DE, Senef JP 1995). The critical free Cu concentration in nutrient media (below which Cu deficiency occurs) ranges from 10-14 to 10-16M. Plants usually have a variable supply of Cu in the soil and the soil solution concentrations range from 10-6 to 10-9 M, but plants still need to solubilize and reduce the metal in a limited manner.
Conclusion
The growth of plants and fungi gets adversely affected by single Cu and Cd and by binary mixture of Cu + Cd. The plants in reference as per the analysis showed that the plants are safe for use, because the concentration of the two concerned metals have been found to be below the levels of toxicity. Over all it could be concluded that the concerned plants and fungi in reference should be consumed to meet the body demand of the cell against the metal starved cell. Further work could be carried out for the plants in reference in order to carry out the relative study and by various other methods. The results mentioned and the study although had been carried out with full zeal, but still other methods may give more conclusive outcomes.
References
- Lata B. Cultivation, mineral nutrition and seed production of Catharanthus roseus (L.) G. Don in the temperate climate zone. Phytochem Rev, 6, 2007, 403-11.
CrossRef - Aslam J, Khan SH, Siddiqui ZH, Fatima Z, Maqsood M, Bhat MA, Nasim SA, Ilah A, Ahmed IZ, Khan SA, Mujib A and Sharma MP. Catharanthus roseus an important drug: its applications and production. Pharma Globale, 2010, 1: 1-16.
- Karthikeyan B, Joe MM, Jaleel CA and Deiveekasundaram M. Effect of root inoculation with plant growth promoting rhizobacteria (PGPR) on plant growth, alkaloid content and nutrient control of Catharanthus roseus (L.) G. Don. Croat. 19, 2010, 205-212.
- Kabesh, P. Senthil kumar, R. Ragunathan and R. Raj Kumar. “Phytochemical Analysis of Catharanthus roseus Plant Extract and its Antimicrobial Activity”. Int. J. Pure App. Biosci. 3 (2), 2015, 162-172.
- Shahin Mardani-Nejada, Ramazan Ali Khavari-Nejada, Sara Saadatmanda, Farzaneh Najafib and Parviz Aberoomand Azarc. “Potent Antioxidant Properties of rolB-transformed Catharanthus roseus (L.) G. Don”. IJP, 15 (2), 2016, 537 – 550.
- Kirk PM, Cannon PF, David JC and Stalpers JA: Ainsworth and Bisky’s Dictionary of fungi,9th Wallingford, UK: CAB International, 2001.
- Hawksworth DL: Mushrooms: the extent of the unexplored potential. Int J Med Mushrooms 3, 2001, 333-337.
CrossRef - Lorenzen K and Anke T: Basidiomycetes as a source for new bioactive natural products. Curr org chem 2, 1998, 329-364.
- Kingston DG and Newman DJ: The search for novel drug lead for predominately antitumor therapies by utilizing Mother Nature’s pharmacophoric libraries. Curr opin Drug Discov Devel, 8, 2005, 207-227.
- Mizuno T: Bioactive biomolecules of mushrooms: food function and medicinal effect of mushroom fungi. Food rev Int 11, 1995, 7-21.
CrossRef - Moncalvo, J.M, Ryvarden,L: A Nomenclatural study of the Ganoderma taceae Donk; Synopsis fungorum 11; Fungiflora: Osle, Norway, 1997, 114.
- Wasser S.P, Weis A.L: Medicinal Mushrooms Ganoderma lucidum(Curtis: Fr), P. karst; Nevo, E.Eds; Peledfus publ House: Haifa, Israel, 1997, 39.
- Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED. A fern that hyperaccumulates arsenic. Nature 409, 2001,
CrossRef - Chen T, Wei C, Huang Z, Huang Q, Lu Q, Fan Z. Arsenic hyperaccumulator Pteris vittata and its arsenic hyperaccumulation. Chinese Science Bulletin 47, 2002, 902-905. .
CrossRef - Tu C, Ma LQ. Effects of arsenic concentration and forms on arsenic uptake by the hyperaccumulator ladder brake. Journal of Environmental Quality 31, 2002, 641-647.
CrossRef - Lombi E, Zhao FJ, Fuhrmann M, Ma LQ, McGrath SP. Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytologist 156, 2002, 195-203.
CrossRef - Zhang W, Cai Y, Tu C, Ma LQ. Arsenic speciation and distribution in an arsenic hyper accumulating plant. The Science of the Total Environment. 300, 2002, 167-177.
- Amin Mir M*, Ahmad Mir B, Firdous S, Hassan U, Gupta A and Kukretee N. “Novel and Easy Accessible Method for the Estimation of Copper Spectrophotometrically in Water and Soil Samples”. Chem Sci J, an open access journal, 7,4, 2016.
- Mildvan, A.S. Metals in enzyme catalysis. In The Enzymes, 2nd edn, (ed. P.D. Boyd), 1970, 445-536.
CrossRef - Chatterjee C, Dube BK, Sinha P, Srivastava P. Detrimental effects of lead phytotoxicity on growth, yield, and metabolism of rice. Commun Soil Sci Plant Anal 35, 2, 2004, 255–265.
CrossRef - Hegedus, A., Erdei,S., Horvath,G. Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress, Plant Science 160, 2001, 1085– 1093.
CrossRef - Sanitá di Toppi L, Gabbrielli R. Response to cadmium in higher plants. Environ. Exp. Bot. 41, 1999, 105–130.
CrossRef - Wagner GJ, Accumulation of cadmium in crop plants and its consequences to human health. Adv. Agron. 51, 1993, 173–212.
CrossRef - Duxbury T, Ecological aspects of heavy metal responses in microorganisms. Adv. Microb. Ecol. 8, 1985, 185-235.
CrossRef - Bingham FT, Page AL, Mahler RJ, Ganje TJ. Yield and cadmium accumulation of forage species in relation to cadmium content of sludge-amended soil. J. Environ. Qual. 5, 1976, 57-59.
CrossRef - Mukherjee A, Sharma A, Talukdeer G. Effects of cadmium on cellular systems in higher organisms. The Nucleus 27, 1984,121-139.
- Obata H, Umebayashi M. Effects of cadmium on mineral nutrient concentrations in plants differing in tolerance for cadmium. J. Plant Nutr. 20, 1997, 97-105.
CrossRef - Das P, Samantaray S, Rout GR. Studies on cadmium toxicity in plants: a review. Environ. Pollution 98, 1997, 29-36.
CrossRef - Moral R, Palacios G, Gomez I, Navarro-Pedreno J, Mataix J. Distribution and accumulation of heavy metals (Cd, Ni and Cr) in tomato plant. Fresenius Environ. Bull. 3, 1994, 395-399.
- Baker DE, Senef JP. In: Alloway BJ (ed), Heavy metals in soils. Blackie Academic and Professional, London. 1995, 179-205.
CrossRef








