Eko Fuji Ariyanto1,2* , Salsabila Irbah Nurazizah3
, Salsabila Irbah Nurazizah3 , Yunisa Pamela1,2
, Yunisa Pamela1,2 , Tenny Putri Wikayani4
, Tenny Putri Wikayani4 , Nurul Qomarilla4
, Nurul Qomarilla4 , Afiat Berbudi5
, Afiat Berbudi5 and Enny Rohmawaty6
and Enny Rohmawaty6
1Division of Biochemistry and Molecular Biology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jl. Raya Bandung Sumedang, KM. 21, Sumedang, Indonesia, 45363
2Research Center for Medical Genetics, Faculty of Medicine, Universitas Padjadjaran, Jl. Eyckman No. 38, Bandung, Indonesia, 40161
3Faculty of Medicine, Universitas Padjadjaran, Jl. Raya Bandung Sumedang, KM.21, Sumedang, Indonesia, 45363
4Cell Culture Laboratory, Faculty of Medicine, Universitas Padjadjaran, Jl. Eckyman No. 38, Bandung, Indonesia, 40161
5Division of Parasitology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jl. Raya Bandung Sumedang, KM. 21, Sumedang, Indonesia, 45363
6Division of Pharmacology and Therapy, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jl. Raya Bandung Sumedang, KM. 21, Sumedang, Indonesia, 45363
Corresponding Author E-mail: fuji@unpad.ac.idDOI : https://dx.doi.org/10.13005/bpj/1990
Abstract
Introduction: Obesity and overweight are considered as one of the most serious problems worldwide. The stimulating factor of preadipocyte differentiation is known to be inhibited by curcumin. This study aims to examine whether lipid accumulation in adipocyte differentiation process using 3T3-L1 cell lines is affected by Curcuma longa, one of the main natural sources of curcumin. Methods: This experimental study used 3T3-L1 preadipocyte cell line. C. longa extracts with different concentrations (0, 5, 10, and 20 ppm) were added. The cells were cultured in DMEM medium containing 10% FBS. Cells were then induced by MDI (methylisobutylxanthine, dexamethasone, insulin) that initiated the adipocyte differentiation process. Medium was then replaced every 2 days. Insulin was added to the cells in the first medium replacement process to optimize glucose uptake and lipogenesis during differentiation process. The optimal adipocyte differentiation in control concentration (0 ppm) was obtained in day 12. Oil Red O solution were used to stain the cells and the cells were observed using microscope. Spectrophotometer was used to measure absorbance value at 550 nm wavelength. Result: Treatment of 5 ppm of C. longa extract significantly increased lipid accumulation as compared to control group (p=0.0151). Addition of 10 ppm C. longa extract increased lipid accumulation (p>0.05), while treatment of 20 ppm C. longa extract reduced lipid accumulation (p>0.05). Conclusion: A significantly increased lipid accumulation was observed following a low dose of C. longa extract. On the other hand, high concentration of C. longa extract decreased lipid accumulation although not statistically significant.
Keywords
3T3-L1 Cell Line, Adipocyte Differentiation, Curcuma longa, Lipid accumulation
Download this article as:| Copy the following to cite this article: Ariyanto E. F, Nurazizah S. I, Pamela Y, Wikayani T. P, Qomarilla N, Berbudi A, Rohmawaty E. Effect of Curcuma longa Extract on Lipid Accumulation during Adipocyte Differentiation Using 3T3-L1 Cell Line. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Ariyanto E. F, Nurazizah S. I, Pamela Y, Wikayani T. P, Qomarilla N, Berbudi A, Rohmawaty E. Effect of Curcuma longa Extract on Lipid Accumulation during Adipocyte Differentiation Using 3T3-L1 Cell Line. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/2Dwsyyw |
Introduction
Accumulation of adipose tissue sufficient to cause health problems is called obesity.3 Obesity and overweight are still considered as the main health problems in the world. Since 1975, obesity has tripled worldwide. More than 1.9 billion adults over the age of 18 are overweight, and of this number, 650 million are obese based on World Health Organization data.1 Obesity, in Indonesia, has become major health issues. It is higher in females than males with the prevalence of 28% and 23.1%, respectively.2
The risk of type 2 diabetes and cardiovascular disease is increased in obese people.4 Obesity is also a risk of metabolic syndrome characterized by insulin resistance, hyperinsulinemia, visceral or intraabdominal adipose buildup, glucose intolerance, low HDL cholesterol levels, hypertension, and hyperinsulinemia.3 Chronic diseases including diabetes, cardiovascular disease, and cancer also occur mainly due to obesity as risk factor.5
In obesity, fat cells in adipocyte tissue can be up to 5 times the normal amount. Preadipocytes in adipose tissue are stimulated by excess nutrients for a long time so that they proliferate and differentiate into mature fat cells. The number of adipocytes increases due to an increase in the size and number of fat cells by hypertrophy and hyperplasia.6 The process of differentiation from preadipocytes to mature adipocytes is called adipogenesis.7 Adipocyte precursor cells can originate from multipotent mesenchyme or fibroblast-like cells.8 In adipogenesis process, there is a change from fibroblast-like preadipocytes or mesenchymal precursor cells that have not differentiated into mature insulin-responsive and lipid-laden adipocyte cells.9, 10
Cell lines that are often used to determine the performance of lipid metabolism, hormone action, and adipogenesis are 3T3-L1 cell lines. In the 3T3-L1 cell line differentiation process there is a change from fibroblasts to cells that are similar to lipid-containing adipocytes.11
Curcumin is known to be able to inhibit the stimulating factor in preadipocyte differentiation of PPARg and C/EBPβ and also increase the inhibitory differentiation factor like Wnt/β-catenin.12 Previous study suggested that the anti-adipogenic effect on adipocyte differentiation is seen in low dose curcumin especially in doses of 15 µM. Cell death/cell apoptosis occurs with high doses by 30 or 50 µM.13
Curcuma longa is one of the main natural sources of curcumin. This study aims to examine the effect of C. longa extract on lipid accumulation in the adipocyte differentiation process using 3T3-L1 cell lines.
Materials and Methods
Research Design
This research was an in vitro experimental laboratory research. The purpose of this study is to determine whether C. longa extract can inhibit lipid accumulation in the differentiation process of adipocytes using 3T3-L1 cell lines.
C. longa Extraction
First, C. longa was selected, washed, and the skin was peeled. Then C. longa was cut into small pieces, dried, and blended until became delicate. Twenty grams of C. longa was put into the flask and was extracted with ethanol.
The filtrate from the first extraction process was put into a distillation flask to continue the process in separating C. longa from the solvent. The distillation process was performed at 80oC for 4 hours to obtain solvent and residue. The remaining water and ethanol that was still present in residue was removed by drying it at 100oC for 4 hours in the oven. The C. longa extract that had been obtained was dissolved to produce concentrations of 5, 10, and 20 ppm.
Culture and Differentiation of 3T3 Cell Line
In the first step of this study, 3T3-L1 preadipocytes were cultured in DMEM (Sigma, USA) medium containing 10% FBS (Sigma, USA) at 37oC, 5% CO2. The cells were cultured for 48 hours or until 100% confluent. Day 0 of adipocyte differentiation was begun when cells in DMEM medium were induced by MDI differentiation cocktail consisting of 0.5 mM methylisobutylxanthine (Sigma, USA), 1 µM dexamethasone (Sigma, USA), and 10 µg/ml insulin (Sigma, USA). Four-well plates were used in this experiment. Each well was added with different concentrations of C. longa extracts (0, 5, 10, and 20 ppm), and cells were then incubated for 48 hours.
After 48 hours, in day 3, medium was then replaced with DMEM containing 10 µg/ml insulin to optimize glucose uptake into the cells and lipogenesis during differentiation process. The cells were incubated for 48 hours. In day 5, medium was then replaced with DMEM. In day 7, the culture medium was replaced again with DMEM. In day 12, the optimal adipocyte differentiation was obtained in concentration control (0 ppm).
Oil Red O Staining
Before staining, the cells were rinsed with PBS (Sigma, USA) twice and fixed with 4% formaldehyde for 10 minutes and formaldehyde was then removed. The cells were rinsed again with PBS twice then were washed by isopropanol 60% for 1 minute. After rinsing and washing process, cells were stained with Oil Red O (Sigma, USA) solution for 15-20 minutes. The cells were then rinsed again with PBS twice. Lipid accumulation was observed using microscope as red stain.
Oil Red O Quantification
The cells were incubated with isopropanol 100% for 5 minutes to dissolve the red stain. The absorbance value was measured at 550 nm wavelength using a spectrophotometer and compared between the wells containing different C. longa extract concentrations.
Statistical analysis
The data were analyzed using graph pad 8.0.1 with one-way ANOVA, followed by Tukey’s post-hoc test and presented in mean±SD.
Results and Discussion
Lipid accumulation in macroscopic and microscopic pictures was indicated by the red stain. The comparison between control and cells treated with different concentration of C. longa extract showed that concentration of 5 ppm appeared to have higher lipid accumulation than control, 10 ppm had comparable lipid accumulation as control group, while 20 ppm concentration showed a reduction of lipid accumulation (Figure 1).
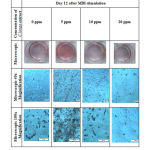 |
Figure 1: Macroscopic and microscopic pictures of 3T3-L1 cell line after Oil Red O staining. |
A quantitative analysis was performed to confirm these findings (Figure 2). The results of absorbance measurement, which represented Oil Red O-stained lipid accumulation, showed that 5 ppm sample had significantly higher absorbance as compared with control group (Figure 2). At 10 ppm concentration, absorbance was showed to be higher than control group despite red-stained lipid accumulation appeared to be comparable with control in Figure 1. The absorbance of 20 ppm concentration only showed a slight decrease as compared with control group which did not very correspond to the obvious difference in red stain observed in Figure 1. Statistical analysis showed significant data between control (0 ppm) and 5 ppm (p=0.0151), 10 ppm and 20 ppm (p=0.0487), and 5 ppm and 20 ppm (p=0.0057). The comparison of control group (0 ppm) to 10 ppm, control to 20 ppm, 5 ppm to 10 ppm, and 5 ppm to 20 ppm showed non-significant results (p>0.05) (Figure 2).
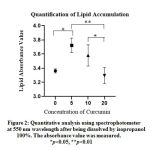 |
Figure 2: Quantitative analysis using spectrophotometer at 550 nm wavelength after being dissolved by isopropanol |
Our study indicated that lower dose of C. longa extract significantly increased lipid accumulation, while, on the contrary, higher concentration of C. longa extract decreased lipid accumulation even though statistically was not significant.
Previous study examining the effect of curcumin in cell adipogenic differentiation in mouse showed that curcumin dosage of 2-10 µm did not obtain substantial morphological changes in 3T3-L1, otherwise cell death occurred in 25 µm dosage.14 This study supported the increasing effect of lipid accumulation data in 5 ppm dosage and decreasing effect of lipid accumulation in 20 ppm. The other reason which might explain increasing effect of 5 ppm C. longa extract in lipid accumulation can be due to type of mouse used in establishing 3T3-L1 cell line. The loss of Nrf2 in C57BL6 mice was seen to impair adipocyte differentiation. Curcumin was predicted to activate Nrf2 so that stabilized C/EBP and PPARC. That mechanism can promote adipocyte differentiation.12
One possible mechanism that can explain the biphasic effect of C. longa extract on lipid accumulation, depending on concentration, is the various compounds present in C. longa extract. C. longa extract was reported to contain numerous active compounds including curcumin, volatile sesquiterpenes, monoterpenes and other volatile substances while the composition might vary due to differences in species, climate and geographical location.15 The increased of lipid accumulation at sample added with 5 ppm extract in this study might be due to the effect of curcuminoids and sesquiterpenoids. These compounds might induce glucose uptake from the culture medium into adipocytes in our experiments and enhance lipogenesis for lipid accumulation. This hypothesis might be in line with previous study which revealed that curcuminoids and sesquiterpenoids inhibited the increase of blood glucose level.16 Another possibility is that addition of low concentration of curcumin might also increase insulin sensitivity as previously reported in a study conducted in C2C12 muscle cell line.17 On the contrary, high concentration of curcumin might induce apoptosis in preadipocytes, and, subsequently, inhibit lipid accumulation as previously reported.13 Moreover, it was also unraveled that metal complexes of curcuminoids might accumulate in cytoplasm and induce apoptosis.18
In this study, we found discrepancy between macroscopic and microscopic pictures and quantitative absorbance level of Oil Red O-stained lipid accumulation. The absorbance of lipid accumulation at 10 ppm was higher than control group (Figure 2) although the red stain in macroscopic and microscopic pictures was comparable with control group (Figure 1). In addition, the absorbance of 20 ppm group only showed a slight decrease as compared with control group (Figure 2), while the difference between these groups in macroscopic and microscopic pictures in Figure 1 was very obvious. This discrepancy can occur due to several factors such as differences in the protocol used in the elution of dye and absorbance measurement.19 During dye elution, we incubated the samples with 100% isopropanol without shaking for 5 minutes to avoid evaporation of isopropanol. We then measured the absorbance at 550 nm. Kraus et al suggested that the optimized protocol was to incubate the samples with 100% isopropanol for 10 minutes on an orbital shaker and to measure the absorbance at 510 nm.19
The limitation of this study is that we did not measure mRNA expression of adipogenic genes such as Pparg, Fabp4 and Adipoq to confirm the differences in the lipid accumulation and degree of differentiation among samples.
Conclusion
This study showed that the addition of C. longa extract in low dose increased lipid accumulation significantly. On the other hand, high concentration of C. longa extract decreased lipid accumulation although not statistically significant.
Acknowledgement
We would like to express our gratitude to Dr. Afiat Berbudi for the kind gift of 3T3-L1 cell line and to Universitas Padjadjaran for the research fund. This study was conducted at Cell Culture Laboratory, Faculty of Medicine, Universitas Padjadjaran, Indonesia.
Conflict of Interest
The authors declared no conflict of interest in this study.
Funding Source
This research was funded by Universitas Padjadjaran Research Grant, No. 3855/UNG.C/LT/2019 for EFA.
References
- World Health Organization. Obesity and overweight 2018 [cited 2019 10 Nov]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Harbuwono DS, Pramono LA, Yunir E, Subekti I. Obesity and central obesity in Indonesia: evidence from a national health survey. Medical Journal of Indonesia. 2018;27(2):114-20.
CrossRef - Kumar V, Abbas AK, Aster JC. Robbins and cotran pathologic of disease. 9th ed. Philadelphia, PA: Elsevier Saunders; 2015. p. 444-50.
- Scherer PE, Hill JA. Obesity, Diabetes, and Cardiovascular Diseases: A Compendium. Circ Res. 2016;118(11):1703-5.
CrossRef - World Health Organization. Obesity [cited 2019 10 Nov]. Available from: https://www.who.int/topics/obesity/en/.
- Ferrier DR. Lippincott’s illustrated reviews: biochemistry. 6th ed. Philadelphia, PA: Wolters Kluwer Health; 2014. p. 350-1.
- Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20(4):242-58.
CrossRef - Hepler C, Vishvanath L, Gupta RK. Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev. 2017;31(2):127-40.
CrossRef - Ruiz-Ojeda FJ, Rupérez AI, Gomez-Llorente C, Gil A, Aguilera CM. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int J Mol Sci. 2016;17(7):1040.
CrossRef - Fessler MB. CO(2) as a Potential Obesogen: A Gas That Will Stick to Your Ribs. Am J Respir Cell Mol Biol. 2017;57(5):499-500.
CrossRef - Morrison S, McGee SL. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte. 2015;4(4):295-302.
CrossRef - Bradford PG. Curcumin and obesity. Biofactors. 2013;39(1):78-87.
CrossRef - Wu LY, Chen CW, Chen LK, Chou HY, Chang CL, Juan CC. Curcumin Attenuates Adipogenesis by Inducing Preadipocyte Apoptosis and Inhibiting Adipocyte Differentiation. Nutrients. 2019;11(10).
CrossRef - Tian L, Song Z, Shao W, Du WW, Zhao LR, Zeng K, et al. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death & Disease. 2018;8(1):e2559-e.
CrossRef - Dosoky NS, Setzer WN. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients. 2018;10(9): 1196.
CrossRef - Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem. 2005;53(4): 959-63.
CrossRef - Mohiti-Ardekani J, Asadi S, Ardakani AM, Rahimifard M, Baeeri M, et al. Curcumin increases insulin sensitivityin C2C12 muscle cells via AKT and AMPK signaling pathways. Cogent Food & Agriculture. 2019;5(1): 1577532.
CrossRef - Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives – A review. Journal of Traditional and Complementary Medicine. 2017;7: 205-33.
CrossRef - Kraus NA, Ehebauer F, Zapp B, Rudolphi B, Kraus BJ, et al. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte. 2016;5(4): 351-8.
CrossRef







