Manuscript accepted on :
Published online on: --
Plagiarism Check: Yes
Reviewed by: Daniela Serban
Second Review by: Manal Hassanein
Chui-Man Lo* , Jie Han
, Jie Han and Emily S. W. Wong
and Emily S. W. Wong
Department of Science, School of Science and Technology, The Open University of Hong Kong, Ho Man Tin, Kowloon, Hong Kong SAR.
Corresponding Author E-mail: cmlo@ouhk.edu.hk
DOI : https://dx.doi.org/10.13005/bpj/2003
Abstract
Aromatheraphy refers to the application of essential oils to treat diseases. Essential oils come from natural plants, with characteristic odor. Apart from aromatherapy, they had been used for thousands of years in many home products, such as cosmetic and mosquito repellents. Due to their different active ingredients, each essential oil has slightly difference in their functions. For example, Chamomile oil extracted from Chamomilla recutita can be used in anti-ulcer, anti-inflammatory, antimicrobial and has a function of sedation; Citronella oil extracted from Cymbopogon nardus is mainly used as mosquito repellent; Jasmine oil extracted from Jasminum officinale can be used for antidepressant and antiseptic; and Geranium oil extracted from Pelargonium graveolens can reduce inflammation, treat acne and alleviate anxiety. In our study, the essential oils were extracted from the corresponding plants by either steam distillation or Soxhlet extraction, and the chemical analysis of their active ingredients were performed by GC-MS. Most of the components in essential oils belong to monoterpenoids, sesquiterpenoids or oxygenated terpenes.
Keywords
Aromatheraphy; Essential oil; Steam distillation; Soxhlet extraction; GC-MS analysis
Download this article as:| Copy the following to cite this article: Lo C. M, Han J, Wong E. S. W. Chemistry in Aromatherapy – Extraction and Analysis of Essential Oils from Plants of Chamomilla recutita, Cymbopogon nardus, Jasminum officinale and Pelargonium graveolens. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Lo C. M, Han J, Wong E. S. W. Chemistry in Aromatherapy – Extraction and Analysis of Essential Oils from Plants of Chamomilla recutita, Cymbopogon nardus, Jasminum officinale and Pelargonium graveolens. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/2GnGA7l |
Introduction
Many essential oils were found to have the bioactivities such as antioxidant, antiviral, antibacterial, antifungal, anti-inflammatory, antidiabetic, antimutagenic and anticarcinogenic.1-4 The term aromatheraphy describes the application of essential oils to treat diseases.5,6 Aromatheraphy involves the massage of aromatic plant extracts into the skin7 or/and inhalation of aromas8 during the therapy,9 and they can be applied on health care settings.10 It has the function to alter human health, mood, mind and cognitive function.8 For examples, anxiety disorders are usually treated pharmacologically and psychologically in conventional cases.11 However, the medicine used in traditional pharmacological treatments are usually have many side-effects12 and they may be addictive.13 Although the effect in aromatherapy is not easy to be measured quantitatively in psychological, mental, spiritual, and social aspects, they are considered to be more safe and have less adverse effects when comparing with conventional drugs.14 Hence, it is becoming popular as an alternative treatment in relieving anxiety symptoms, as aromatheraphy can produce a mild and transient anxiolytic effect.15 For examples, Tsang et al. reported that aromatheraphy can be applied for quelling of anxiety symptoms without adverse effects, and it can be applied as complementary therapy.16 Kutlu et al. reported the study on students with lavender inhalation to reduce examination anxiety.17 Lehrner et al. reported on the use of odor in lavender and orange can alter emotional states and diminish patients’ anxiety in dental office.18 Hadfield reported the use of aromatheraphy massage can affect human autonomic nervous system, and it can reduce the anxiety from cancer patients having malignant brain tumour.19 In addition, apart from reducing anxiety, some of the constituents in essential oils, such as b-caryophyllene,20 carvacrol,21 citral,22 geraniols,23,24 a-humulene,25 D-limonene,26 myrcene,27,28 thymol29,30 and perillyl alcohol,31 were reported to have cytotoxic effect to cancer cell lines.32-34
Essential oils can be regarded as “concentrated hydrophobic liquid containing volatile aroma compound distilled from plants”, as described according to International Organization for Standardization.35 They are complex mixtures belong to the class of terpenes and terpenoids with characteristic aroma that originate from the secondary metabolism of the plants.36 Essential oils can be classified by their difference in extracted parts of plants, odor and functional groups. Extracted parts of plants include leaves, flowers, stems, barks, resins, fruits, roots and seeds.2,37 The odor (also known as aroma or scent) can be classified into floral, citrus, woody, spicy, grassy, minty, pungent, etc.38-40 Functional groups of essential oils can be categorized into monoterpenes, sesquiterpenes, monoterpenols, sesquiterpenols, diterpenols, phenol, phenyl methyl ether, aldehydes, ketones, esters, phenolic compounds and oxides.3,32,41 Most of the components in essential oils belong to terpenoid which are built up from different orientations of isoprene units with the structure CH2=CH–C(CH3)=CH2. Different number of isoprene units can contribute the terpenoid with general formula of (C5H8)n. If there is only one isoprene unit (n = 1), it is called hemiterpenoid with the chemical formula of C5H8. When there are two isoprene units (n = 2), they belong to monoterpenoids (C10H16). For n = 3, they are sesquiterpenes (C15H24). Some terpenoid appear as cyclic structures while some of them are acyclic. Some examples of terpenoids with their chemical structures were shown in Table 1.
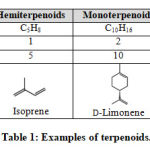 |
Table 1: Examples of terpenoids. |
Apart from the above terpenoid hydrocarbons in Table 1, some oxygenated terpenoids are resulted from additional rearrangements and oxidations. They contain functional groups such as alcohol, aldehyde, and ketone, etc. Some examples of their chemical structures were shown in Table 2.
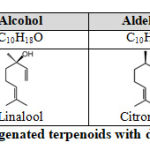 |
Table 2: Examples of oxygenated terpenoids with different function groups. |
Different essential oils have slightly difference in their functions, as their components contain different functional groups. For instance, Chamomile oil has anti-inflammatory and antimicrobial properties, and has a function of sedation.42-46 Citronella oil can repel mosquitoes,47-52 and it was also found to have antimicrobial activities against several oral pathogens.53 Jasmine oil has the function of antidepressant and antiseptic.54-58 Geranium oil can treat acne, reduce inflammation and alleviate anxiety.59-64
There are various methods for extraction of essential oils from natural plants, including steam distillation,65,66 solvent extraction,67,68 Soxhlet extraction,66,69 supercritical fluid extraction,70,71 enfleurage72,73 and cold pressing (expression).74 Steam distillation is a common method which involves the evaporation of volatile components from the plant at a lower temperature with the presence of steam.75 The essential oil can be collected after condensation of the vapour together with steam, and then isolated with water.76 Alternatively, Soxhlet extraction is another common method for obtaining essential oil from plants. Solvent in the flask is heated up and vaporize, and then condense and drip back into the sample thimble. Repeated extraction can be proceeded with the use of the same batch of solvent.69 In our study, we used steam distillation for the extraction of Chamomile, Jasmine and Geranium oils from Chamomilla recutita, Jasminum officinale and Pelargonium graveolens respectively, and Soxhlet extraction for isolation of Citronella oil from Cymbopogon nardus.
In the chemical identification of the extracted essential oils, the most common instrumentation is Gas Chromatography-Mass Spectrometry (GC-MS).36 It is suitable for the volatile samples which can vaporized on heating. The gas chromatography part can separate the components in analyte by the partition between gaseous mobile phase and stationary phase in different retention times. All the components are finally eluted out and identify by the detector. The detector is a mass spectrometer which can breakdown the molecules into ionized fragments and detect these fragments in their characteristic mass-to-charge (m/z) ratio.77
Material and Methods
In our project, four types of common essential oils were chosen for our study. Chamomile, Jasmine and Geranium oils were extracted from dried flowers of Chamomilla recutita, dried flowers of Jasminum officinale and fresh leaves of Pelargonium graveolens respectively by steam distillation. Citronella oil was extracted from fresh leaves and stems of Cymbopogon nardus by Soxhlet extraction. Their chemical components were analyzed by Gas Chromatography-Mass Spectrometry (GC-MS).
Extraction of Essential Oils
General Procedure for Steam Distillation
Flowers or leaves from the plant material (~40 g) was cut and grinded into small pieces with a juice blender. The sample was placed inside a round-bottomed flask, which connected with the distillation apparatus. Water (200 mL) was added inside the round-bottomed flask to generate steam. The sample was heated to 200 oC and distilled for 6 hours. The oil-containing distillate was then collected and dried over anhydrous magnesium sulfate (MgSO4) with further separation by centrifuging at 1300 rpm for 30 minutes. The essential oil was then transferred to an amber glass vial for further analysis.
Steam Distillation of Chamomile: 38.52 g dried flowers of Chamomilla recutita produced 0.23 g blue colour of Chamomile oil in 0.6% recovery.
Steam Distillation of Jasmine: 42.46 g dried flowers of Jasminum officinale produced 0.17 g pale yellow colour of Jasmine oil in 0.4% recovery.
Steam Distillation of Geranium: 40.28 g dried leaves of Pelargonium graveolens produced 0.06 g pale yellow colour of Geranium oil in 0.15% recovery.
Procedure for Soxhlet Extraction of Citronella
Fresh leaves and stems of Cymbopogon nardus (7.94 g) was cut and grinded into small pieces with a juice blender, and then placed into Whatman cellulose extraction thimble (22 mm internal diameter × 80 mm external length). The sample containing thimble was then placed into a Soxhlet’s apparatus. The solvent was n-hexane (250 mL) and the extraction was carried out at 150 oC for 24 hours. The solvent was evaporated at reduced pressure using rotary evaporatory. The extracted essential oil was transferred to an amber glass vial for further analysis. 1.35 g of pale yellow colour of citronella oil was obtained, with 17% recovery.
Analysis of Essential Oils
Preparation of Standard Solutions
Separated sets of standard solutions were prepared for each type of essential oil for GC-MS analysis. 10 mL of the extracted crude oils of Chamomile, Citronella, Jasmine and Geranium were diluted to prepare 1.00 mL of standard solutions with chloroform, ethanol, dichloromethane and n-hexane respectively.
General Procedure for GC-MS Analysis
GC-MS analysis was performed on Agilent 7890B GC system equipped with 5977B mass spectrometer and PAL RSI 85 auto-sampler. Agilent HP-5MS UI column (30 m x 0.25 mm) was used and Helium was used as the carrier gas. The sample injection volume is 1 µL, with flow rate of 1 mL/min for Chamomile, Jasmine and Geranium, and 3 mL/min for Citronella. The selected m/z range is 30 to 550. For the temperature programming of Chamomile, it was hold at 45°C for 2 mins, then 1.5°C/min ramp to 100°C, then 2°C/ min ramp to 200°C and finally 10°C/min ramp to 250°C and hold for 30 mins. For Citronella, it was hold at 50°C for 1 min, then rise 10°C/min to 100°C, 20°C/min to 220°C, and then hold at 220°C for 10 mins. For Jasmine and Geranium, it was programmed from 60 °C, then rise 3°C/min to 240°C.
Results and Discussion
Results indicated that most of the components in Chamomile, Citronella, Jasmine, and Geranium essential oils are all belong to the group of terpenoids, including monoterpenoids, sesquiterpenoids and oxygenated terpenes. The GC-MS analysis of each essential oil is shown below.
GC-MS Analysis for Chamomile (Chamomilla Recutita)
Figure 1 is the GC-MS Chromatogram for chamomile oil (extracted from Chamomilla Recutita), with the major signals assigned with the names and chemical structures of the corresponding components. Their retention times and IUPAC names were shown in Table 3. Chamomile oil mainly contains a-farnesene (C15H24), spathulenol (C15H24O), a-bisabolol (C15H26O), a-bisabolol oxide B (C15H26O2), chamazulene (C14H16) and a-bisabolol oxide A (C15H26O2). a-Farnesene has the chemical formula of C15H24, which belongs to sesquiterpenoids. The other components C15H24O, C15H26O and C15H26O2 are oxygenated terpenes.
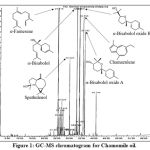 |
Figure 1: GC-MS chromatogram for Chamomile oil. |
Table 3: Main components in Chamomile oil by GC-MS analysis
| Retention Time (min) | Component | Chemical
Formula |
|
| Common name | IUPAC name | ||
| 27.426 | a-Farnesene | 3,7,11-trimethyl-1,3,6,10-dodecatetraene | C15H24 |
| 30.020 | Spathulenol | (1aR,4aR,7S,7aR,7bR)-1,1,7-Trimethyl-4-methylidene-1a,2,3,4a,5,6,7a,7b-octahydrocyclopropa[h]azulen-7-ol | C15H24O |
| 32.309 | a-Bisabolol | 6-methyl-2-(4-methylcyclohex-3-en-1-yl)hept-5-en-2-ol | C15H26O |
| 32.919 | a-Bisabolol oxide B | 2-[5-Methyl-5-(4-methylcyclo-hex-3-en-1-yl)tetrahydrofuran-2-yl]propan-2-ol | C15H26O2 |
| 35.556 | Chamazulene | 7-Ethyl-1,4-dimethylazulene | C14H16 |
| 36.162 | a-Bisabolol oxide A | (3R)-2,2,6-Trimethyl-6-(4-methylcyclohex-3-en-1-yl)oxan-3-ol | C15H26O2 |
GC-MS Analysis for Citronella (Cymbopogon nardus)
GC-MS Chromatogram for Citronella oil (extracted from Cymbopogon nardus) was shown in Figure 2, with their retention times and IUPAC names shown in Table 4. The major components in Citronella oil are D-limonene (C10H16), citronellal (C10H18O), citronellol (C10H20O), geraniol (C10H18O) and eugenol (C10H12O2). D-Limonene has a chemical formula of C10H16, which belongs to the class of monoterpenoids. The other components citronellal, citronellol, geraniol and eugenol are oxygenated terpenes.
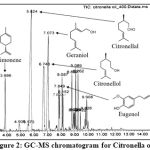 |
Figure 2: GC-MS chromatogram for Citronella oil. |
Table 4: Main components in Citronella oil by GC-MS analysis
| Retention Time (min) | Component | Chemical
Formula |
|
| Common name | IUPAC name | ||
| 3.886 | D-Limonene | 1-Methyl-4-(prop-1-en-2-yl)cyclohex-1-ene | C10H16 |
| 5.824 | Citronellal | 3,7-Dimethyl-oct-6-en-1-al | C10H18O |
| 6.743 | Citronellol | 3,7-Dimethyloct-6-en-1-ol | C10H20O |
| 7.073 | Geraniol | (2E)-3,7-Dimethyl-2,6-octadien-1-ol | C10H18O |
| 7.892 | Eugenol | 2-Methoxy-4-(prop-2-en-1-yl)phenol | C10H12O2 |
GC-MS Analysis for Jasmine (Jasminum officinale)
GC-MS Chromatogram for Jasmine oil (extracted from Jasminum officinale) was shown in Figure 3, with their retention times and IUPAC names shown in Table 5. In Jasmine oil, the major components are benzyl alcohol (C7H8O), linalool (C10H18O), benzyl acetate (C9H10O2), indole (C8H7N), methyl anthranilate (C8H9NO2), a-farnesene (C15H24) and 3-hexenyl benzoate (C13H16O2). a-Farnesene belongs to the class of sesquiterpenoids, while linalool belongs to oxygenated terpenes. Apart from the terpene family, Jasmine oil also contains some other non-terpene aromatic compounds, and some of them are also contain the ester functional group, such as benzyl acetate, methyl anthranilate and 3-hexenyl benzoate. These compounds have pleasant odour for antidepressant and antiseptic function
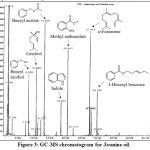 |
Figure 3: GC-MS chromatogram for Jasmine oil. |
Table 5: Main components in Jasmine oil by GC-MS analysis
| Retention Time (min) | Component | Chemical
Formula |
|
| Common name | IUPAC name | ||
| 8.266 | Benzyl alcohol | Phenylmethanol | C7H8O |
| 10.660 | Linalool | 3,7-Dimethylocta-1,6-dien-3-ol | C10H18O |
| 13.205 | Benzyl acetate | Benzyl ethanoate | C9H10O2 |
| 18.493 | Indole | 1H-indole | C8H7N |
| 20.507 | Methyl anthranilate | Methyl 2-aminobenzoate | C8H9NO2 |
| 27.413 | a-Farnesene | 3,7,11-Trimethyl-1,3,6,10-dodecatetraene | C15H24 |
| 29.765 | 3-Hexenyl benzoate | (E)-Hex-3-enyl benzoate | C13H16O2 |
GC-MS Analysis for Geranium (Pelargonium graveolens)
GC-MS Chromatogram for Geranium oil (extracted from Pelargonium graveolens) was shown in Figure 4, with their retention times and IUPAC names shown in Table 6. In Geranium oil, the major components are linalool (C10H18O), isomenthone (C10H18O), a-Terpineol (C10H18O), b-citral (C10H16O), geraniol (C10H18O), a-citral (C10H16O), b-bourbonene (C15H24), caryophyllene (C15H24), d-cadinene (C15H24), 10-epi-γ-eudesmol (C15H26O2) and geranyl tiglate (C15H24O2). There are many isomers of C10H18O and C15H24. b-Bourbonene, caryophyllene and d-cadinene have the chemical formula of C15H24, which belongs to the class of sesquiterpenoids. The other components belong to oxygenated terpenes.
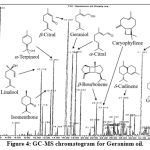 |
Figure 4: GC-MS chromatogram for Geranium oil. |
Table 6: Main components in Geranium oil by GC-MS analysis
| Retention Time (min) | Component | Chemical
Formula |
|
| Common name | IUPAC name | ||
| 10.719 | Linalool | 3,7-Dimethyl-octa-1,6-dien-3-ol | C10H18O |
| 12.813 | Isomenthone | (2S,5S)-5-Methyl-2-propan-2-ylcyclohexan-1-one | C10H18O |
| 13.257 | a-Terpineol | 2-(4-methylcyclohex-3-en-1-yl)propan-2-ol | C10H18O |
| 16.097 | b-Citral | 3,7-Dimethylocta-2,6-dienal | C10H16O |
| 17.144 | Geraniol | 3,7-Dimethyl-2,6-octadien-1-ol | C10H18O |
| 17.953 | a-Citral | 3,7-Dimethyl-2,6-octadien-1-al | C10H16O |
| 22.413 | b-Bourbonene | (1S,2R,6S,7R,8S)-1-methyl-5-methylidene-8-propan-2-yltricyclo[5.3.0.0]decane | C15H24 |
| 23.812 | Caryophyllene | (1R,4E,9S)-4,11,11-Trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | C15H24 |
| 27.989 | d-Cadinene | (1S,8aR)-4,7-dimethyl-1-propan-2-yl-1,2,3,5,6,8a-hexahydronaphthalene | C15H24 |
| 31.661 | 10-epi-γ-Eudesmol | 2-[(2R,4aS)-4a,8-Dimethyl-1,2,3,4,4a,5,6,7-octahydro-2-naphtalenyl]-2-propanol | C15H26O |
| 34.689 | Geranyl tiglate | [(2E)-3,7-dimethylocta-2,6-dienyl] (E)-2-methylbut-2-enoate | C15H24O2 |
Conclusion
Essential oils of Chamomile, Citronella, Jasmine, and Geranium were extracted from the plants of Chamomilla recutita, Cymbopogon nardus, Jasminum officinale and Pelargonium graveolens respectively, using either steam distillation or Soxhlet extraction. Their chemical components were identified by GC-MS. They were found to contain different types of terpenoids (e.g. monoterpenoids, sesquiterpenoids and oxygenated terpenes) as the active ingredients. Due to the presence of these terpenoids with different structures, each type of essential oil has slightly difference in their functions.
Acknowledgement
We would like to express our gratitude to The Open University of Hong Kong for the financial support in this project (R6265 and R6315).
Conflict of Interest
We declare that no conflict of interest was involved in this project.
Funding Source
We would like to thank The Open University of Hong Kong for the financial support in this project (R6265 and R6315).
Reference
- Edris, A. E. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review. Phytotherapy Research, 2007, 21(4): 308-323.
- Shaaban, H. A. E., El-Ghorab, A. H., Shibamoto, T. Bioactivity of Essential Oils and their Volatile Aroma Components: Review. The Journal of Essential Oil Research, 2012, 24: 203-212.
- Zuzarte M. and Salgueiro, L. M., Essential Oils Chemistry, Bioactive Essential Oils and Cancer, Springer International Publishing Switzerland, 2015, Ch. 2: 19-62.
- Sadgrove N. and Jones G., A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture, Agriculture, 2015, 5, 48–102.
- Segen J. C. Dictionary of Alternative Medicine. Stamford, Ct: Appleton and Lange, 1998.
- Ali, B., Al-Wabel, N. A., Shams, S., Ahamad, A., Khan, S. A., Anwar, F. Essential Oils Used in Aromatheraphy: A Systemic Review. Asian Pacific Journal of Tropical Biomedicine, 2015, 5(8): 601-611.
- Vickers, A, Zollman, C. ABC of Complementary Medicine Massage Therapies. BMJ 1999, 319: 1254-1257.
- Gaware, V., Nagare, R., Dhamak, K. B., Khadse, A.N., Kotade, K.B., Kashid, V.A., Laware, R. B. Aromatherapy: Art or Science. International Journal of Biomedical Research, 2013, 4: 74-83.
- Seyyed-Rasooli, A., Salehi, F., Mohammadpoorasl, A., Goljaryan, S., Seyyedi, Z., Thomson, B. Comparing the Effects of Aromatherapy Massage and Inhalation Aromatherapy on Anxiety and Pain in Burn Patients: A Single-blind Randomized Clinical Trial. Burns, 2016, 42(8): 1774-1780.
- Gattefossé, R.-M. Aromatherapy. London: C W Daniel Co Ltd, 1993.
- Schmidt, N.B., Keough, M.E., Hunter, L.R., Funk, A. P. Physical Illness and Treatment of Anxiety Disorders: A Review. Series in Anxiety and Related Disorders: Anxiety in Health Behaviors and Physical Illness. New York: Springer, 2008: 341–366.
- Lippa, A., Czobor, P., Stark, J., Beer, B., Kostakis, E., Gravielle, M., Bandyopadhyay, S., Russek, S. J., Gibbs, T. T., Farb, D. H., & Skolnick, P. Selective anxiolysis produced by ocinaplon, a GABA(A) receptor modulator. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(20): 7380-7385.
- Tyrer, P. Anxiety: A Multidisciplinary Review. London: Imperial College Press, 1999.
- Perry, N., Perry, E. Aromatherapy in the Management of Psychiatric Disorders. CNS Drugs, 2006, 20:257-280.
- Cooke, B., Ernst, E. Aromatherapy: A Systematic Review. British Journal of General Practice, 2000, 50: 493-496.
- Lee, Y.-L., Wu, Y., Tsang, H. W. H., Leung, A. Y., Cheung, W. M. A Systematic Review on the Anxiolytic Effects of Aromatherapy in People with Anxiety Symptoms. The Journal of Alternative and Complementary Medicine, 2011, 17: 101-108.
- Kutlu, A. K., Yilmaz, E., Cecen, D. Effects of Aroma Inhalation on Examination Anxiety. Teaching and Learning in Nursing, 2008, 3: 125–130.
- Lehrner, J., Marwinski, G., Lehr, S., Johren, P., Deecke, L. Ambient Odors of Orange and Lavender Reduce Anxiety and Improve Mood in a Dental Office. Physiology & Behavior, 2005, 86: 92-95.
- Hadfield, N. The Role of Aromatherapy Massage in Reducing Anxiety in Patients with Malignant Brain Tumors. International Journal of Palliative Nursing, 2001, 7(6): 279-285.
- Legault, J., Pichette, A. Potentiating Effect of 𝛽-caryophyllene on Anticancer Activity of 𝛼-humulene, Isocaryophyllene and Paclitaxel. Journal of Pharmacy and Pharmacology, 2007, 59(12): 1643–1647,.
- Jayakumar, S., Madankumar, A., Asokkumar, S., Raghunandhakumar, S., Gokula dhas, K., Kamaraj, S., Josephine Divya, M. G., Devaki, T. Potential Preventive Effect of Carvacrol Against Diethylnitrosamine-induced Hepatocellular Carcinoma in Rats. Molecular and Cellular Biochemistry, 2012, 360(1-2): 51-60.
- Chaouki, W., Leger, D. Y., Liagre, B., Beneytout, J. L., Hmamouchi, M. Citral Inhibits Cell Proliferation and Induces Apoptosis and Cell Cycle Arrest in MCF-7 Cells, Fundamental and Clinical Pharmacology, 2009, 23(5): 549–556.
- Carnesecchi, S., Schneider, Y., Ceraline J., Duranton, B., Gosse, F., Seiler, N., Raul, F. Geraniol, A Component of Plant Essential Oils, Inhibits Growth and Polyamine Biosynthesis in Human Colon Cancer Cells. Journal of Pharmacology and Experimental Therapeutics, 2001, 298(1): 197–200.
- Carnesecchi, S., Langley, K., Exinger, F., Gosse, F., Raul, F. Geraniol, a Component of Plant Essential Oils, Sensitizes Human Colonic Cancer Cells to 5-Fluorouracil Treatment. Journal of Pharmacology and Experimental Therapeutics, 2002, 301(2): 625–630.
- da Silva, S. L., Chaar, J. D. S., Figueiredo, P. D. M. S., Yano, T. Cytotoxic Evaluation of Essential Oil from Casearia sylvestris Sw on Human Cancer Cells and Erythrocytes. Acta Amazonica, 2008, 38(1), 107–112.
- Elegbede, J. A., Elson, C. E., Qureshi, A., Tanner, M. A., Gould, M. N. Inhibition of DMBA-induced Mammary Cancer by the Monoterpene D-Limonene, Carcinogenesis, 1984, 5(5): 661–664.
- Mitić-Ćulafić, D., Žegura, B., Nikolić, B., Vuković-Gaćič, B., Knežević-Vukčević, J., Filipič, M. Protective Effect of Linalool, Myrcene and Eucalyptol Against t-Butyl Hydroperoxide Induced Genotoxicity in Bacteria and Cultured Human Cells. Food and Chemical Toxicology, 2009, 47(1): 260–266.
- do Vale, T. G., Furtado, E. C., Santos Jr., J. G., Viana, G. S. B. Central Effects of Citral, Myrcene and Limonene, Constituents of Essential Oil Chemotypes from Lippia alba (mill.) N.E. Brown. Phytomedicine, 2002, 9(8): 709–714.
- Jaafari, A., Tilaoui, M., Mouse H. A., M’bark, L. A., Aboufatima, R., Chait, A., Lepoivre, M., Zyad, A. Comparative Study of the Antitumor Effect of Natural Monoterpenes: Relationship to Cell Cycle Analysis. Brazilian Journal of Pharmacognosy, 2012, 22(3): 534–540.
- Deb, D. D., Parimala, G., Saravana Devi, S., Chakraborty, T. Effect of Thymol on Peripheral Blood Mononuclear Cell PBMC and Acute Promyelotic Cancer Cell Line HL-60. Chemico-Biological Interactions, 2011, 193(1): 97–106.
- Koyama, M., Sowa, Y., Hitomi T., Lizumi, Y., Watanabe, M., Taniguchi, T., Ichikawa, M., Sakai, T. Perillyl Alcohol Causes G1 Arrest through p15INK4b and p21 WAF1/Cip1 Induction. Oncology Reports, 2013, 29(2): 779–784.
- Gautam, N., Mantha, A. K., Mittal, S. Essential Oils and their Constituents as Anticancer Agents: A Mechanistic View. BioMed Research International, Hindawi Publishing Corporation, 2014.
- Yu, J. Q., Lei, J. C., Zhang, X. Q., Yu, H. D., Tian, D. Z., Liao, Z. X., Zou, G. L. Anticancer, Antioxidant and Antimicrobial Activities of the Essential oil of Lycopus lucidus Turcz. var. hirtus Regel. Food Chemistry, 2011 (126)4: 1593–1598.
- Mitoshi, M., Kuriyama, I., Nakayama, H., Miyazato, H., Sugimoto, K., Kobayashi, Y., Jippo, T., Kanazawa, K., Yoshida, H., Mizushina, Y. Effects of Essential Oils from Herbal Plants and Citrus Fruits on DNA Polymerase Inhibitory, Cancer Cell Growth Inhibitory, Antiallergic, and Antioxidant Activities. Journal of Agricultural and Food Chemistry, 2012, 60(45):11343–11350.
- Aromatic Natural Raw Materials — Vocabulary. International Organization for Standardization, ISO 9235:2013.
- Chamorro, E. R., Zambón, S. N., Morales, W. G., Sequeira, A. F. and Velasco, G. A. Study of the Chemical Composition of Essential Oils by Gas Chromatography, Gas Chromatography in Plant Science, Wine Technology, Toxicology and Some Specific Applications. InTech, 2012: 307-324.
- Hanif, M. A., Nisar, S., Khan, G. S., Mushtaq, Z., Zubair, M. Essential Oils. Essential Oil Research, Springer Nature Switzerland AG, 2019: 3-17.
- Fortineau, A.-D. Chemistry Perfumes Your Daily Life. Journal of Chemical Education, 2004, 81(1): 45-50.
- Sbrollini, M. C. Olfactory Delights. Journal of Chemical Education, 1987, 64(9): 799-801.
- Koyama, S., Heinbockel, T. The Effects of Essential Oils and Terpenes in Relation to Their Routes of Intake and Application. International Journal of Molecular Sciences, 2020, 21: 1558-1593.
- Kumar, Y., Prakash, O., Tripathi, H., Tandon, S., Gupta, M. M., Rahman, L.-U., Lal, R. K., Semwal, M., Darokar, M. P., Khan, F. AromaDb: A Database of Medicinal and Aromatic Plant’s Aroma Molecules With Phytochemistry and Therapeutic Potentials. Frontiers in Plant Science, 2018, 9: 1081-1091.
- Ashnagar, A., Gharib Naseri, N., Alavi, S. Y. Isolation and Identification of the Major Chemical Components in the Capitula of Matricaria chamomilla Grown in Khuzestan Province of Iran. Asian Journal of Chemistry, 2009, 21(7): 4981-4986.
- Gupta, V., Mittal, P., Bansal, P., Khokra, S. L., Kaushik, D. Pharmacological Potential of Matricaria recutita – A Review. International Journal of Pharmaceutical Sciences and Drug Research, 2010, 2(1): 12-16.
- Sharafzadeh, S. and Alizadeh, O., German and Roman Chamomile, Journal of Applied Pharmaceutical Science 01, 2011, 10: 1–5.
- Avonto, C., Rua, D., Lasonkar, P. B., Chittiboyina, A. G. and Khan I. A., Identification of a compound isolated from German chamomile (Matricaria chamomilla) with dermal sensitization potential, Toxicology and Applied Pharmacology, 2017, 318: 16–22.
- Mohamadi Sorme, F., Tabarra, M., Alimadady, H., Rahimi, R., Sepidarkish, M., Karimi, M. Efficacy of Matricaria chamomilla L. in Infantile Colic: A Double Blind, Placebo Controlled Randomized Trial. Journal of Pharmaceutical Research International, 2020, 31(6): 1-11.
- Moor, S., Lenglet, A. and Hill, N. Plant-based insect repellents. Insect Repellents, Principles, Method, Tayler & Francis group, Boca raton, Florida, 2007, 283.
- Kongkaew, C., Sakunrag, I., Chaiyakunapruk, N., and Tawatsin, A. Effectiveness of Citronella preparations in preventing mosquito bites: Systematic review of controlled laboratory experimental studies. Tropical Medicine & International Health, 2011, 16(7): 802–810.
- Solomon, B., Sahle, F. F., Gebre-Mariam, T., Asres, K., Neubert, R. H. H. Microencapsulation of Citronella Oil for Mosquito-repellent Application: Formulation and in vitro permeation studies, European Journal of Pharmaceutics and Biopharmaceutics, 2012, 20: 61-66.
- Rani, N., Wany, A., Vidyarthi, A. and Pandey, D. M. Study of Citronella Leaf Based Herbal Mosquito Repellents Using Natural Binders, Current Research in Microbiology and Biotechnology, 2013, 1(3): 98–103.
- De Toledo, L. G., Ramos, M. A. D. S., Spósito, L., Castilho, E. M., Pavan, F. R.; Lopes, É. D. O., Zocolo, G. J., Silva, F. A. N., Soares, T. H., Dos Santos, A. G., Bauab, T. M., De Almeida, M. T. G. Essential Oil of Cymbopogon nardus(L.) Rendle: A Strategy to Combat Fungal Infections Caused by Candida Species. International Journal of Molecular Sciences, 2016, 17(8): 1252-1267.
- Ameliana, L., Almawadah, A., Wulandari, L. The Effect of Citronella Oil Concentration (Cymbopogon nardus (L. ) Rendle) on the Quality of Shampoo and Antifungal Activity of Candida albicans. Indonesian Journal of Pharmaceutics. (2019), 1(2): 13-17.
- Kamal, H. Z. A., Ismail, T. N. N. T., Arief, E. M., Ponnuraj, K. T. Antimicrobial activities of citronella (Cymbopogon nardus) essential oil against several oral pathogens and its volatile compounds. Padjadjaran Journal of Dentistry, 2020, 32(1): 1-7.
- Toda, H., Mihara, S., Umano, K., Shibamoto, T. (1983). Photochemical studies on Jasmine oil. Journal of Agricultural and Food Chemistry, 31, 554-558.
- Rath, C. C., Devi, S., Dash, S. K., Mishra, R. K. Antibacterial Potential Assessment of Jasmine Essential Oil Against E. Coli. Indian journal of pharmaceutical sciences, 2008, 70(2): 238–241.
- Sayowan, W., Siripornpanich, V., Hongratanaworakit, T., Kotchabhakdi, N., Ruangrungsi, N. The Effects of Jasmine Oil Inhalation on Brain Wave Activies and Emotions. Journal of Health Research, 2013, 27(2): 73-77.
- Dubey, P., Tiwari, A., Gupta, S. K., Watal, G. Phytochemical and Biochemical Studies of Jasminum officinale Leaves. International Journal of Pharmaceutical Sciences and Research, 2016, 7(6): 2632-2640.
- Al-Snafi, A. E. Pharmacology and Medicinal Properties of Jasminum officinale – A Review. Indo American Journal of Pharmaceutical Sciences, 2018, 5(4): 2191-2197.
- Rana, V. S., Juyal, J. P., Amparo Blazquex, M. Chemical Constituents of Essential Oil of Pelargonium graveolens Leaves. International Journal of Aromatherapy, 2002, 12(4): 2160-218.
- Saraswathi, J., Venkatesh, K., Baburao, N., Hilal, M. H., Rani, A. R. Phytopharmacological importance of Pelargonium species. Journal of Medicinal Plants Research, 2011, 5(13): 2587-2598.
- Ghannadi, A., Bagherinejad, M. R., Abedi, D., Jalali, M., Absalan, B., Sadeghi, N. Antibacterial Activity and Composition of Essential Oils from Pelargonium graveolens L’Her and Vitex agnus-castus L. Iranian Journal of Microbiology, 2012, 4(4): 171-176.
- Asharpanah, J., Ramezanloo, F. An Overview on Phytopharmacology of Pelargonium graveolens L. Indian Journal of Traditional Knowledge, 2015, 14(4): 558-563.
- Graҫa, V. C., Ferreira, I. C. F. R. and Santos, P. F. Phytochemical Composition and Biological Activities of Geranium robertianum L.: A Review, Industrial Crops and Products, 2016, 87: 363-378.
- Calamai, A., Palchetti, E., Masoni, A., Marini, L., Chiaramonti, D., Dibari, C., Brilli, L. The Influence of Biochar and Solid Digestate on Rose-Scented Geranium (Pelargonium graveolens L’Hér.) Productivity and Essential Oil Quality. Agronomy, 2019, 9: 260.
- Hamzah, M. H., Man, H. C., Abidin, Z. Z., Jamaludin, H. Comparison of citronella oil extraction methods from Cymbopogon nardus grass by ohmic-heated hydro-distillation, hydro-distillation, and steam distillation. BioResources, 2014, 9(1): 256-272.
- Gu, X., Zhang, Z., Wan, X., Ning, J., Yao, C., Shao, W. Simultaneous Distillation Extraction of Some Volatile Flavor Components from Pu-erh Tea Samples—Comparison with Steam Distillation-Liquid/Liquid Extraction and Soxhlet Extraction. International Journal of Analytical Chemistry, 2009, 276713: 1-6.
- Dawidowicz, A. L., Rado, E., Wianowska, D., Mardarowicz, M., & Gawdzik, J. Application of PLE for the Determination of Essential Oil Components from Thymus vulgaris L. Talanta, 2008, 76(4): 878-884.
- Chrissie, W. The Encyclopedia of Aromatherapy. Vermont: Healing Arts Press, 1996, 16-21.
- Gopalasatheeskumar, K. Significant Role of Soxhlet Extraction Process in Phytochemical Research. Mintage Journal of Pharmaceutical & Medical Sciences, 2018, 7(1): 43-47.
- Puah, C. W., Choo, Y. M., Ma, A. N., Chuah, C. H. Supercritical fluid extraction of palm carotenoids. American Journal of Environmental Science, 2005, 1(4): 264-269.
- Wu, H., Li, J., Jia, Y., Xiao, Z., Li, P., Xie, Y., Zhang, A., Liu, R., Ren, Z., Zhao, M., Zeng, C. Li, C. Essential Oil Extracted from Cymbopogon Citronella Leaves by Supercritical Carbon Dioxide: Antioxidant and Antimicrobial Activities. Journal of Analytical Methods in Chemistry, 2019, 1-10.
- Oktavianawati, I., Letisya, N., Citra, P., Utari, D., Winata, I., Handayani, W., Nugraha, A. Essential Oil Composition of Rose Flowers from Karangpring Village Jember District Extracted by Distillation and Enfleurage. Jurnal ILMU DASAR, 2019, 20(2): 67-74.
- Dawidowicz, A.L., E. Rado, D. Wianowska, M. Mardarowicz and J. Gawdzik, Application of PLE for the Determination of Essential Oil Components from Thymus Vulgaris L. Talanta, 2008, 76: 878-884.
- Rassem, H. H. A., Nour, A. H., Yunus, R. M., Techniques For Extraction of Essential Oils From Plants: A Review, Australian Journal of Basic and Applied Sciences, 2016, 10(16): 117–127.
- Gakuubi, M. M., Steam Distillation Extraction and Chemical Composition of Essential Oils of Toddalia Asiatica L. and Eucalyptus Camaldulensis Dehnh. Journal of Pharmacognosy and Phytochemistry, 2016, 5(2): 99–104.
- Li, Y., Fabiano-Tixier, A.-S., and Chemat, F. Essential Oils: From Conventional to Green Extraction, Essential Oils as Reagents in Green Chemistry, Springer, Briefs in Green Chemistry for Sustainability, 2014, Chapter 2: 9–20.
- Marriott, P. J., Shelliea, R., Cornwell, C. Gas Chromatographic Technologies for the Analysis of Essential Oils. Journal of Chromatography A, 2001, 936: 1–22.







