Momo A1, Obianime AW1, Uche FI2,3, Berebon DP4 and Odimegwu DC4*
1Department of Pharmacology, Faculty of Basic Medical Sciences, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria.
2Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria.
3School of Pharmacy, Institute for Science and Technology in Medicine, Staffordshire, Keele University, UK.
4Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Enugu State, Nigeria, 410001, Nigeria.
Corresponding Author E-mail: damian.odimegwu@unn.edu.ng
DOI : https://dx.doi.org/10.13005/bpj/1980
Abstract
There is wide claims on the folkloric use of the leaves of Triumfetta rhomboidea Jacq (TR) for most reproductive function among men in Nigeria. The effects of aqueous extracts of TR leaves on hormonal and male fertility function was investigated in male guinea pigs. The crude extract of TR was subjected to various serological and histological tests. Hormonal Parameters – testosterone, luteinizing hormone (LH), follicle stimulating hormone (FSH), and prolactin were assayed based on the solid-phase enzyme linked Immunosorbent Assay (ELISA). Semen analysis was carried out for the determination of the concentration of spermatozoa, sperm motility, percentages of abnormal sperm cells (sperm morphology) and debris. The testis, liver and kidney of each animal were stained with haematoxylin and eosin (H and E) and subjected to histological examination. The results indicated that the aqueous extract of TR significantly (p < 0.05) increased the basal level of serum testosterone with little or no effect on the serum levels of LH, FSH and prolactin of the male guinea pig. The administration of 400 mg/kg of TR extract significantly (p < 0.05) increased the sperm count from 61.25 1.25 (control animals) to 81.75±2.02 (14 days) and 98.75 ± 4.2 (28 days). However, 25 - 400 mg/kg of the extract administered over the different duration of the study had no significant (p < 0.05) effect on the sperm motility, sperm morphology and sperm debris respectively. TR over different times of exposures (7 – 28 days) caused mild, moderate to severely diffused and generalized macrovesicular steatosis in a time - dependent alterations of the various organs histology compared to the controls. The study shows that the aqueous extract of TR may be useful in treating fertility problems associated with oligospermia (low sperm count) and low testosterone level.
Keywords
Fertility; Hormonal; Histological Parameters; Male Guinea Pig; Sperm; Triumfetta rhomboidea.
Download this article as:| Copy the following to cite this article: Momo A, Obianime A. W, Uche F. I, Berebon D. P, Odimegwu D. C. Aqueous Extract of Triumfetta rhomboidea Jacq Leaves Modulates Hormones and Upregulates Male Fertility Function in Guinea Pig. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Momo A, Obianime A. W, Uche F. I, Berebon D. P, Odimegwu D. C. Aqueous Extract of Triumfetta rhomboidea Jacq Leaves Modulates Hormones and Upregulates Male Fertility Function in Guinea Pig. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/31eu6pT |
Introductionn
Triumfetta rhomboidea Jacq (TR) (Family: Tiliaceae) is an under shrub, widely distributed in tropical and subtropical India, Ceylon, Malay Peninsula, China, Africa and in America1,2. It is a perennial herb having important roles in ancient therapy3. Taxonomically, TR is a stout herb, ovate – rounded, 3 – lobed and entire leaves with yellow flowers, 5- 10 stamens, capsules pubescent and spine globrous4.
Various parts of TR plants are widely used in most parts of Nigeria whether directly as folk remedies or indirectly as pharmaceutical preparation for certain diseases. Ethnomedically, TR juice and paste is used in urinary trouble & dysentery5, bark and leaves are used in Jaundice-Hepatitis, diarrhoea, asthma and inflammation, infusion of root is used in anxiety6 and root is used in leucorrhoea7.
Earlier scientific investigation suggested that the plant possesses antibacterial, antiulcer, ecbolic, cytotoxic, antimicrobial, antitumor and antioxidant, antidiabetic, diuretic8. Currently, there is a growing interest in the pharmacological evaluation of various plants used in Nigeria ethnomedicinal plants. The reasons for the upsurge in use of traditional medicine being (i) the strong association of people with local flora and their belief on traditional knowledge regarding plants as medicine, (ii) easy availability of local medicinal plants, (iii) relatively poor access to allopathic drugs and their high cost and (iv) lower economic profile of the rural people9, more herbal traditional healers versed in the use of local medicinal plants and failure of most last line drugs due to prevalence of multi-drug resistance organisms due to antibiotic abuse.
Presently, the scientific community is engrossed in elucidating the role of several therapeutic modalities, currently considered as elements of complementary and alternative medicine, on the control of certain diseases1. However, despite the numerous medicinal uses attributed to TR, there are little pharmacognostical and pharmacological reports on leaf, root and stem of this plant10.
Quite very fewer number of plant extracts or compounds have been found to have effects on the levels of luteinizing hormone (LH), follicle stimulating hormone (FSH) and testosterone levels in humans11.
Currently, there are limited data on the scientific basis for hormonal and fertility utility potentials from leaf extract of TR. Thus, the hormonal, fertility and histological property of the plant is so less explored. In this present work, preliminary evaluation of reproductive hormones such as serum testosterone (T), luteinizing hormone (LH), follicular stimulating hormone (FSH), inhibin and also prolactin are evaluated. Thus the present study seeks to present a baseline data on the hormonal, fertility and histological parameters to justify the use of TR in folklore and to define its cellular-toxicological effects.
Materials and Methods
Plant Material and Extracts
The fresh leaves of TR were collected from a local garden in Orlu Local Government Area of Imo State. The plant material was taxonomically identified and authenticated by a taxonomist, Mr. H. D Onyeachusim in the Department of Botany, University of Port Harcourt where voucher specimen was also deposited.
Preparation/Extraction of Plant Material
The collected fresh leaves were air-dried for 14 days under shade until a constant weight was obtained. It was pulverized with a mechanical grinder. The dried powdered leaves (660gm) were extracted exhaustively in distilled water using the soxhlet extraction apparatus. The filtrate was concentrated and evaporated to dryness in vacuum at 40 ºC, using a rotary evaporator. The solid yield was calculated (48.5% w/w), stored in a capped container in a refrigerator at 4 ºC, for further experiment. During the experiment, the required amount of the crude extract was calculated, weighed out and dissolved in the required and measured distilled water to give the concentration and dose required for the experiment (25, 50, 100, 200 and 400 mg/kg).
Animals
Eighty-four (84) healthy male guinea pigs weighing between 450 – 600 gm were obtained from the animal house of the Department of Pharmacology of the University of Port Harcourt. The animals were randomly distributed into cages and allowed to acclimatize with the new environment for 14 days in a well ventilated room at a room temperature of 28 ± 2o C under natural lighting condition. The animals were housed four per cage and provided with water and food ad libitum. The Study was approved under the ethical regulation of the University of Port Harcourt, and all the animals used in this study were handled in accordance with international guidelines for care and use of laboratory animals in Biomedical Research as promulgated by the Canadian Council of Animal Care12.
Animals Experimental
The eighty-four (84) animals were weighed and randomly divided into two (2) sets. Set A (n = 80) contains the Test animals and set B (n = 4) contains the Control Animals. The set A animals were divided into 4 groups according to the treatment period – 7days (Group 1), 14 days (Group 2), 21 days (Group 3), and 28 days (Group 4). Each group was further subdivided into 5 subgroups of four animals (n = 4) and orally administered, using oral cannula, with single doses of 25mg/kg (for subgroup 1), 50mg/kg (for subgroup 2), 100 mg/kg (for subgroup 3), 200 mg (for subgroup 4), and 400 mg/kg (for subgroup 5) of the aqueous extract of TR daily at 12.00 midday for 7days (Group 1), 14 days (Group 2), 21days (Group 3) and 28 days (Group 4) respectively. The set B animals received only distilled water (0.3mls) daily for 28 days. At the end of each treatment period, the animals from each group were anaesthetized with diethyl-ether and blood samples were collected by cardiac puncture with a 21G needle fixed on 5ml syringe for the measurement of biochemical parameters.
Assay of Hormonal Parameters
The blood was collected into lithium heparinized bottle, centrifuged for 15min at 3000 rpm and serum was separated and assayed for luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone and prolactin using the Biocheck LH, Biocheck FSH, Immunospec testosterone and Biocheck prolactin test kits respectively. All these tests are based on the solid-phase enzyme linked Immunosorbent Assay (ELISA).
All assays were done in triplicates. Briefly, for a particular hormone, the respective immunoassay test kit was used. Fifty microlitre (50µl) of the respective hormonal reference standard and corresponding test sample were measured into the appropriate microtitre wells. Similarly, 100µl of corresponding hormonal antibody-enzyme conjugate reagents was measured into the wells. This was gently mixed for 30 seconds and incubated at room temperature for 45 minutes. The incubated mixture was removed by flicking the plate contents into the sink.
The microtitre wells were subsequently rinsed and flicked five (5) times with phosphate buffered saline. Aliquot (100µl) of the TMB reagent was measured into each well, gently mixed for 10 seconds, and incubated at room temperature in the dark for 20-30 minutes. Finally, the reaction was stopped by the addition of 100µl of STOP solution (2M H2SO4) to the well (Test tube), gently mixed for 30 seconds, and the optical density was read at 450 nm within 15 minutes to give the absorbance (GM 2000).
The concentration (C) of the test sample was calculated as follows:
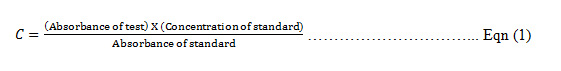
Measurement of semen parameters
The specimens were collected by aspirations. This involved making incisions on the caudal epididymis and aspirating the semen into a polyester capillary tube. The sample was flashed into an aliquot of 1ml of diluents. Semen analysis was carried out immediately using Neubauer Haemocytometer; for the determination of the concentration of spermatozoa, sperm motility, percentages of abnormal sperm cells (sperm morphology) and debris as described13 in line with standard laboratory techniques14.
Motility was determined by counting the motile, sluggish and immotile spermatozoa in at least ten randomly selected fields. The percentage of motile sperm is calculated from the mean percentage motility for all the fields counted i.e. number of motile sperm cells divided by the total number of counted cells.
The morphology of the spermatozoa was assessed, after appropriate staining technique, on the basis of relative comparison between normal and abnormal ones. The sperm count and percentage debris were also determined. The percentage of abnormal sperm morphology was calculated from the following formula=
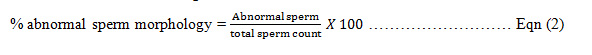
Histopathological Studies
The testis, liver and kidney of each animal were carefully isolated, washed in Phosphate buffered saline (PBS) and fixed in 10% formalin. Each of these organs was processed in an automatic tissue processor, and embedded in paraffin wax. Sections of 5 – 6 µm were cut on a rotary microtome by serial sectioning until the entire thickness of the organ was sectioned. Staining was done by haematoxylin and eosin (H and E) staining method. The organs were viewed microscopically and photomicrograph taken using AmScope™ version 3.7 digital light microscope (USA).
Statistical Analysis
Data were expressed as mean ± SEM. Comparison between data of control and treated groups of guinea pigs were performed using one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05. All statistical analysis was done using Graph Pad Prism version 7.
Results
Effects of oral administration of aqueous extracts of TR leaves on the Hormonal parameters of the male guinea pig
The effects of the aqueous extract of TR at different doses (25 – 400 mg/kg) over time (7 – 28 days) on the serum testosterone, follicle stimulating Hormone (FSH) luteinizing Hormone (LH) and prolactin levels of the male guinea pig are shown in Figure 1, 2, 3 and 4. These results indicate that the aqueous extract of TR significantly ( p < 0.05) increased the basal level of serum testosterone while it had little or no effect on the basal serum levels of LH, FSH and prolactin of the male guinea pig.
The serum testosterone level was significantly increased (p < 0.05) in the experimental animals by the aqueous extract in a dose-dependent manner when compared to the serum testosterone levels in the control animals. These effects were also time-dependent over the 28 days’ period of administration (Figure 1). The increase in serum Testosterone is highest in animals treated with the 400 mg/kg dose for 28 days while the lowest increase is seen in animals treated with the lowest dose level of 25 mg/kg for 7 days. However, both levels are significantly (p < 0.05) higher than the levels in the untreated (control) animals.
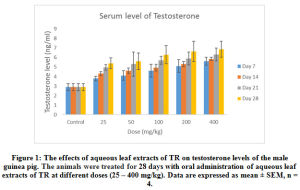 |
Figure 1: The effects of aqueous leaf extracts of TR on testosterone levels of the male guinea pig. The animals were treated for 28 days with oral administration of aqueous leaf extracts of TR at different doses (25 – 400 mg/kg). Data are expressed as mean ± SEM, n = 4. |
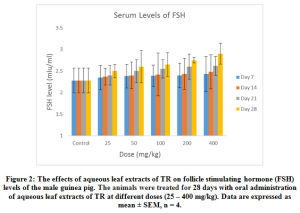 |
Figure 2: The effects of aqueous leaf extracts of TR on follicle stimulating hormone (FSH) levels of the male guinea pig. The animals were treated for 28 days with oral administration of aqueous leaf extracts of TR at different doses (25 – 400 mg/kg). Data are expressed as mean ± SEM, n = 4. |
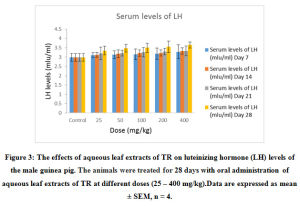 |
Figure 3: The effects of aqueous leaf extracts of TR on luteinizing hormone (LH) levels of the male guinea pig. The animals were treated for 28 days with oral administration of aqueous leaf extracts of TR at different doses (25 – 400 mg/kg). Data are expressed as mean ± SEM, n = 4. |
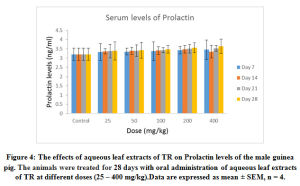 |
Figure 4: The effects of aqueous leaf extracts of TR on Prolactin levels of the male guinea pig. The animals were treated for 28 days with oral administration of aqueous leaf extracts of TR at different doses (25 – 400 mg/kg). Data are expressed as mean ± SEM, n = 4. |
Effect of Oral Administration of aqueous extracts of TR leaves on semen parameters of the male guinea pig
As shown in Figure 5, a 25 – 400 mg/kg of the aqueous extract of TR caused a significant (p < 0.05) dose-and-time dependent increase in the sperm count in all the experimental groups compared to the control sperm count except in the group treated with the aqueous extract for 7 days, where there was a non–significant (p < 0.05) increase. Maximal response was obtained with the 400mg/kg dose administered on the 28 day. The administration of 400 mg/kg of TR extract significantly (p < 0.05) increased the sperm count from 61.25 1.25 (control animals) to 81.75±2.02 (14 days) and 98.75 ± 4.2 (28 days).
Furthermore, 25- 400 mg/kg of the extract administered over the different duration of the study had no significant (p < 0.05) effect on the sperm motility (Figure 6), sperm morphology (Figure 7) and sperm debris (Figure 8) respectively.
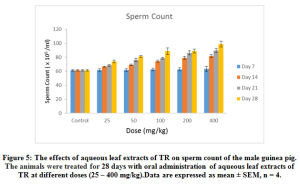 |
Figure 5: The effects of aqueous leaf extracts of TR on sperm count of the male guinea pig. The animals were treated for 28 days with oral administration of aqueous leaf extracts of TR at different doses (25 – 400 mg/kg). Data are expressed as mean ± SEM, n = 4. |
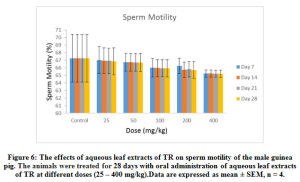 |
Figure 6: The effects of aqueous leaf extracts of TR on sperm motility of the male guinea pig. The animals were treated for 28 days with oral administration of aqueous leaf extracts of TR at different doses (25 – 400 mg/kg). Data are expressed as mean ± SEM, n = 4. |
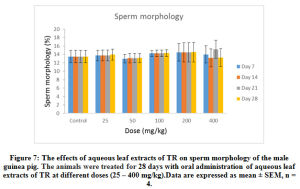 |
Figure 7: The effects of aqueous leaf extracts of TR on sperm morphology of the male guinea pig. The animals were treated for 28 days with oral administration of aqueous leaf extracts of TR at different doses (25 – 400 mg/kg). Data are expressed as mean ± SEM, n = 4 |
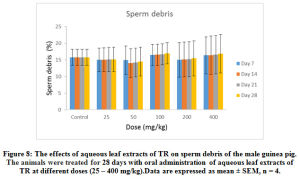 |
Figure 8: The effects of aqueous leaf extracts of TR on sperm debris of the male guinea pig. The animals were treated for 28 days with oral administration of aqueous leaf extracts of TR at different doses (25 – 400 mg/kg). Data are expressed as mean ± SEM, n = 4. |
Effects of oral administration of the aqueous extracts of TR on some Histological Parameters of the male guinea pig
A photomicrographs showing dose-dependent effects of 7 days’ administration of aqueous extract of TR (50 – 400mg/kg) on the histology of the liver of the male guinea pig is shown in Figure 9 (a – e). Histopathological examinations of sections of the liver revealed that aqueous extract of TR at various doses (25 – 400 mg/kg) and at different times of exposures (7 – 28 days) caused mild to moderate to severely diffused and generalized macrovesicular steatosis during seven (7) days administration (Figure 9 b and c). The control group exhibited normal architecture of the liver (Figure 9 a). However, at the highest dose (400 mg/kg), there was formation of fibrosis with massive infiltration of the liver parenchyma by chronic inflammatory cells (Figure 9 e). Administration of (200 mg/kg) of TR over 28 days also caused time-dependent alterations of the liver histology compared to the control (Figure 9 d).
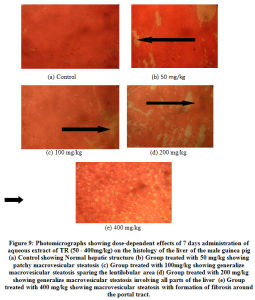 |
Figure 9: Photomicrographs showing dose-dependent effects of 7 days administration of aqueous extract of TR (50 – 400mg/kg) on the histology of the liver of the male guinea pig. |
Furthermore, time dependent effects of TR over a 28 days’ administration of 200 mg/kg of the aqueous extract is shown in (Figure 10). The histopathological examination of section of the kidney (Figure 11) and Testes (Figure 12) did not show any apparent gross structural derangement.
 |
Figure 10: Photomicrograph showing Time dependent effects of 200 mg/kg of aqueous extract of TR over 28 days’ administration. |
 |
Figure 11: Photomicrographs showing the effects of TR (400 mg/kg) administered for 28 days on the kidney of the male guinea pig (a) Control, showing Normal kidney with glomerulus and Tubules (b) Kidney treated with 400 mg/kg of aqueous extract of TR, showing normal glomerular and Renal tubules. |
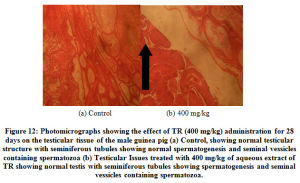 |
Figure 12: Photomicrographs showing the effect of TR (400 mg/kg) administration for 28 days on the testicular tissue of the male guinea pig. |
Discussion
This study was carried out to investigate the effects of 28 days daily oral administration of different doses of the aqueous leaf extract of TR on some Biochemical, hormonal, semen and histological parameters of the male guinea pig.
The semen parameters, studied here are the sperm count, sperm motility, sperm morphology and sperm debris. These parameters to a large extent determines the quantity and quality of sperm and invariably affect their fertilizing potentials15.
In this study all the doses (25 – 400 mg/kg) of the aqueous leaf extract of TR administered over 28 days significantly (p < 0.05) increased the sperm count dose and time dependently. However, the extract did not cause any significant (p < 0.05) effect on the other semen parameters studied; sperm motility, sperm morphology and percentage of sperm debris. Reactive oxygen species (ROS) such as free radicals may reduce sperm count, quality and motility; hence plants with antioxidant activities1 may enhance sperm quality16, improve various processes of male reproductive function such as spermatogenesis and steroidogenesis17. The results of this study show that the aqueous extract of this plant increases sperm count; however, sperm count alone does not secure fertilizing capacity. Sperm should be actively and progressively motile since they have to move from the cervix to the fallopian tube where fertilization of the ovum occurs. The morphological characteristics of the sperm is also important.
This study also shows that the aqueous extract of TR causes an increase in the level of testosterone but has little or no effect on the serum levels of Luteinizing Hormone (LH), Follicle stimulating Hormone (FSH) and prolactin. A normal serum FSH level does not guarantee the presence of intact spermatogenesis but abnormal serum FSH and inhibin are indicative of impaired spermatogenesis18. The increase in the level of testosterone was found to be statistically significant at (p < 0.05) (ANOVA). The selective statistically significant increase (p < 0.05) in testosterone level could probably be due to either a direct stimulation of the testicular interstitial cells (Leydig cells) in the seminiferous tubules to produce more testosterone or inhibition of the breakdown or metabolism of plasma testosterone by the aqueous extract.
The increase in testosterone level may have contributed to the significant increase seen in the sperm count of the animal as spermatogenesis is controlled by Testosterone19.
This is so because optimum level of testosterone is required for normal sex drive in adult male. Plant extracts that increase blood testosterone concentrations are very useful in modifying sexual functions in animals especially those suffering from hypotestosteronemia while those exhibiting LH, and FSH will be potential agents in developing male contraceptives20. Thus, the Testosterone-increasing effect of this extract may account for its use as libido enhancer as claimed by traditional medicine practitioners in Eastern and other parts of Nigeria. This finding agrees with earlier works21,22 which though working on different plant extracts, observed that significant increase in serum testosterone concentration enhanced copulatory performance of male rats, contribute to the improvement in sexual function, libido and penile erection.
Finally, Histopathological results of the liver sections showed that aqueous extract of TR caused various degrees of pathologic changes in the liver of the male guinea pig. This ranges from mild to severely diffuse macrovesicular steatosis. The higher doses produced fibrosis with massive infiltration of the liver parenchyma by chronic inflammatory cells. These changes were dose and time dependent. The disorder observed in the liver of the guinea pig also known as fatty Liver disease, is a reversible condition where large vacuoles of triglyceride fat accumulate in liver cells via the process of steatosis (abnormal retention of lipids within a cell).
From the foregoing, the aqueous extract of TR may contain constituents that adversely affect fat metabolism in the liver of the male guinea pig; hence the pathological picture seen. Histological reports on sections of the kidney and testicular tissues suggest that their functions are not affected by any of the constituents of the aqueous extracts of TR.
Conclusion
This study revealed that the aqueous leaf extract of TR selectively increase the sperm count dose-and-time dependently and does not have any significant effect on the level of the other sperm parameters (motility, morphology, and debris) of the male guinea pig studied. It further revealed that the aqueous extract increased the serum testosterone level dose-and-time dependently and does not have any significant effects on the level of the other serum sex hormones studied (LH, FSH and prolactin) in the male guinea pig. The above therefore suggest that the aqueous extracts of these leaves has spermatogenetic and testosterone – increasing effects in the male guinea pig. From the foregoing, the aqueous extract may be useful in treating fertility problems associated with Oligospermia (low sperm count) and low testosterone level. However great caution must be taken in view of the hepatotoxic effect observed possibly by the co – administration of a hepatoprotective agent. This study therefore supports the claims on the folkloric use of the leaves of this plant to improve libido, sex drive and reproductive function among men in Nigeria.
The present report embodied in this work is further significant being the first time that fertility enhancement property of TR is scientifically evaluated and validated. Therefore, further studies are recommended to further isolated, map, and define the efficacy of fertility compounds to be isolated from the medicinal plant.
Conflict of Interest
The authors declare no conflict of interest.
Refrences
- Sivakumar P, Kumar S. R, Sivakumar T, Nethaji R and Vijyabaskaran M. Antitumor and Antioxidant activities of Triumfetta rhomboidea against Dalton’s Ascites Lymphoma bearing Swiss albino mice. Res J Med Sci., 2008; 2 (4): 203 – 208.
- Gupta S, Shaival K. R, Vikas G. Pharmacognostical studies of the plant drug Triumfetta rhomboidea Jacq. Global Journal of Pharmaceutical Education and Research., 2016; 5 Issues 1-2.
- Odimegwu D. C, Uche I. F, Ozioko C. L, Ogbuanya, C. E., Gugu, T. H and Esimone O. C. In vitro antimicrobial evaluation of methanol extract of Triumfetta rhomboidea leaves against some clinical bacterial isolates. J. Biol. Chem. Sci., 2011; 5 (5): 1970 – 1977.
CrossRef - Singh V. P and Dinesh Jadhav (2011). Ethnobotany of Bhil tribe – A case study among the Bhil of Ratlam District (Madhya Pradesh). Scientific Publishers (India). E – ISBN: 978-93-87307-36 – 0.
- Biswas A, Bari M. A, Roy M and Bhadra S. K. Inherited folk pharmaceutical knowledge of Tribal people in the Chittagong Hill tracts, Bangladesh, Ind J Trad Knowl., 2010; 9 (1): 77 – 89.
- Hamill F. A, Apio S, Mubiru N. K, Bukenya-Ziraba R, Mosango M, et al. Traditional herbal drugs of Southern Uganda, II: literature analysis and antimicrobial assays, J Ethnopharmacol., 2003; 84 (1): 57-78.
CrossRef - Yadav J. P, Kumar S and Siwach P. Folk medicine used in gyneocological and other related problems by rural population of Haryana, Ind J Trad Knowl., 2006; 5 (3): 323-326.
- Sahoo H. B, Mandal P. K, Sagar R and Bhattamisra S. K. Evaluation of lactogenic activity of Triumfetta rhomboidea root: Validating its traditional usage. Journal of Experimental and Integrative Medicine., 2016; Volume 6 Issue 1. DOI: 10.5455/jeim. 160216.or.146 www.jeim.org.
CrossRef - Ayinde B. A, Omogbai E. I and Amaechina F. C. Pharmacognosy and Hypotensive Evaluation of Ficus Exasperata Vahl (Moraceae) Leaf. Acta Poloniae Pharmaceutica – Drug Res & Nat Drugs., 2007; 64, 6, 543-546.
- Gupta S, Shaival K. R and Vikas G. Pharmacognostical studies of the plant drug Triumfetta rhomboidea Jacq. Global Journal of Pharmaceutical Education and Research., 2016; Vol.5 Issue 1-2.
- Abdillahi, H. S. and Van Staden J. South African plants and male reproductive healthcare: Conception and contraception. Journal of Ethnopharmacology., 2012; 143:475 – 480.
CrossRef - Canadian Council on Animal Care (2017): Guide to the Care and Use of Experimental Animals Volume 1, 2nd Edition. E.D. Olfert, B.M. Cross and A.A. McWilliam (editors).
- Ochei J and Kolhatkar A. (2000). Medical laboratory science and practice. Theory and practice. TaTa McGraw-Hill (1st Edn).
- WHO (2010). WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed. Geneva, Switzerland: WHO Press.
- Aladakatti R. H, Ahmed R. N and Ghoseawar G. Sperm parameters changes induced by Azadirachta indica root on albino rats. J Basic and Clin physiol pharmacol., 2002; 12(1): 3 – 9.
CrossRef - Glenville, M. The nutritional approach of male factor infertility. Dragons Tale. 2008; 18, 4 –5.
- Sheweita A. S, Tilmisany M. A and Al-Sawaf Mechanisms of male infertility: role of antioxidants. Current Drug Metabolism., 2005; 6, 1–7.
CrossRef - Sikka S. C and Hellstrom W. J. G. Current updates on laboratory techniques for the diagnosis of male reproductive failure. Asian Journal of Andrology., 2016; 18, 392–401.
CrossRef - Stephen FS. (2001). Male infertility: Overview: Assessment, Diagnosis and Treatment (US) 1st
- Abdillahi H. S and Staden J. V. South African plants and male reproductive healthcare: Conception and contraception. Journal of Ethnopharmacology.,2012; 143: 475–480.
CrossRef - Yakubu M.T and Afolayan A. J. Effect of aqueous extract of Bulbine natalensis Baker stem on the sexual behaviour of male rats. International Journal of Andrology., 2009; 32, 629–636.
CrossRef - Gauthaman K, Adaikan P.G and Prasad R.N. Aphrodisiac properties of Tribulus terrestris extract (Protodiosan) in normal and castrated rats. Life Sciences., 2003; 71, 1385–1396.
CrossRef








