Aamina M1 , Ahmad Alhowail2
, Ahmad Alhowail2 , Maha Aldubayan2
, Maha Aldubayan2 , Rabbani SI1,2*
, Rabbani SI1,2*
1Department of Pharmacology, Al-Ameen College of Pharmacy, Bangalore, India.
2Department of Pharmacology and Toxicology, College of Pharmacy, Qassim University, Buraydah, Kingdom of Saudi Arabia.
Corresponding Author E-mail : syedrabbani09@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1993
Abstract
The effect of ethanolic extract of Terminalia chebula was tested on hematological complications induced by doxorubicin (DXR) in male and female rats. Hematological parameters were evaluated by estimating the hemoglobin content and total leucocytes count. The antioxidant assay was done by finding the serum catalase level. The results of the study was analyzed by Two-way Anova and Bonferroni tests and p<0.05 was considered to indicate the significance of the results. Our data indicated DXR at 2.5 mg/kg significantly (p<0.001) decreased the hemoglobin content, total WBC count and serum catalase level compared to control groups in both male and female rats. Administration of Terminalia chebula extract at 0.5 and 1 g/kg significantly (p<0.05) improved the level of hemoglobin content and total WBC count and elevated the catalase concentration compared to DXR. The results suggest that co-administration of Terminalia chebula can prevent the hematological complications of doxorubicin possibly by elevating the antioxidant status.
Keywords
Terminalia chebula; Doxorubicin; Hematological Complications; Oxidative Stress
Download this article as:| Copy the following to cite this article: Aamina M, Alhowail A, Aldubayan M, Rabbani S. I. Ameliorative Effect of Terminalia Chebula on Hematological Complications Induced by Doxorubicin. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Aamina M, Alhowail A, Aldubayan M, Rabbani S. I. Ameliorative Effect of Terminalia Chebula on Hematological Complications Induced by Doxorubicin. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/32ti4ub |
Introduction
Doxorubicin (DXR) is one of the most effective and widely used anticancer drugs. Its dose-dependent anticancer activity was discovered over forty years ago. Doxorubicin interacts with DNA by intercalation and inhibits macromolecular biosynthesis. The mechanism involves inhibition in the progression of topoisomerase II, an enzyme that relaxes supercoils in DNA for transcription. Doxorubicin stabilizes the topoisomerase II complex after it has broken the DNA chain for replication, prevents the DNA double helix from being resealed and thereby impedes the process of replication.1
In spite of its high antitumor efficacy, DXR’s use in chemotherapy has been largely limited due to its cardiac, hematological, renal, pulmonary and testicular toxicities. The hematological side effects results due to suppression of bone marrow leading to anemia, increased bleeding tendencies and susceptibility for infections.2
DXR is reported to cause an imbalance between free oxygen radicals and antioxidants. The disturbance in oxidant-antioxidant systems which has been demonstrated with lipid peroxidation (LPO) and protein oxidation results with tissue injury. Suppression of growth and formation of free radicals are suggested to be the major pathways for the DXR-toxic effects.3
Traditional medicinal plants have many therapeutic properties, are accessible by all the communities of the world and are economically effective means of treatments for many diseases. Terminalia chebula (Haritaki) has been extensively used in Ayurveda, Unani & Homeopathy medicine & has become cynosure of modern medicine. It belongs to the family Combretaceae4. The herb is generating enormous curiosity among the research fraternity across the globe because of its reported medicinal properties like anti-oxidant, antibacterial, antifungal, anti-neoplastic, antiviral, anti-diabetic, cardio protective, immunomodulatory etc.5
Earlier studies suggests that Terminalia chebula is effective in the treatment of gum bleeds and possess anticancer and antioxidant properties6. However, its role in prevention of complications associated with doxorubicin chemotherapy is not studied extensively. Since the data from the previous research suggests that co-administration of an antioxidant have the potential to minimize the side effects of DXR7, this study was designed to evaluate the effect of ethanolic extract of Terminalia chebula in reducing the hematological adverse effects of DXR in rats.
Materials and Method
Chemicals
A gift sample of ethanolic extract of Terminalia chebula (TCE) was obtained from Sami Labs, Bangalore, India. The powder form of the extract was weighed, dissolved in distilled water and administered orally depending on the dose and body weight of the animals.
Animals
Eight week old healthy, laboratory bred, Wistar rats (male and female) weighing 160 ± 10 g were maintained under standard laboratory conditions (24o ± 2o C, 12:12 h light / dark cycle) and provided water and pellet food ad libitum. The experiments were conducted in CPCSEA (Committee for the purpose of control and supervision of experiments on animals, Chennai, India) approved animal house after obtaining the prior approval from the Institutional Animal Ethics Committee.
Administration of Doxorubicin
Doxorubicin (DXR) was obtained as a research sample from GETWELL Pharmaceuticals, Gurgaon, India. A solution of DXR was prepared by dissolving the required amount of DXR in distilled water as per dosage. A freshly prepared DXR (2.5 mg/kg) was administered by the intra- peritoneal route. On alternate days, each animal received a dose of DXR for a period of 12 days.8
Dosage, Treatment and Sampling
The experiment was done separately on male and female rats, comprising of 6-8 animals. The animals were grouped as normal control (saline – 5 ml/kg, 28 days), positive control (DXR, 12 days), negative control (TCE – 1 g/kg, daily for 28 days) and treatment group (DXR + TCE – 0.25, 0.5 and 1 g/kg9, daily for 28 days).
To find the effect of pre and post treatment of TCE, two additional groups were tested. In the pre-treatment, TCE – 0.5 mg/kg was administered daily for 16-days followed by 12-days of DXR (6-doses on alternate days). In the post-treatment group the schedule was reversed such as 12-days of DXR followed by 16-days of TCE (0.5 mg/kg, daily).
Hematological Parameters
Hemoglobin Estimation
(Sahli’s method): Blood is mixed with N/10 HCl resulting in the conversion of Hb to acid hematin which is brown in color. The solution is diluted till it’s color matches with the brown colored glass of the comparator box. The concentration of Hb is read directly (Gram percent).10
Total WBC Estimation
In order to count WBC, the lysing of erythrocytes should be done. For this 1.5-2% acetic acid solution with a small quantity of crystal violet was used as diluting fluid. Acetic acid destroys the erythrocytes, leaving behind the leucocytes. Crystal violet was used to stain the nucleolus of the leucocytes. This enables the leucocytes to be easily identifiable, and to be counted. The number of leucocytes in a diluted fluid can be counted by using a Neubauer counting chamber and represented as cells/cu mm of blood.11
Serum Catalase Activity
It was estimated by the method of Clairborne et al. The procedure involves the breakdown of H2O2 that can be measured at 240 nm in unit time. The catalase activity is indicated as mM of hydrogen peroxide consumed per minute.12
Statistical Analysis
Two-way ANOVA and Bonferroni comparison was done for all groups. Two comparisons were made- normal control v/s DXR and DXR v/s treatment groups. All values with significance p< 0.05 are shown with an asterisk or superscript.
Results
Effect of TCE on Hemoglobin Content
Our observations from figure-1 indicated that doxorubicin (DXR) significantly (p<0.001) reduced the hemoglobin content compared to the control animals. The administration of TCE at 0.5 g/kg showed significant (p<0.05) elevation in the hemoglobin level in female rats and when the higher dose of TCE (1 g/kg) was tested, the hemoglobin content was found to be elevated (p<0.01) in both male and female rats compared to DXR-treated rats. In the pre and post-treatment groups, an enhanced hemoglobin level was observed when TCE (0.5 g/kg) was tested in post-administration animals. However, the highest tested dose of TCE (1 g/kg) in normal animals did not change significantly the hemoglobin content.
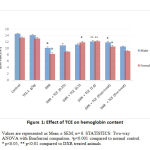 |
Figure 1: Effect of TCE on hemoglobin content |
Effect of TCE on Total WBC Count
The analysis of total WBC count indicated that administration of DXR significantly (p<0.001) reduced the count in both male and female rats compared to the control data. TCE at 0.5 g/kg enhanced (p<0.05) the total WBC level in both male and female rats and further increase in the dose of TCE (1 g/kg) exhibited more enhancement (p<0.01) in the level of WBC count compared to DXR animals. Administration of TCE (0.5 g/kg) after the DXR-treatment (post treatment group) significantly (p<0.05) increased the WBC count compared to DXR values in both male and female rats (Figure-2).
 |
Figure 2: Effect of TCE on total WBC count |
Effect of TCE on Serum Catalase Levels
The serum estimation of SOD levels were found to be significantly (p<0.001) reduced after the administration of DXR compared to the control animals. The higher tested doses of TCE (0.5 and 1 g/kg) exhibited significant (p<0.05) elevation of SOD levels compared to DXR. Both pre and post-treatment group’s analysis revealed that TCE did not produce significant variation in the levels SOD compared to DXR group. The testing of TCE (1 g/kg) in normal animals also did not display any significant change in the levels of SOD (Figure-3).
 |
Figure 3: Effect of TCE on serum catalase level |
Conclusion
In this research, it was observed that Terminalia chebula at 0.5 and 1 g/kg improved the hemoglobin content, total WBC count and catalase levels suppressed by doxorubicin. Terminalia chebula being an antioxidant also found to possess anticancer, immune-stimulatory, hematinic and wound healing properties. The co-administration of Terminalia chebula with DXR has the merits of enhancing the prognosis of DXR chemotherapy. More research in this direction could provide effective measures in addressing the issues of DXR-mediated complications in cancer chemotherapy patients.
References
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 56:185-229 (2004).
CrossRef - Singal PK, Deally CMR, Weinberg LE. Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol. 19(8): 817–828 (1987).
CrossRef - Liu LL, Li QX, Xia L, Li J, Shao L. Differential effects of dihydropyridine calcium antagonists on doxorubicininduced nephrotoxicity in rats. Toxicol. 231(1):81–90 (2007).
CrossRef - Gupta PC. Biological and pharmacological properties of Terminalia chebula Retz (Haritaki) – An overview. Int J Pharm Pharm Sci. 4: 62-68 (2012).
- Fundter, JM.. Terminalia chebula In Lemmens, R.H.M.J. & Wulijarni-Soetjipto, N. (Eds.): Plant Resources of South-East Asia. No. 3: Dye and tannin-producing plants. Prosea Foundation, Bogor, Indonesia. pp 122-125 (1992).
- Gandhi NM, Nair CK. Radiation protection by Terminalia chebula: Some mechanistic aspects Mol Cell Biochem. 277: 43-8 (2005).
CrossRef - Li W, Xu B, Xu J, Wu XL. Procyanidins Produce Significant Attenuation of Doxorubicin-Induced Cardiotoxicity via Suppression of Oxidative Stress. Basic Clin Pharmacol Toxicol. 104: 192-197 (2009).
CrossRef - Viswanatha Swamy AH, Wangikar U, Koti BC, Thippeswamy AH, Ronad PM, Manjula DV. Cardioprotective effect of ascorbic acid on doxorubicin-induced myocardial toxicity in rats. Indian J Pharmacol. 43(5): 507-11 (2011).
CrossRef - Sharma P, Prakash T, Kotresha D, Ansari MA, Sahrm UR, Kumar B, Debnath J, Goli D. Antiulcerogenic activity of Terminalia chebula fruit in experimentally induced ulcer in rats. Pharm Biol. 49(3): 262-8 (2011).
CrossRef - Wintrobe MM. ‘Clinical Hematology’ 7th Philadelphia: Lea and Febiger. pp 114-115 (1975).
- Schalm, O.W, Jain N.C and Caroll, E.J: Textbook of Veterinary Haematology, 2nd Edition, Published by Lea and Febiger, Philadelphia. pp 129-250 (1975).
- Clairborne A. Catalase activity. In: Greewald AR, editor. Handbook of methods for oxygen Radical Research.Florida: CRC Press. pp. 237–242 (1995).
- Doroshow JH. Anthracycline Antibiotic-Stimulated Superoxide, Hydrogen Peroxide and Hydroxyl Radical Production by NADH Dehydrogenase. Can Res. 43: 4543-4551 (1983).
- Al-Sowayan NS, Mahmoud NH. The protective effect of grape seed extract on cardiotoxicity induced by doxorubicin drug in male rats. Adv Biosci Biotechnol. 5: 1078-1089 (2014).
CrossRef - Chattopadhyay RR, Bhattacharyya SK. Plant Review: Terminalia chebula. Rev. 23: 145-150 (2007).
CrossRef - Isacson D, Bingefors K. Epidemiology of analgesic use: a gender Eur J Anaesthesiol Suppl. 26: 5-15 (2002).
CrossRef - Cheng HY. Antioxidant and free radical scavenging activities of Terminalia chebula, Pharm. Bull. 26(9): 1331—1335 (2003).
CrossRef








