Hany Salah Mahmoud1 , Wael M. Elsaed2,3
, Wael M. Elsaed2,3 , Howayda E. Khaled4
, Howayda E. Khaled4 , Tamer Mohamed Ezzat5
, Tamer Mohamed Ezzat5 and Menna Allah I. El Menyawi6*
and Menna Allah I. El Menyawi6*
1Center of Scientific Foundation for Experimental Studies and Research, Ismailia, Egypt, 41511
2Department of Anatomy and Embryology, Faculty of Medicine, Taibah University, Madinah, Saudi Arabia
3Department of Anatomy and Embryology, Faculty of Medicine, Mansoura University, Mansoura, Egypt
4Zoology Department, Faculty of Sciences, Suez University, Egypt
5Department of Internal Medicine and Nephrology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
6Medical Physiology Department, Faculty of Medicine, Suez Canal University, Egypt, 41511
Corresponding Author E-mail : mennaelmenyawi@med.suez.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/2000
Abstract
Background: Camel urine has been widely used in desert area traditional medicine. It was considered as an alternative medicine to cure variable diseases. This present study aimed to investigate the ameliorative effect of camel urine against cisplatin induced subacute and chronic urinary tract toxicity in male Albino rats. Methodology: Thirty two rats were assigned randomly into four groups, eight rats each. Group I served as control, group II received only camel urine, group III injected with cisplatin and group IV injected with cisplatin and treated with camel urine for 2 months. Blood sera were collected at 1 month and 2 months interval for creatinine, urea and uric acid estimation. Moreover, urinary bladder and kidney were subjected to histochemical (masson's trichrome) and histopathological examinations. Results: There was significant (P<0.05) improvement in renal functions (creatinine, urea and uric acid) in cisplatin group treated with camel urine. Also, camel urotherapy induced significant histopathological improvement in urinary bladder and renal tissues deteriorated by the cisplatin. Marked reduction in fibrous tissue formation was detected in kidney and urinary bladder in cisplatin group treated with camel urine. Conclusion: Camel urine proved its safety when used alone and also its palliative and ameliorative effect in renal toxicity induced by cisplatin.
Keywords
Camel; Cisplatin; Kidney; Urine; Urinary Bladder
Download this article as:| Copy the following to cite this article: Mahmoud H. S, Elsaed W. M, Khaled H. E, Ezzat T. M, Menyawi M. A. I. E Ameliorative Effect of Camel Urotherapy to Cisplatin Induced Urinary Tract Subacute and Chronic Toxicity in Male Albino Rats. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Mahmoud H. S, Elsaed W. M, Khaled H. E, Ezzat T. M, Menyawi M. A. I. E Ameliorative Effect of Camel Urotherapy to Cisplatin Induced Urinary Tract Subacute and Chronic Toxicity in Male Albino Rats. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/2CilIfP |
Introduction
Cisplatin is a potent well known chemotherapy agent used to treat a broad spectrum of malignancies (Sonoda et al., 2019). It was the first FDA-approved platinum compound for cancer treatment in 1978 (Kelland, 2007). Cisplatin has been used for treatment of various cancers including; bladder, head, neck, lung, ovarian (Desoize and Madoulet, 2002) and testicular cancers (Facchini et al., 2018). It is effective against various types of cancers including; carcinomas, germ cell tumors (Schaffrath et al., 2017), lymphomas, and sarcomas. Its mode of action depends on its ability to crosslink with the purine bases on the DNA; interfering with DNA repair mechanisms, causing DNA damage, and subsequently inducing apoptosis in cancer cells (Dasari and Bernard Tchounwou, 2014).
However, it has a nonspecific cytotoxic effect (Manohar and Leung, 2018) that also acts on normal cells causing adverse reactions, mainly renal dysfunction (Hayes et al., 1977). The prevalence of cisplatin nephrotoxicity is still high, occurring in approximately one-third of patients who have undergone cisplatin therapy (Oh et al., 2014). Long term use of cisplatin may lead to a permanent reduction of renal function by inducing tubular cell damage and death (Soni et al., 2018). It is manifested by vasoconstriction in the renal microvasculature, decrease in glomerular filtration rate, and tubular epithelial cell toxicity (Yao et al., 2007).
These adverse effects are owing to activation (Soni et al., 2018; Holditch et al., 2019) of several apoptic pathways (Tsuruya et al., 2003). Cisplatin also induces production of reactive oxygen species (ROS) (So et al., 2007) which has been implicated in its direct cellular toxicity (Choi et al., 2015). ROS is an important factor in the apoptosis pathway owing to distortion in the mitochondrial membrane potential (Ježek and Hlavatá, 2005). Consequently, ROS damages the respiratory chain, which ultimately triggers the apoptotic process (Suganya et al., 2019).
The camel urotherapy (CU) is widely used in the Arabian Peninsula (Al-Yousef et al., 2012) to treat various diseases (Alhaidar et al., 2011), as it has an unusual and unique biochemical composition (Ahamad et al., 2017). These unusual biochemical constituent may be acquired through the camel’s consumption of plants in the desert that possess several boiactive substances. Some of these desert plants have demonstrated to have strong antifungal and antibacterial activities (Zaki et al., 1984; Kaul et al., 1976). The camel urine was proven to have minimal traces of ammonia and urea that are recognized to be responsible for toxicity and bad smell of urine. Moreover, the camel urine contains about 10 folds more mineral salts than human urine (Al-Yousef et al., 2012). It contains huge quantities of K and Na, which can reverse electrolyte imbalance as well as large amounts of zinc that has various curative properties (AI-Attas, 2009). Moreover, camel urine was proven to repress inflammatory angiogenesis in murine animal model (Alhaider et al., 2014a; Alhaider et al., 2014b) and possess cytotoxic effect (Al-Harbi et al., 1996). Therefore, possessing anti- cancer effect (Alkhamees and Alsanad, 2017). It has antioxidative effect attributed mainly to its high level of creatinine and uric acid levels (Alkhamees and Alsanad, 2017) which considered as a powerful antioxidants (Giovannini et al., 2006). Uric acid (UA) is a powerful scavenger of free radicals and provides about 60% of free-radical scavenging capacity in plasma (Fabbrini et al., 2014). It also increases non enzymatic antioxidant capacity (Neubauer et al., 2019). The antioxidative effect of creatinine referred to its ability to buffer cellular ATP levels, decrease intracellular Ca2+ accumulation (Tarnopolsky, 2011) and reactive oxygen species formation that consequently reduces tissue oxidative damage (Sestili et al., 2011). Based on this mechanism of action, the camel urotherapy could oppose apoptotic and oxidative induced by cisplatin effect. The camel produces special antibodies contain two heavy chains without light chains (Alhaider et al., 2012) which make them accessible to pass easily through blood brain barrier, breast’s milk and renal glomeruli (Romli et al., 2017). So, the camel urotherapy could also attenuate the immunosuppressive effect of cisplatin in cancer patients. Furthermore, it is basic with a pH 7.8 and also has potent antiplatelet activity against ADP-induced and arachidonic acid-induced platelet aggregation (Ali et al., 2019; Alhaider et al., 2012). This study aimed to assess the ameliorative effect of camel urotherapy on cytotoxic effect of cisplatin on urinary tract in male albino rats.
Materials and Methods
Animals
Thirty two Spargue Dawley male rats were bought from the Laboratory of Animal House, Faculty of Pharmacy, Mansoura University, Egypt. The weight of the rats ranged from 220 to 230 g. Before the starting of the study, the rats were left for 2 weeks as a routine program to be adapted to the surrounding environment. Three rats per cage were kept in a room with saw dust covered floor and controlled temperature (25 ± 2 °C). The rats were permitted for unrestricted admission to standard diet and water. The procedures of this experimental animal care, Faculty of Pharmacy, institutional Rresearch (Nanji et al., 1985) Board of Mansoura University, Egypt. The Ethics Committee of the Experimental Animal Care Society and Institutional Research Board (IRB) at Mansoura University approved the experimental procedures.
Preparation of Camel Urine Sample
Camel urine samples were taken from eight years old female camels in Marsa Matrouh desert, Egypt. Camels were fed on mountains weeds and foliage. At first, about 250 – 300 ml urine samples were collected directly into stainless steel containers, and then transferred into suitable glass vials. The urine samples were kept at −80°C until further use.
Experimental Design
The rats were assigned randomly into four groups, each group had 8 rats. Group I received only distilled water, group II received only camel urine (2ml in the morning and 2 ml in the evening every day for 2 months), group III injected with 7 mg/kg cisplatin (Mylan, lot n˚/Batch n˚: 187061) i.p once/ day for 2 months, group IV injected with 7 mg/kg cisplatin once/ day and treated with camel urine received 7 ml/kg cisplatin i.p and 2ml in the morning and 2 ml in the evening of camel urine for 2 months.
Kidney Function Test
Blood samples were collected in coagulated test tubes at the beginning of the study, after 1 month` and 2 months from 4 rats for each duration. The coagulated blood samples were centrifuged at 1200 – 1500 rpm. Clear sera were obtained and stored at – 20˚C. Serum uric acid, urea, and creatinine levels were measured by using a quantitative enzymatic assay (Elitech diagnostics, Sees, France, Urease-GLDH. Kinetic, and Spinreact, respectively) according to the manufacturer’s protocol. The results were standardized using commercial standard solutions.
Tissue Samples
The kidneys and urinary bladder of each experimental animal was excised immediately after scarification. In order to eliminate blood contamination, the kidney was immersed in ice phosphate buffer saline (PBS) and dried by filter paper. Neutral buffered formalin (10%) was used to fix the kidney and urinary bladder tissue for histopathology and histochemical examinations.
Histopathology and Histochemistry
Kidney sections were fixed in formalin (10%) and dehydrated in ascending concentrations of ethyl alcohol (70–100%) and then managed and stained with Hematoxylin and Eosin (H&E) for histopathology (Bancroft et al., 1996). Other 5 µm paraffin embedded kidney sections of kidneys in all groups, after 2 months, were stained with Masson trichrome for collagen detection.
Results
Kidney Function Tests
The activity of creatinine showed significant increase in group III than control (group I) and camel urine gavaged control (group II). The administration of camel urine twice daily to cisplatin treated rats (group IV) significantly (P<0.05) ameliorated the increased creatinine activity than group III either after 1 month or 2 months. Also the levels of urea and uric acid followed the same trend, whereas group II showed significant (P<0.05) increase in their levels than control. Administration of camel urine to cisplatin treated rats significantly reduced urea and uric acid than cisplatin group (group III) as shown in Table (1).
Table 1: Creatinine, Urea, and Uric acid (Mean± SEM) levels after 1 month and 2 months of study among 4 experimental groups
| Duration | Group I | Group II | Group III | Group IV | |
| Creatinine | 1 month | 0.66 ±0.03a | 0.64 ±0.03 a | 1.61± 0.15b | 1.16 ± 0.03c |
| 2 months | 0.90±0.06a | 0.80±0.06a | 2.10±0.13b | 1.00±0.03a | |
| Urea | 1 month | 30.15 ± 1.27a | 28.6 ± 1.12a | 83.00 ± 5.40 b | 51.48 ± 1.44 c |
| 2 months | 0.90±0.06 a | 0.80±0.06 a | 128.50±21.31b | 45.60±3.20c | |
| Uric acid | 1 month | 1.77 ± 0.05a | 1.76 ± 0.05a | 2.79 ± 0.09b | 2.22 ± 0.07c |
| 2 months | 1.80±0.05 a | 1.80±0.05 a | 3.20±0.20 b | 1.90±0.08 a |
Different superscripts (a, b and c) in the same row indicates significance at P < 0.05.
Histopathology
The cross section of kidney of group I of control rats revealed normal glomeruli with normal capillary tuft and intact basement membranes along with patent urinary space. Renal tubules were also normal. Group II, control camel urine group, showed normal kidney tissue architecture. After month of the experimental study, mild cast formation observed in the group II. Whereas, group III showed expansion and proliferation of the glomerular tuft, cast formation and tubular damage along with early fibrosis around glomeruli and in renal medulla. The group IV showed moderate improvement of the kidney tissue (Fig. 1). After 2 months, cisplatin treated group (group III) revealed massive renal tubular lesions of both proximal and distal convolutes tubules. The lesions were consisting of degeneration, pyknotic nuclei, loss of brush borders, necrosis of some tubular cells and hyaline cast formations inside tubular lumens. The camel urine treated group (group IV) showed pronounced improvements, except for some epithelial degenerative changes and mild to moderate cystic dilatations of some tubules (Fig. 2).
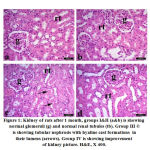 |
Figure 1: Kidney of rats after 1 month, groups I&II (a&b) is showing normal glomeruli (g) and normal renal tubules (tb). |
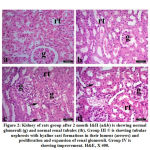 |
Figure 2: Kidney of rats group after 2 month I&II (a&b) is showing normal glomeruli (g) and normal renal tubules (tb). |
At the end of second month the stained sections with masson’s trichrome of both renal medulla and urinary bladder revealed different degree of fibrosis in Group III. The treated group IV showed marked amelioration of fibrosis (Fig. 3 and Fig. 4).
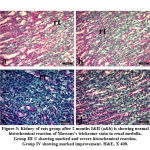 |
Figure 3: Kidney of rats group after 2 months I&II (a&b) is showing normal histochemical reaction of Massons’s trichrome stain in renal medulla. |
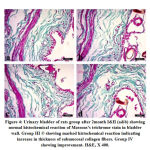 |
Figure 4: Urinary bladder of rats group after 2month I&II (a&b) showing normal histochemical reaction of Massons’s trichrome stain in bladder wall. |
Discussion
Cisplatin is a widely used chemotherapy for the solid tumors (Dasari and Bernard Tchounwou, 2014). One of the drastic effects of cisplatin is nephrotoxicity (Manohar and Leung, 2018). In this study, the effect of camel urine gavage on cisplatin induced nephrotoxicity was assessed after 1 month and 2 months.
Present results revealed higher serum creatinine in cisplatin treated group comparing with control at 1 and 2 months. This results were compatible with Oh et al., (2014) who induced nephrotoxicity by injecting the rats with cisplatin.
On the same line the levels of serum urea and uric acid were significantly increased in cisplatin group than control. The increase in serum uric acid matched with (Nanji et al., 1985) and (Sen et al., 2018) who found that cisplatin causing increase in blood urea nitrogen, serum creatinine and uric acid levels. Urea is well-thought-out the principal nitrogenous metabolic product formed during protein metabolism (Renugadevi and Prabu, 2009). Uric acid is reflected the final product created from an exogenous purines pool as well as endogenous purine metabolism (Maiuolo et al., 2016). Creatinine is an itemization product of muscular creatine phosphate, which is normally formed at a constant rate that matched to muscle mass, and well-thought-out as a measure of renal function (Gowda et al., 2010). The serum creatinine, urea, and uric acid may produce perturbation of the glomeruli filtration rate, and promote their serum levels which is linked to renal damage and considered as nephrotoxicity indicator. The promoted level of serum urea concentrations may elevate after injury of renal parenchymal. Hyperuricemia is well thought-out as a prognostic factor to kidney, which may infer the physical response to an increased production of endogenous oxygen species as uric acid scavenges peroxynitrite molecules (Renugadevi and Prabu, 2009; Rajakrishnan et al., 2017) . Levels of serum creatinine is known as a potent sign of the first phase of any kidney disease than the uric acid and urea levels (Renugadevi and Prabu, 2009). The most important organ in the body is the kidney via which the toxic materials are eliminated from the body of the living body. The analysis of the urine is a major way to establish whether kidney function is going on properly or not (Farooqui et al., 2017). The urea level in the blood is also considered as an indicator of kidney function. Cisplatin produced destruction of the proximal and distal convoluted tubules performing the kidney hemodynamics, declined the reabsorption, promoted the vascular resistance, and resulted in an increment in the BUN levels (Hassan et al., 2010).
The administration of camel urine to cisplatin treated rats significantly decreased serum creatinin, urea and uric acid than cisplatin group. This decrease could be attributed to the beneficial effects of camel urine ingredients that ameliorated higher creatinine, urea and uric acid levels in serum. Moreover, the camel urine has been previously proven to possess antioxidant activity (Mahmoud et al., 2019). The oxidative stress is a major causal factor and mechanism that underlies cisplatin-induced nephrotoxicity (Soni et al., 2018). This effect was confirmed by the observed renal histopathological lesions including tubular nephrosis and necrosis either in cortical region or medulla. Mashhadi et al., (2014) found obvious degeneration in renal tubules and glomeruli in rats received cisplatin .The pathological cascade of cisplatin-tempted renal damage can be timidly divided into three chief occasions, which may overlap at certain times: initial cytotoxic, inflammatory and fibroproliferative events (Taguchi et al., 2005).The in vivo machineries of cisplatin induced nephrotoxicity are multifaceted and include oxidative stress, inflammation, apoptosis, and fibrogenesis (Lieberthal et al., 1996) . Reactive oxygen species (ROS) act directly on cell constituents, including proteins, lipids, and DNA, beside destruction to their structure (Abdel-Daim et al., 2014); (Madkour and Abdel-Daim, 2013). Renal injuries caused by subacute cisplatin administration in the present study may be attributed to the production of fibrogenic factors and embarrassment of cellular proliferation as previously mentioned by (Ibrahim et al., 2016). This was evidenced in the current study by increase fibrous tissue staining in Massons’s trichrome sections. Parallel to our results Quesada et al., (2018) and Francescato et al., 2018) found that renal tissue of cisplatin treated rats had different degrees of fibrosis than control. All above mentioned perturbations increased serum renal product injury markers; creatinine, urea and uric acid in cisplatin group. Functionally, the urine camel could improve the cisplatin induced kidney functions perurbations as well as histopathological lesions and urinary tract fibrosis. However, scarce in vivo studies were available concerning the mechanisms underlying such effect. Therefore further studies should be done to clarify such mechanisms. Camel urine seems to be safe as there were no histopathological lesions nor renal blood biomarkers alterations were found in control group administered camel urine only. Therefore it could be recommended for its safety effect at this dose range.
Conclusion
Taking into account these results, the camel urotherapy has a significant ameliorative effect against the chronic and subacute deteriorative effect of cisplation on the urinary tract. It enhances the renal function tests, reduces the damaging effect of cisplatin on the kidney and urinary bladder tissue. Its ameliorative effect on reducing fibrosis formation exhibits markedly in renal and bladder tissue sections. Further studies could be needed for thorough explanation of other mechanisms of actions of camel urotherapy.
Conflict of Interest
The authors of this paper have no conflict of interest regarding this paper.
Funding Source
There is no funding source
References
- Sonoda, H., Oshikawa-Hori, S., Ikeda, M. An Early Decrease in Release of Aquaporin-2 in Urinary Extracellular Vesicles After Cisplatin Treatment in Rats. Cells., 2019; 8, 139.
CrossRef - Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer., 2007; 7, 573–584.
CrossRef - Desoize, B., Madoulet, C. Particular aspects of platinum compounds used at present in cancer treatment. Crit. Rev. Oncol. Hematol., 2002; 42, 317–325.
CrossRef - Facchini, G., Rossetti, S., Cavaliere, C., D’Aniello, C., Di Franco, R., Iovane, G., Grimaldi, G., Piscitelli, R., Muto, P., Botti, G., Perdonà, S., Veneziani, B.M., Berretta, M., Montanari, M. Exploring the molecular aspects associated with testicular germ cell tumors: a review. Oncotarget., 2018; 9.
CrossRef - Schaffrath, J., Schmoll, H.-J., Voigt, W., Müller, L.P., Müller-Tidow, C., Mueller, T. Efficacy of targeted drugs in germ cell cancer cell lines with differential cisplatin sensitivity. PLoS ONE 12., 2017; e0178930.
CrossRef - Dasari, S., Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. European Journal of Pharmacology., 2014; 740, 364–378.
CrossRef - Manohar, S., Leung, N. Cisplatin nephrotoxicity: a review of the literature. J. Nephrol., 2018; 31, 15–25.
CrossRef - Hayes, D.M., Cvitkovic, E., Golbey, R.B., Scheiner, E., Helson, L., Krakoff, I.H. High dose cis-platinum diammine dichloride: amelioration of renal toxicity by mannitol diuresis., 1977; Cancer 39, 1372–1381.
CrossRef - Oh, G.-S., Kim, H.-J., Shen, A., Lee, S.B., Khadka, D., Pandit, A., So, H.-S. Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolyte Blood Press., 2014;12, 55.
CrossRef - Soni, H., Kaminski, D., Gangaraju, R., Adebiyi, A. Cisplatin-induced oxidative stress stimulates renal Fas ligand shedding. Renal Failure., 2018; 40, 314–322.
CrossRef - Holditch, S.J., Brown, C.N., Lombardi, A.M., Nguyen, K.N., Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. IJMS., 2019; 20, 3011
CrossRef - Tsuruya, K., Ninomiya, T., Tokumoto, M., Hirakawa, M., Masutani, K., Taniguchi, M., Fukuda, K., Kanai, H., Kishihara, K., Hirakata, H., Iida, M. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int., 2003 63, 72–82.
CrossRef - So, H., Kim, H., Lee, J.-H., Park, C., Kim, Y., Kim, E., Kim, J.-K., Yun, K.-J., Lee, K.-M., Lee, H.-Y., Moon, S.-K., Lim, D.J., Park, R. Cisplatin Cytotoxicity of Auditory Cells Requires Secretions of Proinflammatory Cytokines via Activation of ERK and NF-κB. JARO., 2007; 8, 338–355.
CrossRef - Choi, Y.-M., Kim, H.-K., Shim, W., Anwar, M.A., Kwon, J.-W., Kwon, H.-K., Kim, H.J., Jeong, H., Kim, H.M., Hwang, D., Kim, H.S., Choi, S. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS ONE., 2015; 10, e0135083.
CrossRef - Yao, X., Panichpisal, K., Kurtzman, N., Nugent, K. Cisplatin nephrotoxicity: A Review. The American Journal of the Medical Sciences., 2007; 334, 115–124.
CrossRef - Ježek, P., Hlavatá, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. The International Journal of Biochemistry & Cell Biology., 2005; 37, 2478–2503.
CrossRef - Suganya, M., Gnanamangai, B.M., Govindasamy, C., Elsadek, M.F., Pugazhendhi, A., Chinnadurai, V., Selvaraj, A., Ravindran, B., Chang, S.W., Ponmurugan, P. Mitochondrial dysfunction mediated apoptosis of HT-29 cells through CS-PAC-AgNPs and investigation of genotoxic effects in zebra (Danio rerio) fish model for drug delivery. Saudi Journal of Biological Sciences., 2019; 26, 767–776.
CrossRef - Al-Yousef, N., Gaafar, A., Al-Otaibi, B., Al-Jammaz, I., Al-Hussein, K., Aboussekhra, A. Camel urine components display anti-cancer properties in vitro. Journal of Ethnopharmacology., 2012; 143, 819–825.
CrossRef - Alhaidar, A., Abdel Gader, A.G.M., Mousa, S.A. The Antiplatelet Activity of Camel Urine. The Journal of Alternative and Complementary Medicine., 2011; 17, 803–808.
CrossRef - Ahamad, S.R., Alhaider, A.Q., Raish, M., Shakeel, F. Metabolomic and elemental analysis of camel and bovine urine by GC–MS and ICP–MS. Saudi Journal of Biological Sciences., 2017; 24, 23–29.
CrossRef - Zaki, D., Abdelaziz, M., El-Gengeihy, S. and Morsi, N.. Antimicrobial potentialities of some Egyptian desert plants. Herb Hung., 1984; 23, 73-84.
- Kaul, V., Nigam, S. and Dhar, K. Antimicrobial activities of the essential oils of Artemisiaabsinthium Linn, Artemisia vestitia Wall, and Artemisia vulgaris Linn. Indian J Pharmacol., 1976; 38 21-2 .
- Al-Attas, A. Determination of essential elements in milk and urine of camel and in Nigella sativa seeds. Arab J.Nucl.Sci.Appl., 2009; 42, 59-67.
- Alhaider, A., Abdel gader, A. G. M., Almeshaal, N. and Saraswati, S.. Camel milk inhibits inflammatory angiogenesis via downregulation of proangiogenic and proinflammatory cytokines in mice. APMIS., 2014a; 122, 599-607.
CrossRef - Alhaider, A., Gader, A. G. M. A., Almeshal, N. and Saraswati,
- . Camel urine inhibits inflammatory angiogenesis in murine sponge implant angiogenesis model. Biomedicine & Aging Pathology., 2014b;4, 9-16.
CrossRef - Al-Harbi, M., Qureshi, S., Ahmed, M., Raza, M., Baig, M. and Shah, A. . Effect of camel urine on the cytological and biochemical changes induced by cyclophosphamide in mice. J Ethnopharmacol., 1996; 52, 129-137.
CrossRef - Alkhamees, O.A., Alsanad, S.M. A REVIEW OF THE THERAPEUTIC CHARACTERISTICS OF CAMEL URINE. AJTCAM., 2017; 14, 120–126.
CrossRef - Giovannini, I., Chiarla, C., Giuliante, F., Pallavicini, F., Vellone, M., Ardito, F., Nuzzo, G., 2006. [No title found]. Crit Care 10, 421.
CrossRef - Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes., 2014; 63(3):976-981.
CrossRef - Neubauer K, Kempinski R, Matusiewicz M, Bednarz-Misa I, Krzystek-Korpacka M. Nonenzymatic Serum Antioxidant Capacity in IBD and Its Association with the Severity of Bowel Inflammation and Corticosteroids Treatment. Medicina (Kaunas, Lithuania). 2019 Apr; 55(4).
CrossRef - Tarnopolsky MA. Creatine as a therapeutic strategy for myopathies.Amino Acids., 2011; 40:1397-1407.
CrossRef - Sestili P, Martinelli C, Colombo E, et al. Creatine as an antioxidant. Amino Acids. 2011;40 (5):1385-1396.
CrossRef - Ali, A., Baby, B., Vijayan, R. From Desert to Medicine: A Review of Camel Genomics and Therapeutic Products. Front. Genet., 2019; 10, 17.
CrossRef - Alhaider, A.A., Bayoumy, N., Argo, E., Gader, A.G.M.A., Stead, D.A. Survey of the camel urinary proteome by shotgun proteomics using a multiple database search strategy., 2012; Proteomics 12, 3403–3406.
CrossRef - Romli, F., Abu, N., Khorshid, F.A., Syed Najmuddin, S.U.F., Keong, Y.S., Mohamad, N.E., Hamid, M., Alitheen, N.B., Nik Abd Rahman, N.M.A. The Growth Inhibitory Potential and Antimetastatic Effect of Camel Urine on Breast Cancer Cells In Vitro and In Vivo. Integrative Cancer Therapies ., 2017; 16, 540–555.
CrossRef - Nanji, A.A., Mikhael, N.Z., Stewart, D.J. Increase in serum uric acid level associated with cisplatin therapy. Correlation with liver but not kidney platinum concentrations. Arch. Intern. Med., 1985; 145, 2013–2014.
CrossRef - Sen, S., Chakraborty, R., Kalita, P. Dillenia indica fruit prevents cisplatin-induced kidney injury in experimental rats through modulation of oxidative stress, marker enzyme, and biochemical changes. Nutrire., 2018; 43, 15.
CrossRef - Renugadevi, J., Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology ., 2009; 256, 128–134.
CrossRef - Dasari, S., Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol., 2014 ;740, 364–378.
CrossRef - Maiuolo, J., Oppedisano, F., Gratteri, S., Muscoli, C., Mollace, V. Regulation of uric acid metabolism and excretion. International Journal of Cardiology., 2016; 213, 8–14.
CrossRef - Gowda, S., Desai, P.B., Kulkarni, S.S., Hull, V.V., Math, A.A.K., Vernekar, S.N. Markers of renal function tests. N Am J Med Sci., 2010; 2, 170–173.
- Rajakrishnan, R., Lekshmi, R., Benil, P.B., Thomas, J., AlFarhan, A.H., Rakesh, V., Khalaf, S. Phytochemical evaluation of roots of Plumbago zeylanica L. and assessment of its potential as a nephroprotective agent. Saudi Journal of Biological Sciences., 2017; 24, 760–766.
CrossRef - Farooqui, Z., Ahmed, F., Rizwan, S., Shahid, F., Khan, A.A., Khan, F. Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomedicine & Pharmacotherapy., 2017; 85, 7–15.
CrossRef - Hassan, I., Chibber, S., Naseem, I. Ameliorative effect of riboflavin on the cisplatin induced nephrotoxicity and hepatotoxicity under photoillumination. Food Chem. Toxicol., 2010; 48, 2052–2058.
CrossRef - Mahmoud, H.S., Elsaed, W.M., Gabr, S.A. Camel Urotherapy and Hepatoprotective Effects Against Carbon Tetrachloride-induced Liver Toxicity. International J. of Pharmacology., 2019; 15, 696–705.
CrossRef - Mashhadi, M.A., Arab, M.R., Azizi, F., Shahraki, M.R. Histological Study of Toxic Effects of Cisplatin Single Dose Injection on Rat Kidney. Gene Cell Tissue 1, 2014.
CrossRef - Taguchi, Takashi, Nazneen, A., Abid, M.R., Razzaque, Mohammed S. Cisplatin-Associated Nephrotoxicity and Pathological Events, in: Razzaque, M.S., Taguchi, T. (Eds.), Contributions to Nephrology. KARGER, Basel., 2005; pp. 107–121.
CrossRef - Lieberthal, W., Triaca, V., Levine, J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. American Journal of Physiology-Renal Physiology., 1996; 270, F700–F708.
CrossRef - Abdel-Daim, M.M., Abd Eldaim, M.A., Mahmoud, M.M.. Trigonella foenum-graecum protection against deltamethrin-induced toxic effects on haematological, biochemical, and oxidative stress parameters in rats. Can. J. Physiol. Pharmacol., 2014; 92, 679–685.
CrossRef - Madkour, F.F., Abdel-Daim, M.M. Hepatoprotective and Antioxidant Activity of Dunaliella salina in Paracetamol-induced Acute Toxicity in Rats. Indian J Pharm Sc., 2013; 75, 642–648.
CrossRef - Ibrahim, A., Eldaim, M.A.A., Abdel-Daim, M.M. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology., 2016; 68, 1039–1048.
CrossRef - Quesada, A., O’Valle, F., Montoro-Molina, S., Gomez-Morales, M., Caba-Molina, M., González, J.F., de Gracia, M.C., Osuna, A., Vargas, F., Wangensteen, R. 5-aminoisoquinoline improves renal function and fibrosis during recovery phase of cisplatin-induced acute kidney injury in rats. Bioscience Reports., 2018; 38, BSR20171313.
CrossRef - Francescato, H.D.C., Almeida, L.F., Reis, N.G., Faleiros, C.M., Papoti, M., Costa, R.S., Coimbra, T.M. Previous Exercise Effects in Cisplatin-Induced Renal Lesions in Rats. Kidney Blood Press Res., 2018; 43, 582–593.
CrossRef







