Khot Anant1*, S P Chaukimath2, Janagond Ajit3 and Hugar Leela1
1Department of Pharmacology, BLDE(DU), Shri. BM Patil Medical College, Vijayapura, India.
2Department of Psychiatry, BLDE(DU), Shri. BM Patil Medical College & RC, Vijayapura, India.
3Department of Dermatology, BLDE(DU), Shri. BM Patil Medical College & RC, Vijayapura, India.
Corresponding Author E-mail: anantkhot04@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2029
Abstract
Cutaneous adverse drug reactions (CADRs) are common, comprise approximately 2-3% of all the ADRs. Most of them are mild, self-limiting. Severe and potentially life-threatening eruptions occur in approximately 1 in 1000 hospitalised patients. Which carry a high degree of morbidity & mortality. Hence early detection, evaluation and monitoring of ADRs in particular CADRs are essential. As the pattern of CADRs is changing every year with the introduction of new medications & evolving prescription practices. To determine the pattern of various types of CADRs & to identify causative drug implicated in our setup, this study was carried out. A retrospective analysis of the CADRs retrieved from the Pharmacovigilance centre database, reported spontaneously between 25th Aug 2015 to 31st Oct 2019. The CADRs obtained were categorized according to their morphology & the suspected drugs were grouped according to ATC classification. Causality, severity & preventability assessment was done by using pretested scales. 70 patients had CADRs with male to female ratio of 1:2. Urticaria (37.14%) was the most common CADR & 5.7% of the CADRs were severe. Anti-infectives for systemic use (48.6%) was predominantly involved in the causation of CADRs. Most of the CADRs belong to a possible category, 75% of them were either recovered or recovering at the time of reporting & only 25% of the CADRs are preventable. Pattern of ACDRs & the drugs causing them are slightly different in our population as compared to other previous studies. Which emphasizes the need for robust ADR monitoring system in our setup.
Keywords
Cutaneous adverse drug reactions; Pharmacovigilance; Urticaria
Download this article as:| Copy the following to cite this article: Anant K, Chaukimath S. P, Ajit J, Leela H. A Study of Cutaneous Adverse Drug Reactions; Clinical/Morphological Pattern and Causative Agents Reported in an ADR Monitoring Centre in a Tertiary Care Hospital of North Karnataka. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Anant K, Chaukimath S. P, Ajit J, Leela H. A Study of Cutaneous Adverse Drug Reactions; Clinical/Morphological Pattern and Causative Agents Reported in an ADR Monitoring Centre in a Tertiary Care Hospital of North Karnataka. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/3k60ceO |
Introduction
Cutaneous Adverse Drug Reaction (CADR) is any undesirable change in the structure or function of the skin, its appendages or mucous membrane & encompasses all adverse events related to drug eruption, regardless of the aetiology.1 The prevalence of CADRs is about 0.16 to 1.35%, as most of the reactions often are underreported. A study in Germany showed that up to 68.2% of physician who suspected an adverse drug reaction (ADR), did not actually report these events to the appropriate authorities. This may be due to the tendency to report only severe ADR cases.2 Cutaneous reactions are common, comprise approximately 2-3% of all the ADRs.3 Most of the CADRs are mild, self-limiting and usually resolve after the offending agent has been discontinued. Severe and potentially life-threatening eruptions occur in approximately 1 in 1000 hospitalized patients & the mortality rates for Stevens-Johnson syndrome (SJS), Stevens-Johnson syndrome – Toxic Epidermal Necrolysis (SJS‐TEN) overlap, toxic epidermal necrolysis (TEN) & drug‐induced hypersensitivity syndrome (DIHS) are approximately 5–10%, 30%, 50% and 10% respectively.4
Atopy, genetic variations in drug metabolism, HLA variation, comorbidities, underlying disease, active viral infection, immune status of the patient, and concomitant intake of other drugs can alter the rate, presentation, course, and the outcome of CADRs. Along with that, only about 50% of drug reactions can be detected in the premarketing trials.5 Hence early detection, evaluation and monitoring of ADRs, in particular, CADRs is essential in order to prevent the morbidity & mortality due to severe CADRs.6
As the pattern of CADRs is changing every year with the introduction of new medications & evolving prescription practices, understanding its precise nature may help narrow down the search for the offending drug.7 To determine the pattern of various types of CADRs & to find out the causative drug(s) implicated or suspected in the causation of CADRs in our setup, this study was carried out
Materials and Methods
A retrospective analysis of the CADRs retrieved from the Pharmacovigilance centre (BMPMC) database, reported spontaneously by the Health Care Professional’s working in BLDE(DU)’s SBM Patil Medical College Hospital in Vijayapura. The suspected CADRs were diagnosed by either treating consultants or the dermatologist & the relevant details of each CADRs were collected in the spontaneous ADR reporting form designed by NCC (National Coordinating Center). After due completion, CADR details were entered into the Vigiflow. The PDF (Portable Document Format) of ICSR (Individual Case Safety Report) generated was also utilized for data entry. The data pertains to the period extending from 25th Aug 2015 to 31st Oct 2019, an Institutional Ethical Clearance was obtained before starting the study.
The CADRs gathered were categorized according to their morphology8 & the suspected drugs were grouped according to the Anatomical Therapeutic Chemical (ATC) classification of drugs.9 Causality assessment was done by using World Health Organization- Uppsala Monitoring Centre (WHO-UMC) causality assessment system,10 the severity was assessed by using the criterion developed by Hartwig et.al.,11 & the preventability was assessed by using Schumock-Thornton criteria.12 The data collected was analysed & inferences were drawn by using various statistical methods. SPSS Version 20 has been used for data entry & analysis
Results
A total of 70 patients had CADRs, out of which 23 were males & 47 were females (Male to female ratio was 1:2). The mean age of patients suffering from CADRs was 35.71±19.87 years, minimum age of the patient who had CADR was 14 months & maximum was 95 years. The age range in which maximum CADRs were seen between 21-40 years.
Clinical/morphological presentation of CADRs
The proportions of various CADRs are shown in Table 1. The most common CADR was urticaria, followed by maculopapular eruptions & the remaining morphological pattern were as shown in table no.1
Table 1: Showing distribution of various CADRs
| TYPE OF CADR | FREQUENCY |
| Urticaria | 26 (37.14%) |
| Maculopapular eruptions | 20 (28.57%) |
| SJS-TEN overlap | 07 (10%) |
| SJS | 05 (7.14%) |
| TEN | 03 (4.28%) |
| Alopecia | 02 (2.85%) |
| Bullous Fixed Drug Eruptions (FDE) | 02 (2.85%) |
| Acute generalized exanthematous pustulosis (AGEP) | 01 (1.42%) |
| Lichenoid drug eruptions | 01 (1.42%) |
| Acneiform eruptions | 01 (1.42%) |
| Petechial haemorrhages | 01 (1.42%) |
| Skin atrophy | 01 (1.42%) |
15 patients had severe CADRs in the form of SJS, SJS-TEN overlap & TEN variants of epidermal necrolysis. Few representative cases are as shown below. Figure 1. Showing the lesions of a patient who had SJS-TEN overlap, following the ingestion of Tab. Ofloxacin 200 mg + Ornidazole 500 mg Injectable form of this fixed-dose combination (FDC) has been banned in India since 2017.13 Incidentally, the patient had similar complaints 2 years back, the details of the drug or the condition was not available with the patient. Figure 2, showing the Bullous fixed drug eruption following the ingestion of Tab. Amoxicillin 500 mg + Clavulanic acid 125 mg (Tab. Erox CV 625) & Figure 3, showing the urticarial rashes over the abdomen following infusion of Linezolid in a 13-year-old girl.
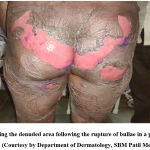 |
Figure 1: Showing the denuded area following the rupture of bullae in a patient with SJS-TEN overlap (Courtesy by Department of Dermatology, SBM Patil Medical College) |
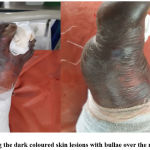 |
Figure 2: Showing the dark coloured skin lesions with bullae over the right foot (Dorsum) |
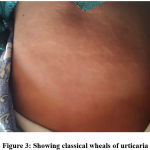 |
Figure 3: Showing classical wheals of urticaria |
Causative drugs
Nature of the drugs implicated in causing CADRs have been shown in Table no.2. According to that, Anti-infectives for systemic use was the predominant group involved in causation of CADRs as per the ATC classification.
Table 2: Showing different anatomical groups (Level 1 as per ATC) involved in causing CADRs
| ATC main group (1st level) | Frequency |
| Alimentary tract and metabolism | 8 (11.4%) |
| Blood and blood-forming organs | 4 (5.7%) |
| Cardiovascular system | 1 (1.4%) |
| Systemic hormonal preparations, excluding. Sex hormones & Insulins | 1 (1.4%) |
| Anti-infectives for systemic use | 34 (48.6%) |
| Musculoskeletal system | 6 (8.6%) |
| Nervous system | 13(18.6%) |
| Respiratory system | 01 (1.4%) |
| Various | 02 (2.9%) |
Among the Anti-infectives for systemic use- 3rd generation cephalosporins (J01DD-4th level) mainly Ceftriaxone & Fluoroquinolones (J01MA), mainly Levofloxacin & FDC of Ofloxacin with Ornidazole were the most common culprits.14 2 Patients also had CADR following ingestion of Cotrimoxazole & 2 due to Linezolid infusion. One patient had developed TEN, following the ingestion of Ornidazole/ Azithromycin/ Fluconazole-Combi kit. This Combi kit has been banned in India, since March 2016. One patient was maintained on a stable dose of Tenofovir + Lamivudine + Efavirenz (TLE) regimen, was changed to Tab. Nevirapine due to shortage of TLE drugs & that resulted in SJS. Another patient had a rash (SJS), after the ingestion of antiretroviral FDC (Zidovudine + Lamivudine + Nevirapine). In about 3 cases, Inj. Ceftriaxone & Inj. Metronidazole was started & stopped at the same time. Hence it was difficult to identify an offending drug.
Causality assessment
According to the WHO-UMC causality assessment system, 48 (68.6%) CADRs were categorised as possible with regarding the suspected drugs & 21 (30%) were categorised as probable & only one was fulfilling the criteria of “Certain”.
Preventability & severity assessment
Among all the CADRs-only 25.71% of CADRs were preventable according to the Schumock-Thornton criteria & the remaining were not preventable.
According to the criterion developed by Hartwig et al., CADRs were classified as,
Mild – 30 cases (42.9%)
Moderate- 36 cases (51.4%)
Severe -4 cases (5.7%)
Although, SJS-TEN spectrum CADRs are considered as severe CADRs. But according to the criterion developed by Hartwig et al., only four patients required Intensive medical care. Hence, 11 cases here were categorically put into CADRs of moderate severity.
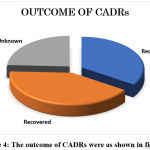 |
Figure 4: The outcome of CADRs were as shown in figure 4 |
The outcome of CADRs
75% of the CADRs were either recovered or recovering at the time of reporting. Unfortunately, one patient had died because of Acute Myocardial Infarction, though it was a fatal outcome, not attributed to ADR.
Discussion
The present study was carried to determine the pattern of CADRs in our setup; a total of 70 CADRs were reported during the study period. The mean age of patients suffering from CADRs was 35.71±19.87 years. The age range in which maximum CADRs were seen between 21-40 years. Which is in accordance with the study carried out by Pudukadan D et al.,15 & Dimri D et al.,16 In our study, there was female preponderance about the occurrence of CADRs. Which was similar to most of the previous studies, but opposite to the research carried out by Agrawal et al.,
The most common CADR was Urticaria. Which accounted for 37.14% of all CADRs in our study. Which was in conformity with the survey by Al-Raaie et al.,17 & opposite to the study by Agrawal, et al. According to them, the most common CADR was FDE. The second most common CADR was Maculopapular rash or eruptions (28.57%), which was similar to the study carried out by Pudukadan D et al. & opposite to the research carried out by Janardhan B et al., according to them the Maculo-Papular eruptions were the most common CADR, followed by urticaria.18 This variation could be due to different patterns of drug usage and distinct ethnic group characteristics. Along with that, 15 (21.42%) patients had epidermal necrolysis variants. Which carry a high degree of morbidity, economic burden & mortality.19
Anti-infectives for systemic use was the most common anatomical group, suspected to be involved in the causation of CADRs. Which was similar to the previous studies such as Jha N et al., & Sharma R et.al.,20 But, opposite to the research conducted by Al-Raaie et al., According to them nonsteroidal anti-inflammatory agents (NSAIDs)-M01A (3rd level, Pharmacological subgroup) OR Musculoskeletal system (Level 1, anatomical group-as per ATC classification), was predominantly involved in the causation of CADRs. Within Anti-infectives for systemic use, 3rd generation Cephalosporins (ATC code-J01DD) were incriminated in the causation of CADRs.21 Different patterns of drug usage in the populations studied can explain this variation. Also, we lack uniform quality of medications throughout India & availability of standard treatment guidelines. The primary indications for the above category of drugs were fever, Urinary tract infection & Acute Gastroenteritis.
The nervous system was the second most common group, incriminated in the causation of CADRs. Which was similar to a study carried out by Sharma VK et al.,22 & opposite to the research carried out by Sharma S et al.,23 & Modi A et.al.,24
According to the WHO-UMC causality assessment system, most of the CADRs were categorised as “Possible”, because the dechallenge was interrupted by the symptomatic treatment of CADRs. Rechallenge was not performed on the ethical ground; hence it was difficult to assign a category of “Certain”. Therefore, few of them were categorised as “Probable”. Among all the CADR’s -only ¼ th (25.71%) of them were preventable, which was similar to the study carried out by Modi A et.al. Most of the CADRs were mild to moderate in severity & only 4 (5.7%) patient had SCARs. Which was similar to the study carried out by Sharma R et.al. 75% of the CADRs were either recovered or recovering at the time of reporting with or without symptomatic treatment & outcome was unknown in remaining patients. This was due to the lack of follow up. Limitation of this study was the sample size, maybe due to under-reporting & at times the patients will consult a general practitioner for an ADR, there is no way how we can track them.
Conclusion
Pattern of ACDRs & the drugs causing them are slightly different in our population from others. May due to availability of even high-risk drugs without prescription & being an economically weak area, which makes them take medicines issued by the pharmacist or people working in a pharmacy. Which emphasises the need for more extensive ADR monitoring in our setup & it will be useful in generating our data. For physicians, it is always a challenge to weigh the benefits & risks of each therapeutic decision because there are no reliable tools for diagnosing ADRs. Further studies in this area are needed for early recognition & prevention of morbidity due to CADRs.
Acknowledgement
We want to thank Dr Akram A Naikwadi, Professor & Head, Department of Pharmacology, the Medical & Nursing Superintendent of Shri. BM Patil Medical College Hospital for their constant support & cooperation. We also would like to thank, NCC-IPC, PvPI for providing training & technical support to our centre
Conflict of interest
None
Funding source
No funding was applied
References
- Nayak S, Acharjya B. Adverse cutaneous drug reaction. Indian J Dermatol. 2008;53(1):2–8.
- Talib N, Leelavathi M, Hamzah Z. Common adverse cutaneous drug reaction patterns and the causative drugs in Malaysia. South African Family Practice. 2015 Jul 4;57(4):227–30.
- Agrawal A, Ghate S, Gupta AK, Dhurat R. Clinical spectrum of cutaneous adverse drug reactions. Indian J Drugs Dermatol 2018; 4:61-6.
- Chung WH, Wang CW, Dao RL. Severe cutaneous adverse drug reactions. J Dermatol. 2016;43(7):758–66
- Jha N, Alexander E, Kanish B, Badyal DK. A Study of Cutaneous Adverse Drug Reactions in a Tertiary Care Center in Punjab. Indian Dermatol Online J. 2018 Oct;9(5):299–303.
- Duong TA, Valeyrie-Allanore L, Wolkenstein P et.al. Severe cutaneous adverse reactions to drugs. Lancet 2017; 390: 1996–2011
- Jayanthi CR, Bedwal A, Rajarathna K. Cutaneous adverse drug reactions from a teaching hospital in Bengaluru: An observational study to determine the spectrum and outcome. Natl J Physiol Pharm Pharmacol 2017;7(5):476-481.
- Micheletti RG, Rosenbach M, Wintroub BU et.al. Cutaneous Drug Reactions. In: Jameson JL, Kasper DL, Longo DL, Fauci AS, Hauser SL, Loscalzo J editors. Harrison’s Principles of Internal Medicine. 20thed.New York: McGraw-Hill; 2018.p.362-72
- WHOCC – Structure and principles [Internet]. [cited 2019 Nov 19]. Available from: https://www.whocc.no/atc/structure and principles/
- Organization WH. The use of the WHO-UMC system for standardized case causality assessment. Uppsala Monit Cent. 2005;(3):2–7.
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992; 49:2229‑32
- Schumock GT, Thornton JP, authors. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992; 27:538 [PubMed]
- Banneddrugs.pdf [Internet]. [cited 2019 Nov 19]. Available from: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadConsumer/banneddrugs.pdf
- Wong SX, Tham MY, Goh CL, Cheong HH, Chan SY. Spontaneous cutaneous adverse drug reaction reports—An analysis of a 10-year dataset in Singapore. Pharmacol Res Perspect. 2019;7(2):1–10.
- Pudukadan D, Thappa DV. Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India. Indian J Dermatol Venereol Leprol2004; 70:20-4.
- Dimri D, Raina RS, Thapliyal S, Thawani V. Retrospective Analysis of Pattern of Cutaneous Adverse Drug Reactions in Tertiary Hospital of Pauri Garhwal. J Clin Diagn Res. 2016 May;10(5):FC01–6.
- Al-Raaie F, Banodkar DD. Epidemiological study of cutaneous adverse drug reactions in Oman. Oman Med J. 2008;23(1):21–7.
- Janardhan B, Shailendra D. Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital. Int JRes Dermatol 2017; 3:74-8.
- Shah SP, Desai MK, Dikshit RK. Analysis of Cutaneous Adverse Drug Reactions at a Tertiary Care Hospital – a Prospective Study. Trop J Pharm Res 2011; 10(4):517-522 doi: http://dx.doi.org/10.4314/tjpr.v10i4.18
- Sharma R, Dogra D, Dogra N. A study of cutaneous adverse drug reactions at a tertiary centre in Jammu, India. Indian Dermatol Online J. 2015; 6:168–71.
- Tian XY, Liu B, Shi H, Zhao ZR, Zhou XP, Zhang T, et al. Incidence of adverse cutaneous drug reactions in 22,866 Chinese inpatients: a prospective study. Arch Dermatol Res. 2015;307(9):829–34.
- Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: clinical pattern and causative agents–a 6-year series from Chandigarh, India. J Postgrad Med 2001 Apr 1;47(2):95.
- Sharma S, Jayakumar D, Palappallil DS. Pharmacovigilance of Cutaneous Adverse Drug Reactions among Patients Attending the Dermatology Department at a Tertiary Care Hospital. Indian Dermatol Online J. 2019 Aug 28;10(5):547–54.
- Modi A, Desai M, Shah S, Shah B. Analysis of Cutaneous Adverse Drug Reactions Reported at the Regional ADR Monitoring Center. Indian J Dermatol. 2019;64(3):250.








