Nutakki Ravikumar* and Chilaka Naga Kavitha
and Chilaka Naga Kavitha
Department of Pharmacology, GITAM Institute of Pharmacy, GITAM (Deemed to be University), Visakhapatnam, India, 530045
Corresponding Author E-mail : nutakkiravi@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1948
Abstract
Immune dysregulation arbitrated through inequity of T helper sub-types is linked in both type 1 diabetes and asthma inductions. The imbalance of T helper sub-types leads to unbalance of immune cytokines in these conditions. Re-establishing of immune balance, in other words, re-balance of all arms of immune system is crucial in treating comorbid conditions associated with type 1 diabetes and asthma. Curcumin’s role on dysregulated immune function in these comorbid conditions is not evident. This study was focused to reveal the immune modulatory effect of curcumin in comorbid diabetic asthma mice. Male Balb/c mice were made type 1 diabetic by intravenous injection of 80 mg/kg alloxan on day 0. The mice were sensitized twice on day 3 and 8 with intraperitoneal injection of ovalbumin emulsion (50 µg ovalbumin blended with 2.5 mg alum/sensitization). Allergic asthma was induced by daily intranasal challenge of ovalbumin (100 µg ovalbumin/25 µl of sterile saline) on days 13-15. Oral curcumin treatment (100 & 200 mg/kg) was given each day from day 3 to day 15. Nasal hyperresponsiveness was recorded on day 16 soon after the intranasal ovalbumin challenge. Blood, bronchoalveolar lavage fluid and lungs were collected 1 h after recording of nasal hyperresponsiveness for further analysis. Curcumin treated mice showed significantly less numbers of eosinophils, interleukin-4 levels with high interferon-gamma to interleukin-4 ratio in blood and bronchoalveolar lavage fluids. In addition, curcumin condensed allergic airway inflammation by obstructing the infiltration of inflammatory cells and the metaplasia of mucus cells. Furthermore, curcumin 200 mg/kg reduced blood glucose levels in these mice. In conclusion, curcumin alleviated allergic asthma by modulating immune dysfunction along with glucose reducing property in comorbid mice.
Keywords
Curcumin; Diabetic Asthma; Eosinophils; Immune Dysfunction; Ovalbumin; T Helper Cells
Download this article as:| Copy the following to cite this article: Ravikumar N, Kavitha C. N. Therapeutic Potential of Curcumin on Immune Dysregulation in Comorbid Diabetic Asthma in Mice . Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Ravikumar N, Kavitha C. N. Therapeutic Potential of Curcumin on Immune Dysregulation in Comorbid Diabetic Asthma in Mice . Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/2B8tkkf |
Introduction
T helper (Th) cell sub-types work synergistically to balance the immune equilibrium in healthy immune system and the supremacy of T helper 1 (Th1) can down-regulate T helper 2 (Th2) and vice versa [1]. Inequity of Th1/Th2 cells is noticed in immune dysregulated conditions; autoreactive Th1 cells in type 1 diabetes (T1D) and autoreactive Th2 cells in asthma [2]. Participation of few common immune dysregulatory mechanisms may be involved in the concurrent presence of these two conditions [3]. However, the co-occurrence of T1D and asthma is still provocative due to their opposing regulatory dependence of Th1 and Th2 on each other [1,4]. Interactions of genetic susceptibility and environmental triggers at individual’s young age may govern the immune dysregulation and development of these clinical conditions [4-6].
Cytokine disparity due to over expression of either autoreactive Th1 or Th2 cells along with several other inflammatory cells and defective auto tolerance play an important role in asthma exacerbations and T1D pathogenesis [7-11]. Functionally, Th1 cells secrete interferon-gamma (IFN-γ) and Th2 cells secrete interleukin-4 (IL-4) and the Th1/Th2 cytokine ratio is used to depict the major Th sub-type [12,13]. High levels of IFN-γ in T1D and over secretions of IL-4, interleukin-5 (IL-5), interleukin-13 (IL-13) in asthma have been observed [14-18]. In case of patients with both T1D and asthma, an intermediary and distinctive cytokine secretary pattern that combines the features of both diseases is noticed [4]. However, data paucity is present from animal studies that denote the differences in Th1/Th2 paradigm and cytokine profile under comorbid conditions of both T1D and allergic asthma. Our recent study results in ovalbumin induced allergic asthma in diabetic mice demonstrated the dysregulated Th1/Th2 paradigm and an intermediary cytokine profile than in mice without diabetes [19]. We believe that understanding immune dysregulation mediated through Th1/Th2 immune mechanisms in comorbid diabetic asthma will help identifying future immunomodulatory agents that may be effective at treating asthma patients with associated autoimmune diabetes.
Curcumin is a polyphenolic compound that possesses strong antioxidant properties along with anti-inflammatory effects and is widely utilized in Asian countries for treatment of inflammation. Curcumin has shown to attenuate allergic inflammations, asthma and food allergy models in mice [20-22]. Further, curcumin demonstrated reduction of abnormalities associated with diabetes made by alloxan and streptozotocin [23,24]. In addition, curcumin has shown to interact with several immune mediators thus showcasing immunomodulation in several different cancer types [25-27]. Also, curcumin has demonstrated immunomodulatory activities in osteoclastic diseases [28] and against pathogenic Vibrio alginolyticus [29]. It has been thought that the complex interface between inflammation, oxidative stress and immunity appears to contribute to the immune modulation potential of curcumin [30]. Yet, its role on immune dysregulation in comorbid conditions of both T1D and asthma has not been attempted so far. Therefore, it was thought worthwhile to investigate curcumin for its potential as immunomodulator in comorbid mice with T1D and allergic asthma.
Materials and Methods
Animals
Male Balb/c mice having age between 6-8 weeks were obtained from the Vivo Biotech Ltd, India. Mice were housed individually and acclimated for at least 5 days before the experimentation. Mice were maintained on a 12 hour light/dark cycle in an environment with controlled temperature (19-25 0C), relative humidity (30-70%) and air changes (15-20 per hour). The animal procedures were handled as per the approved protocol (VB/IAEC/07/2019/360/Mouse/Balb/c) by the Institutional Animal Ethics Committee (IAEC) of Vivo Biotech Ltd. Mice were given free access to food and water throughout the study duration.
Experimental Groups and Treatment
Post acclimatization, the mice were grouped into six groups with n=6 per group based on their body weight: (1) non-diabetic asthma + vehicle; (2) non-diabetic asthma + curcumin 100 mg/kg; (3) non-diabetic asthma + curcumin 200 mg/kg; (4) diabetic asthma + vehicle; (5) diabetic asthma + curcumin 100 mg/kg; (6) diabetic asthma + curcumin 200 mg/kg. Asthma was induced in both non-diabetic and diabetic animals as per the procedures mentioned below. Curcumin (Cat No. C1386) was purchased from Sigma-Aldrich, India. Curcumin was dissolved in 1% dimethylsulphoxide (1% DMSO) for its administration into animals and was dosed orally on daily basis for 13 days as per the experimental design shown in Figure 1. Vehicle animals received 1% DMSO per kg. body weight orally. Daily body weights and clinical signs were noted until end of the study.
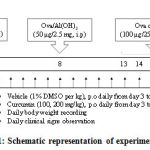 |
Figure 1: Schematic representation of experimental design |
Alloxan Diabetes
Diabetic group mice were fasted for 3 h on day 0 before they were injected with 80 mg/kg of alloxan monohydrate (Sigma-Aldrich, India) via tail vein injection as per the procedure described earlier with slight modifications [31]. Alloxan was dissolved freshly in sterile water. Post 72 h of alloxan injection, the induction of T1D was confirmed by measuring the blood glucose using AccuCheck Aviva glucometer (Roche Diagnostics) in the sample drawn from tail prick. The blood glucose level >250 mg/dl was considered as diabetic for the study.
Ovalbumin Asthma
The mice in above groups were sensitized twice on days 3 and 8 with an intraperitoneal (i.p) injection of 50 µg ovalbumin (Ova) emulsified in 2.5 mg of aluminium hydroxide (Al(OH)3) in 0.2 ml of PBS. Sensitized mice were challenged with 100 µg Ova in 25 µl of sterile saline (4% w/v Ova) via intranasal on days 13-15 one hour later to compound treatment, as described previously with modifications to induce allergic asthma [32,33].
Evaluation of Nasal Hyperresponsiveness (NHR)
Nasal hyperresponsiveness as a measure of upper airway hyperreactivity was evaluated on day 16, twenty four hours after last compound treatment and immediately after intranasal Ova (4% w/v Ova) challenge. The number of nose rubbings in 10 minutes immediately after Ova challenge were counted for each mouse as described elsewhere [34].
Measurement of Differential Leukocyte Count (DLC) and Blood Glucose Levels
After NHR evaluation, a small sample of blood was drawn via tail prick and random blood glucose was estimated using AccuCheck Aviva glucometer (Roche Diagnostics). Further blood was withdrawn via retro orbital plexus and the aliquot in ethylene diamine tetra acetic acid (EDTA) tubes was used to count total leukocytes by hematology analyser (Medonic CA620). The differential leukocytes were counted manually after Giemsa staining of blood smear. Absolute differential leukocyte counts were derived by multiplying the percentage of differential leukocyte count (DLC) with total leukocyte count. The aliquot in serum separators was centrifuged at 10000 rpm for 5 minutes at 4 0C and the serum separated was stored at -80 0C for cytokine estimations using enzyme-linked immunosorbent assay (ELISA) kit.
Bronchoalveolar Lavage Fluid (BALF)
The mice were euthanized by CO2 asphyxiation immediately after blood collection. The chest cavity was opened and a tracheal cannula was inserted. Two infusions of 0.8 ml of phosphate buffered saline (PBS) into lungs were performed to collect the maximum amount of bronchoalveolar lavage fluid (BALF). Soon after collection, BALF was centrifuged at 2500 rpm for 10 minutes at 4 0C and the supernatant was collected and stored at -80 0C for cytokine estimations using ELISA kit. A 250 µl of PBS was added to the lavage pellet and total leukocytes were counted with Medonic CA620 hematology analyser. The differential leukocyte counts in BALF were estimated manually after staining of BALF smear with Geimsa and the absolute DLCs in BALF were derived by multiplying percent DLC with total leukocytes in BALF.
Histology of Lung
Lungs (n=2) in each group were collected in 10% formalin and embedded in paraffin blocks. A 4 µm sections were made and stained with hematoxylin & eosin (H & E). The H & E stained lung sections were observed for inflammatory cell aggregates and mucus cell metaplasia around airways as a measure of airway inflammation by a pathologist who is unaware of treatment details of each group. The mucus cell metaplasia was graded as follows: 0=no difference; 1=scattered mucus cells; 2=aggregates of mucus cells; 3=monolayer of mucus cells; and 4=multi-layered mucus cells. Gross observation of spleen and other internal organs was carried during necropsy and spleen weights were noted.
Enzyme-Linked Immunosorbent (ELISA) Assay for Th1 and Th2 Cytokines in Serum and BALF
The level of IFN-γ as Th1 responsive cytokine and IL-4 and IL-10 as Th2 responsive cytokines was assessed by ELISA method in both serum and BALF, following kit’s manual (Invitrogen Mouse Th1/Th2 Elisa kit, cat # 88-7711-44). The assay was conducted in 96-well format and the ODs were obtained by reading the plate using Micro plate reader (Multiskan GO, Thermo Scientific). The unknown concentrations were derived from extrapolation of the standard curve constructed using mouse IFN-γ (range: 15-2000 pg/ml), mouse IL-4 (range: 4-500 pg/ml) and mouse IL-10 (range: 30-4000 pg/ml).
Statistical Analysis
All statistical analysis and graphs were generated by using GraphPad Prism 8.1 software. Data were analysed using One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test or unpaired student’s t-test wherever applicable and p<0.05 was considered as significant.
Results and Discussion
Curcumin Reduces Alloxan-Induced Hyperglycemia in Diabetic Asthma Mice
Balb/c mice presented significant rise in blood glucose at 72 h (day 3) after single alloxan injection with a concomitant decrease in body weight, suggesting T1D state in these mice (Table 1). Curcumin treatment at 200 mg/kg dose exhibited significant decrease in alloxan-induced hyperglycemia in diabetic asthma mice when paralleled to vehicle treated diabetic asthma mice (Figure 2). However, curcumin at 100 mg/kg dosage did not show notable decrease in blood glucose of diabetic asthma mice compared to vehicle treatment (Figure 2). Also, curcumin at 200 mg/kg dose significantly lowered the percent body weight loss (-10.46% vs -15.81%) than vehicle treated mice (Table 2), suggesting the improvement of type 1 diabetic condition. The antidiabetic potential by curcumin observed in this study is in congruence with its reported effect. Curcumin was stated to enhance the recovery and role of pancreatic islet cells, which led to the alleviation of hyperglycemia in alloxan model of diabetes [23]. Also, it has been shown that curcumin protected cyclophosphamide induced rapid onset of autoimmune diabetes by inhibiting the pancreatic lymphocyte proliferation and leukocyte infiltration in NOD mice [35]. Further, curcumin at 200 mg/kg significantly reduced the relative spleen weight versus non-diabetic asthma vehicle treated mice (0.43 vs 0.52; Table 2). The reduced spleen size could be due to the inhibitory role of curcumin on lymphocyte and splenocyte proliferation mediating through its immunosuppressive activity after immunogenic stimuli [36].
Table 1: Hyperglycaemia and body weights in diabetic asthma mice on day 3 after alloxan injection
| Group | Random blood glucose (mg/dl) | Body weight (g) |
| Diabetic asthma + vehicle | 412.00 ± 52.31**** | 22.10 ± 0.41^^^ |
| Diabetic asthma + curcumin 100 mg/kg | 406.67 ± 51.16**** | 23.21 ± 0.60^ |
| Diabetic asthma + curcumin 200 mg/kg | 408.67 ± 55.38**** | 22.34 ± 0.28^^^ |
Data are presented as mean ± SEM (n=6).
****p<0.0001 versus random blood glucose before alloxan treatment (106.00 ± 9.37)
^p<0.05, ^^^p<0.001 versus body weight before alloxan treatment (24.86 ± 0.34)
Table 2: Percent body weight change from basal and relative spleen weights in non-diabetic and diabetic asthma mice on day 16 after curcumin treatment
| Group | % Body weight change | Relative spleen weight (mg) |
| Non-diabetic asthma + vehicle | 2.49 ± 0.75 | 0.52 ± 0.04 |
| Non-diabetic asthma + curcumin 100 mg/kg | 2.55 ± 1.03 | 0.50 ± 0.02 |
| Non-diabetic asthma + curcumin 200 mg/kg | 1.04 ± 1.28 | 0.43 ± 0.02* |
| Diabetic asthma + vehicle | -15.81 ± 1.16 | 0.31 ± 0.04 |
| Diabetic asthma + curcumin 100 mg/kg | -13.59 ± 2.95 | 0.31 ± 0.04 |
| Diabetic asthma + curcumin 200 mg/kg | -10.46 ± 0.99## | 0.24 ± 0.03 |
The percent body change was calculated using formula: (Body weight on day 16 – body weight on day 3) / body weight on day 3 * 100.
Data are presented as mean ± SEM (n=6).
*p<0.05 versus non-diabetic asthma vehicle; ##p<0.01 versus diabetic asthma vehicle
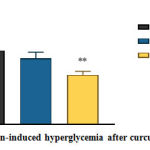 |
Figure 2: Alloxan-induced hyperglycemia after curcumin treatment. |
The random blood glucose was measured using glucometer on day 16 from blood withdrawn via tail prick.
Data are presented as mean ± SEM (n=6).
**=p<0.01 against diabetic asthma vehicle.
Curcumin Inhibits Nasal Hyperresponsiveness after Ovalbumin Challenge
The allergen-induced allergic hyperreactivity of upper airways was assessed by measuring the number of nose rubbings as a measure of nasal hyperresponsiveness immediately after challenge with ovalbumin. Untreated non-diabetic and diabetic mice presented almost similar NHR after Ova challenge. The nasal rubbing frequency increased due to intranasal Ova challenge was reduced after curcumin treatment in both non-diabetic and diabetic asthma mice. The significant reduction of frequency of nasal rubbings was achieved with curcumin treatment at 200 mg/kg in case of non-diabetic asthma mice compared to no treatment group. Curcumin reduced NHR in diabetic mice with insignificant change achieved compared to vehicle. The NHR lowering potential was almost similar in both dose groups of curcumin (Figure 3). Curcumin has been shown to inhibit the discharge of allergic mediators like histamine and leukotrienes by inhibiting the mast cell degranulation, which anchors the mediation of allergic hyperresponses in airways during asthmatic stimuli. Suppression of histamine discharge from rat peritoneal mast cells in in-vitro set-up has suggested the inhibition of mast cell dependent specific and non-specific allergic responses by curcumin [37]. Also, similar results were reported in another mouse study showcasing the anti-inflammatory role of curcumin in allergic inflammation via inhibition of immunoglobulin E (IgE) mediated activation of mast cells [38].
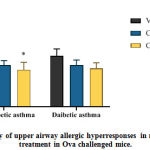 |
Figure 3: Frequency of upper airway allergic hyperresponses in mice after curcumin treatment in Ova challenged mice. |
The number of nasal rubbings per mice was counted for 10 minutes immediately after last Ova challenge.
Data are presented as mean ± SEM (n=6).
*p<0.05 versus non-diabetic asthma vehicle.
The effect of curcumin on Ova-induced allergic airway inflammation was studied by measuring the inflammatory cell numbers in blood, BALF and H & E stained lung sections from samples collected 1 h after last Ova challenge in sensitized non-diabetic and diabetic mice. Curcumin treatment at both 100 and 200 mg/kg doses demonstrated significant lowering of Th2 responsive eosinophils in BALF (Figure 4a) and blood (Figure 4b) in both non-diabetic asthma and diabetic asthma mice. Eosinophil reduction by curcumin has been widely reported and our results are in harmony through the reported data [39,40]. Eosinophil reduction associated with reductions in total IgE as well as in Ova-specific IgE was noticed after curcumin treatment in Ova induced asthma model that led to the inhibition of local mucosal inflammation of lung airways [39].
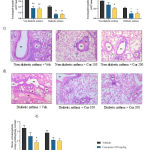 |
Figure 4: Decreased infiltration of Th2 responsive inflammatory cells in BALF |
Eosinophil cell numbers in a) BALF and b) blood collected 1 h after last Ova challenge. c) mucus cell metaplasia in bronchioles (BV) and perivascular (BV) inflammatory cell aggregates in non-diabetics, d) mucus cell metaplasia and perivascular inflammatory cell aggregates in diabetics, and e) average grading of mucus cell metaplasia per lung section obtained 1h after last Ova-challenge from sensitized mice.
Absolute differential leukocyte counts in BALF and blood were determined by Giemsa staining. Lung tissue inflammation was assessed by H & E staining of lung sections (magnification, x100).
Inflammatory cell aggregation around blood vessel (BV) and mucus cell metaplasia of bronchiole (BR) in lung sections was shown by arrow. Vehicle, Curcumin at 100 mg/kg and 200 mg/kg are represented as Veh, Cur 100 and Cur 200 respectively in lung section figures.
Data are presented as mean ± SEM (n=4 in BALF and n=6 in blood for eosinophil data; n=2 (3 lung sections/animal) for average graded mucus metaplasia).
*p<0.05 and **p<0.01versus non-diabetic vehicle; #p<0.05 and ##p<0.01 versus diabetic asthma vehicle.
Further, histopathological observation of H & E stained lung sections revealed that both doses of curcumin reduced the severity of mucus cell metaplasia and perivascular inflammatory cell aggregation in both non-diabetic (Figure 4c) and diabetic (Figure 4d) asthma mice after Ova challenge. Several literature documented the inhibition of airway inflammation of lungs with curcumin treatment and proposed the involvement of nuclear factor-kappa B (NF-κB) and its inhibition by curcumin to alleviate the inflammation of lung airways [20,39,40]. Nuclear factor-kappa B (NF-κB) activation is evident in the pathogenesis of asthma and agents that aggravate asthma development are known to activate NF-κB. Rapid activation of NF-κB has been observed predominantly in pulmonary epithelial cells in Ova induced pulmonary inflammation [41]. Inhibition of NF-κB via the blockade of p65 and p50 translocation has been shown to inhibit lymphocyte proliferation and production of cytokines that are responsible for eosinophil production, thereby inhibiting the allergic airway inflammation and hyperresponses [39]. In addition, curcumin treatment at 200 mg/kg showed significantly lower graded mucus cell metaplasia per lung section in non-diabetic sensitized mice after Ova challenge compared to vehicle treatment. Mucin 5AC (MUC5AC) and monocyte chemoattractant protein-1 (MCP-1), which are responsible for mucus hypersecretion and goblet cell metaplasia have shown to be decreased after curcumin treatment [20]. Also, reduced metaplasia grading observed with curcumin treatment in diabetic asthma mice too, but the changes were insignificant compared to vehicle (Figure 4e).
Curcumin Modulates Th1/Th2 Cytokine Balance in Ova-induced Allergic Asthma in Both Non-Diabetic and Diabetic Mice
In order to know the effect of curcumin on Th1/Th2 immune balance, the Th1 and Th2 cytokines were estimated in BALF and serum samples collected post 1 h of last Ova challenge. The cytokines estimated were IFN-γ as a Th1 mediated cytokine secretion and IL-4 and IL-10 as Th2 mediated cytokine secretion. The BALF and serum cytokine levels indicated a significant up-regulation of Th2 responsive IL-4 after Ova sensitization and challenge in both non-diabetic and diabetic mice compared to normal controls. The levels of IL-4 in normal control animals of our previous experiment were used for comparison purpose. The treatment of curcumin at 200 mg/kg dose in these mice significantly lowered the IL-4 levels in both BALF (Figure 5b) and serum (Figure 5e) leading to a significant increase in Th1/Th2 (IFN-γ/IL-4) ratios, respectively in these fluids (Figure 5g and 5h). The extent of Th1/Th2 ratio increase was more in case of diabetic asthma mice after curcumin treatment than in non-diabetic asthma mice (Figure 5g and 5h). The shift of Th1/Th2 ratio towards increased number was greatly attributed to the lowered IL-4 levels in the denominator after curcumin treatment. This upward trend in Th1/Th2 cytokine balance after curcumin treatment indicated the anti-inflammatory role of curcumin on overactive Th2 responsive cytokines in these mice. Immunomodulatory effect of curcumin that mediated through altering Th1/Th2 balance has been witnessed in a mouse model of food allergy, which is again a Th2 dominant autoimmune disease [21]. Turmeric extract treatment have revealed to regulate immune responses towards Th1 from Th2 dominant immune responses as measured by an increase in IFN-γ and decrease in IL-4 levels in splenocytes isolated from Ova-immunized mice [21]. Further, curcumin at 100 mg/kg dose also showed reductions in IL-4 in BALF (Figure 5b) and serum (Figure 5e) of both non-diabetic and diabetic asthma mice compared to vehicle but to an insignificant level. Interferon-gamma and IL-10 levels were not affected greatly by curcumin treatment although there were little decrease observed when compared to vehicle treatment in these mice (Figure 5c and 5f).
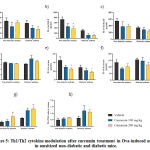 |
Figure 5: Th1/Th2 cytokine modulation after curcumin treatment in Ova-induced |
a-c) BALF levels of IFN-γ, IL-4, IL-10 respectively at 1 h after last Ova challenge. d-f) Serum levels of IFN-γ, IL-4, IL-10 respectively at 1 h after last Ova challenge.
g & h) Th1/Th2 ratio in BALF & serum, respectively.
Data are presented as mean ± SEM (n=4 for BALF and n=6 for serum).
*p<0.05versus non-diabetic asthma vehicle; #p<0.05versus diabetic asthma vehicle.
Conclusion
The study results exposed the anti-inflammatory and immunomodulatory role of curcumin in diabetic asthma as well as in non-diabetic asthma mice. It showed significant inhibition of all allergic asthma parameters such as low levels of BALF and blood eosinophilia, IL-4, less accumulation of inflammatory cells and mucus cell metaplasia around airways in these mice. Curcumin reduced NHR significantly in non-diabetic asthma mice and insignificantly in diabetic asthma mice. Moreover, curcumin modified the dysregulated Th1/Th2 immune balance towards a favourable immune response, ultimately leading to the alleviation of allergic inflammation in the lungs of these mice. Additionally, curcumin at 200 mg/kg improved glycemia and decreased body weight loss in comorbid diabetic asthma mice. Nevertheless, curcumin modulated the immune dysregulation mediated through altered Th1/Th2 balance in comorbid mice leading to the alleviation of allergic asthma and diabetes in these mice.
Acknowledgement
The authors are grateful to the Vivo Biotech Ltd. (Hyderabad, India) for providing the animals and laboratory research facilities for this research work. The authors are also thankful to GITAM Institute of Pharmacy, GITAM (Deemed to be University), Visakhapatnam for providing support to the research work.
Conflict of Interest
Authors have no conflict of interest.
References
- Kidd P, 2003. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Alternative Medicine Review, 8(3):223-46.
- Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM and Nevalainen J, 2018. The association between asthma and type 1 diabetes: A paediatric case-cohort study in Finland, years 1981-2009. International Journal of Epidemiology, 47(2):409-16.
- Rottem M and Shoenfeld Y, 2003. Asthma as a paradigm for autoimmune disease. International Archives of Allergy and Immunology, 132:210-14.
- Rachmiel M, Bloch O, Bistritzer T, Weintrob N, Ofan R, Koren-Morag N and Rapoport MJ, 2006. Th1/Th2 cytokine balance in patients with both type 1diabetes mellitus and asthma. Cytokine, 34:170-76.
- Stene LC and Nafstad P, 2001. Relation between occurrence of type 1 diabetes and asthma. The Lancet, 357:607-08.
- Zoka A, Muzes G, Somogyi A, Varga T, Szeman B, Al-Aissa Z, Hadarits O and Firneisz G, 2013. Altered immune regulation in type 1 diabetes. Clinical and Developmental Immunology, article ID. 254874.
- Araujo LM, Lefort J, Nahori MA, Diem S, Zhu R, Dy M, Leite-de-Moraes MC, Bach JF, Vargaftig BB and Herbelin A, 2004. Exacerbated Th2-mediated airway inflammation and hyperresponsiveness in autoimmune diabetes-prone NOD mice: A critical role for CD1d-dependent NKT cells. European Journal of Immunology, 34:327-35.
- Chou FC, Chen HY, Chen SJ, Fang MC and Sytwu HK, 2013. Rodent models for investigating the dysregulation of immune responses in type 1 diabetes. Journal of Diabetes Research, 1-8.
- Bach JF and Chatenoud L, 2001. Tolerance to islet autoantigens in type 1 diabetes. Annual Review of Immunology, 19:131-61.
- Mahajan S and Mehta AA, 2006. Role of cytokines in pathophysiology of asthma. Iranian Journal of Pharmacology and Therapeutics, 5(1):1-14.
- Rabinovitch A and Suarez-Pinzon WL, 2007. Role of cytokines in the pathogenesis and therapy of type 1 diabetes. Cell Biochemistry and Biophysics, 48:159-63.
- Vaseghi H and Jadali Z, 2016. Th1/Th2 cytokines in type 1 diabetes: Relation to duration of disease and gender. Indian Journal of Endocrinology and Metabolism, 20(3):312-16.
- Salvi SS, Babu KS and Holgate ST, 2001. Is asthma really due to a polarized T cell response toward a helper T cell type 2 phenotype? American Journal of Respiratory and Critical Care Medicine, 164:1343-46.
- Xiangyang Q, Weijun C, Liegang L, Ping Y and Bijun X, 2006. Effect of a Siraitia grosvenori extract containing mogrosides on the cellular immune system of type 1 diabetes mellitus mice. Molecular Nutrition & Food Research, 50:732-38.
- Guo Y, Xiao Z, Wang Y, Yao W, Liao S, Yu B, Zhang J, Zhang Y, Zheng B, Ren B and Gong Q, 2018. Sodium butyrate ameliorates streptozotocin-induced type 1 diabetes in mice by inhibiting the HMGB1 expression. Frontiers in Endocrinology, 9:1-9.
- Park JH, Jung JH, Yang JY and Kim HS, 2013. Olive leaf down-regulates the oxidative stress and immune dysregulation in streptozotocin-induced diabetic mice. Nutrition Research, 33:942-51.
- Mazzarella G, Bianco A, Catena E, De Palma R and Abbate GF, 2000. Th1/Th2 lymphocyte polarization in asthma. Allergy, 55(suppl. 61):6-9.
- Levine SJ and Wenzel SE, 2010. The role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes: Are we getting closer? Annals of Internal Medicine, 152(4):232-37.
- Ravikumar N and Kavitha ChN, 2019. Role of dexamethasone on immune dysregulation mediated through Th1/Th2 cytokine balance in mice challenged with type 1 diabetes and allergic asthma. International Journal of Research in Pharmaceutical Sciences, 10(4):3042-54.
- Zhu T, Chen Z, Chen G, Wang D, Tang S, Deng H, Wang J, Li S, Lan J, Tong J, Li H, Deng X, Zhang W, Sun J, Tu Y, Luo W and Li C, 2019. Curcumin attenuates asthmatic airway inflammation and mucus hypersecretion involving a PPARγ-dependent NF-kB signaling pathway in vivo and in vitro. Mediators of Inflammation, article ID. 4927430.
- Shin HS, See HJ, Jung SY, Choi DW, Kwon DA, Bae MJ, Sung KS and Shon DH, 2015. Turmeric (Curcuma longa) attenuates food allergy symptoms by regulating type 1/type 2 helper T cells (Th1/Th2) balance in a mouse model of food allergy. Journal of Ethnopharmacology, 175:21-29.
- Chong L, Zhang W, Nie Y, Yu G, Liu L, Lin L, Wen S, Zhu L and Li C, 2014. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1-GATA3 signaling pathway. Inflammation, 35(5):1476-85.
- Miao MS, Tian S and Guo L, 2014. Effect of curcumin on diabetes in an alloxan mice model. Advanced Materials Research, 1051:363-67.
- Zhang D, Fu M, Gao SH and Liu JL, 2013. Curcumin and diabetes: A systemic review. Evidence-Based Complementary and Alternative Medicine, article ID. 636053.
- Giordano A and Tommonaro G, 2019. Curcumin and cancer. Nutrients, 11(2346):1-20.
- Varalakshmi Ch, Ali AM, Pardhasaradhi BVV, Srivastava RM, Singh S and Khan A, 2008. Immunomodulatory effects of curcumin: In-vivo. International Immunopharmacology, 8(5):688-700.
- Yadav VS, Mishra KP, Singh DP, Mehrotra S and Singh VK, 2005. Immunomodulatory effects of curcumin. Immunopharmacology and Immunotoxicology, 27(3):485-97.
- Yang C, Zhu K, Yuan X, Zhang X, Qian Y and Cheng T, 2020. Curcumin has immunomodulatory effects on RANKL-stimulated osteoclastogenesis in vitro and titanium nanoparticle-induced bone loss in vivo. Journal of Cellular and Molecular Medicine, 24:1553-67.
- Elgendy MY, Hakim AS, Ibrahim TB, Soliman WS and Ali SE, 2016. Immunomodulatory effects of curcumin on Nile Tilapia, Oreochromis niloticus and its antimicrobial properties against Vibrio alginolyticus. Journal of Fisheries and Aquatic Science, 11:206-15.
- Boroumand N, Samarghandian S and Hashemy SI, 2018. Immunomodulatory, anti-inflammatory and, antioxidant effects of curcumin. Journal of Herbmed Pharmacology, 7(4):211-19.
- Pettersson US, Christoffersson G, Massena S, Ahl D, Jansson L, Henriksnas J and Phillipson M, 2011. Increased recruitment but impaired function of leukocytes during inflammation in mouse models of type 1 and type 2 diabetes. PLos One, 6(7):e22480.
- Carvalho VF, Barreto EO, Arantes ACS, Serra MF, Ferreira TPT, Jannini-Sa YAP, Hogaboam CM, Martins MA and Silva PMR, 2018. Diabetes downregulates allergen-induced airway inflammation in mice. Mediators of Inflammation, 1-11.
- Fernandez-Rodriguez S, Ford WR, Broadley KJ and Kidd EJ, 2008. Establishing the phenotype in novel acute and chronic murine models of allergic asthma. International Immunopharmacology, 8:756-63.
- Zhang Z, Shi L, Pang W, Liu W, Li J, Wang H and Shi G, 2016. Dietary fiber intake regulates intestinal microflora and inhibits ovalbumin-induced allergic airway inflammation in a mouse model. PLos One, 11(2):e0147778.
- Castro CN, Tabarrozzi AEB, Winnewisser J, Gimeno ML, Noguerol MA, Liberman AC, Paz DA, Dewey RA and Perone MJ, 2014. Curcumin ameliorates autoimmune diabetes. Evidence in accelerated murine models of type 1 diabetes. Clinical and Experimental Immunology, 177:149-60.
- Ranjan D, Chen C, Johnston T. D, Jeon H and Nagabhushan M, 2004. Curcumin inhibits mitogen stimulated lymphocyte proliferation, NF-κB activation, and IL-2 signaling. Journal of Surgical Research, 121(2):171-77.
- Choi YH, Yan GH, Chai OH and Song CH, 2010. Inhibitory effects of curcumin on passive cutaneous anaphylactic response and compound 48/80-induced mast cell activation. Anatomy & Cell Biology, 43:36-43.
- Li X, Lu Y, Jin Y, Son JK, Lee SH and Chang HW, 2014. Curcumin inhibits the activation of immunoglobulin E-mediated mast cells and passive systemic anaphylaxis in mice by reducing serum eicosanoid and histamine levels. Biomolecules & Therapeutics, 22(1):27-34.
- Oh SW, Cha JY, Jung JE, Chang BC, Kwon HJ, Lee BR and Kim DY, 2011. Curcumin attenuates allergic airway inflammation and hyperresponsiveness in mice through NF-κB inhibition. Journal of Ethnopharmacology, 136:414-21.
- Paul P, Majhi S, Mitra S and Banerjee ER, 2018. Immuno-modulatory and therapeutic effect of curcumin in an allergen-sensitized murine model of chronic asthma. Journal of Clinical & Cellular Immunology, 9(3):1-9.
- Poynter ME, Irvin CG and Janssen-Heininger YM, 2002. Rapid activation of nuclear factor-kappa B in airway epithelium in a murine model of allergic airway inflammation. American Journal of Pathology, 160:1325-34.







