Ashwaq S. Ali1 , Bashir S. H1
, Bashir S. H1 and Khaled A. Abdelshafeek*1 ,2, 3
and Khaled A. Abdelshafeek*1 ,2, 3
1Department of Chemistry, Faculty of Science, Albaha University, Albaha, Saudi Arabia
2Chemistry of Medicinal Plants Department, Pharmaceutical and Drug Industries Division, National Research Centre, Dokki, Giza, Egypt
3Albaha University, Faculty of Science and Arts in AlMukhwah, Department of Chemistry, Saudi Arabia
Corresponding Author E-mail: khabdelhady@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1927
Abstract
The purpose of this study is to investigate some chemical constituents and biological activities of different extracts of Withania somnifera (WS) growing in Albaha region KSA. The volatile oils of both leaves and stems were extracted by hydrodistillation method and analyzed using Gas Chromatography/ Mass spectrometry (GC/MS) technique. The pet ether extracts of different parts (leaves, stems and seeds) of the plant were fractionated into acetone insoluble fraction, unsaponifiable matter and fatty acids which were identified using (GC/MS) analysis. All defatted plant powder of each organ were extracted with methanol (80 %) which subsequently fractionated using (chloroform, ethyl acetate and n-butanol) respectively. Flavonoidal content of all extracts was determined using the standard Aluminum chloride spectrophotometric method while phenolic content was determined by using Folin-Ciocalteau spectrophotometric method. The results of the antimicrobial activity of different showed that, the extracts of leaves and stems only showed activity against gram positive bacteria while, all gram negative bacteria are resistant to all extracts except ethyl acetate extract of leaves.
Keywords
Antimicrobial Activity; Folin-Ciocalteau; GC/MS; Lipid Constituents; Volatile Oils; Withania somnifera
Download this article as:| Copy the following to cite this article: Ali A. S, Bashir S. H, Abdelshafeek K. A. Isolation, Identification of Some Chemical Constituents and Antimicrobial Activity of Different Extracts from Withania somnifera Growing at Albaha Region, KSA. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Ali A. S, Bashir S. H, Abdelshafeek K. A. Isolation, Identification of Some Chemical Constituents and Antimicrobial Activity of Different Extracts from Withania somnifera Growing at Albaha Region, KSA. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/31zVmjz |
Introduction
The plant natural products plays an important role in treating human diseases. Whereas human beings since ancient times interested to study and isolated active compounds from plants.1 Withania somnifera has been an important medicine plant belonging to Solanaceae family. It can be found naturally distributed in many areas on the world mostly the drier regions, subtropical zones of the Mediterranean region and northern Africa to south west Asia. W. somnifera has been known for it’s ability to promote public health and mental health over the years without toxicity or side effects.2,3 Several groups of chemical constituents have been extracted, isolated and identified from this plant such as steroidal lactones, alkaloids, flavonoids, tannins, saponins, reducing sugars and terpenoids.4-6 Active constituents are present in this plant which explains it’s medicinal properties such as (Tropin, Anaferine, Anahygrine and Withanine) besides withanolides (withaferin A and withanolide D).7 W. somnifera also considered as a good source for fatty acids.8 Many researchers documented the biological and pharmaceutical properties of W. somnifera and proved it’s activity as antimicrobial, anti-inflammatory, antioxidant, antiulcer, antidepression, used in treatment of various kinds of cancers such as colon, lung, skin, blood and breast cancer and cardio protective.9,10 Additionally, the plant has a great potential in treatment of burns, wounds and dermatological diseases, have a beneficial effects in preventing brain and neurological disorders such as anxiety, Alzheimer, Parkinson and Schizophrenia.11-14
By reviewing the available literature, there is no data about the lipid constituents of the plant so, the present study has focused to investigate some chemical constituents and antimicrobial activity for different extracts of leaves, stems and seeds of W. somnifera .
Materials and Methods
Plant Material
WS plant was collected from Albaha region, Saudi Arabia during December 2018. The plant parts leaves, stems and seed were separated. All parts were air dried for two weeks. subsequently, ground each part to get a fine powder.
Extraction of Volatile Oil
About 180 g of plant powder (leaves and stems) of WS were subjected to hydrodistillation method15 using Clevenger apparatus for about 4 hours. The obtained volatile oils were analyzed using GC/MS analysis with the following conditions: about 200 mg of each oil were transferred to a glass test tube 15ml, mixed with 1 ml methanol, vortexes for 2 min, and filtered through 0.22 μm Nylon membrane. A portion from the clear extract was transferred to auto sampler vial, and a volume of 1.5 μl was injected. instrument: Clarus 500 GC/MS Perkin Elmer model. The oven was programmed as follows: Initial temperature was 43 °C (hold 5 min) to 65 °C (rate 8 °C/min, hold 3.0 min), to 100 °C (rate 4 °C/min, hold 8.5 min), to 280 °C (rate 15 °C/min / hold 2 min). The injector temperature was 265 °C, and the injection volume was 1.5 µL, and the split ratio was 50:1. Samples were acquired by applying a total MS scan from 40 to 500 m/z (500 scan/sec). NIST 2008 was used to characterize the eluted compounds.
Extraction of Lipid Constituents
About 500 g of powdered material (leaves, stems and seeds) of WS were extracted with Petroleum ether (40-60 °C) using soxhelt apparatus. The pet. ether extract passed over fuller’s earth to remove the colored pigments in case of leaves and stems extracts, after then dehydrated by passing on an anhydrous sodium sulfate, filtered and evaporated under vacuo at 40 °C. Boiling acetone was added to the pet. ether residues, separately, for leaves and stems only and left overnight. The formed white precipitate (acetone insoluble fraction) was filtered off. The acetone soluble fraction of leaves, stems and pet. ether extracts of seeds were saponified by refluxing for 5 hours with alcoholic KOH. The alcoholic solution for each part was concentrated and diluted with cold distilled water. Then, the unsaponifilable matter was extracted by shaking with chloroform (three times). In addition, the aqueous sap solution of leaves, stems and seeds were acidified and extracted several times with chloroform to extract the fatty acids which were dissolved in methanol containing HCl. Each mixture refluxed for 4 hrs., concentrated, extracted with chloroform and evaporated to obtain the fatty acid methyl esters. All lipid constituents were subjected to GC/MS analysis. which were performed using the following conditions: Apparatus: Perkin Elmer Clarus 600 gas chromatograph inked to a mass spectrometer. An aliquot of the extract was injected into the Elite-5MS column of 30 m, 025 lm film thickness, 025 lm internal Diameter capillary column using the following temperature program. The GC/MS system starts with the initial oven temperature of 40°C, holds for 2 min, then increases to 200°C with a 5°C min1 rate of increase and holds for 2 min. From 200°C, the temperature increases at a rate of 5°C min1 to 300°C and holds for 2 min. The injector temperature was maintained at 280°C. The interface temperature was 240°C and the source temperature was 220°C. The vacuum of the system was maintained at 111e5 and the electron energy was 70 eV. Helium was used as a mobile phase at a 10 ml /min flow rate. Mass spectral detection was performed in electron ionization mode by scanning at 40–600 m/z. Finally, unknown compounds were identified by comparing the spectra with those recorded in the National Institute of Standard and Technology (2005) and WILEY (2006) libraries (Coates 2000; Linstrom and Mallard, 2005). The total time required for analyzing a single sample was 58 min.
Fractionation of Methanolic Extracts
The defatted powdered material (leaves, stems and seeds) of WS were macerated in aqueous methanol (80%) for three days with continuous stirring and filtered. The combined alcoholic extracts were evaporated under reduced pressure at 45 °C and hot distilled water was added to the alcoholic extract for each part. Partition the aqueous filtrate for each part with chloroform, ethyl acetate and butanol sequentially.
Determination of Total Phenolics and Flavonoidal Content
The total phenolic content (TPC) of different extracts of WS leaves and stems (Butanol BE-1, BE-2, Chloroform CE-1, CE-2, Ethyl acetate EE-1, EE-2 and Methanol ME-1, ME-2) were determined by using Folin-Ciocalteau spectrophotometric method.16 Total flavonoidal content (TFC) of different extracts of WS leaves and stems were determined by using the standard Aluminium chloride spectrophotometric method.17
Determination of Antimicrobial Activity
The antimicrobial activity of different extracts was performed at Albaha regional research laboratory, Albaha, Saudi Arabia. It carried out against two type of gram positive bacteria ( Staphylococcus aureus ( ATCC 25923) and Enterococcus faecalis ( ATCC 29212)), two gram negative bacteria Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853) and one fungal strain Candida albicans (ATCC 10231). Susceptibility screening test was carried out by disc diffusion method.18 Amoxicillin and Fluconazole were used as standard drugs.
Results and Discussion
Volatile Oils of WS Leaves and Stems
The volatile oil of both leaves and stems was obtained as a pale yellow oil with characteristic odor which was analyzed using GC/MS as in figure (1 and table 1). The data revealed that, both oils are consist of a mixture of thirty six and thirty seven compounds respectively with 9-Octadecenamide as a major compound (42.81% and 44.49% respectively) . The alcoholic compounds present in leaves are more than in stems. Hydrocarbons were present relatively high in stems more than leaves. As well as aromatics, sesquiterpenes , diterpenes, ketones and aldehydes were present in a small percentage in both. Two ester compounds namely methyl palmitate and isooctyl laurate were present in volatile oil of leaves, while only one ester (isooctyl laurate) was exist in volatile oil of stems. Sulfur compounds were present in volatile oil for stems only as butyl octadecyl sulfite and octadecyl 2-pentenyl sulfite. By reviewing the available literature, it was found that these data were reported for the first time for this plant.
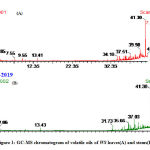 |
Figure 1: GC-MS chromatogram of volatile oils of WS leaves(A) and stems(B) |
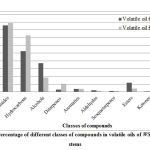 |
Figure 2: Percentage of different classes of compounds in volatile oils of WS leaves and stems |
Table 1: GC/MS data for volatile oil of WS leaves and stems
| No. | Compound | Rt
(min) |
Percentages | M+ | B.P. | Fragments | |
| Leaves | Stems | ||||||
| 1 | Benzene | 2.77 | — | 0.52 | 78 | 78 | 26(5), 39(15), 51(22) |
| 2 | Cyclohexane | 2.86 | 2.35 | 1.39 | 84 | 56 | 41(55), 69(30), 84(82) |
| 2 | Pentanal | 3.10 | 0.71 | — | 86 | 44 | 29(42), 41(30), 58(48) |
| 3 | 2-Methylbutanal | 2.73 | — | 0.21 | 86 | 41 | 27(42), 29(95), 39(35) |
| 4 | Toluene | 4.71 | 0.31 | — | 92 | 91 | 39(15), 65(12), 92(55) |
| 5 | 2-Ethylfuran | 3.30 | 0.98 | — | 96 | 81 | 29(10),53(45), 67(15) |
| 6 | Methyl cyclohexane | 3.87 | — | 0.24 | 98 | 83 | 27(16), 39(18), 41(40) |
| 7 | Heptane | 3.46 | — | 0.78 | 100 | 43 | 15(10), 29(50), 57(50) |
| 8 | 3-Methylhexane | 3.07 | 1.26 | 0.70 | 100 | 43 | 29(25), 57(55), 71(59) |
| 9 | Hexanal | 5.55 | 0.27 | 0.37 | 100 | 44 | 29(35), 41(67), 56(82) |
| 10 | 3-Hexen-1-ol | 7.55 | 1.74 | — | 100 | 67 | 29(15), 41(75), 55(38) |
| 11 | 3-Ethyl-2,2-dimethyloxirane | 9.45 | 0.94 | — | 100 | 59 | 31(40), 41(57), 85(22) |
| 12 | 2-Methyl- 2-pentanol | 3.95 | — | 0.27 | 102 | 59 | 29(10), 41(22), 45(50) |
| 13 | 2-Methylheptane | 5.06 | 0.23 | 0.21 | 114 | 43 | 29(18), 57(86),70(20) |
| 14 | 3-Methylheptane | 5.30 | 0.49 | 0.39 | 114 | 43 | 29(40), 41(55), 57(72) |
| 15 | 4-Hydroxy-4-methyl- 2-pentanone | 6.83 | 0.40 | 0.37 | 116 | 43 | 39(10), 59(35), 83(5) |
| 16 | 2-Pentylfuran | 12.90 | — | 0.47 | 138 | 81 | 27(10), 41(10), 53(18) |
| 17 | 2,5-Dimethyl-3,4-hexadiol | 10.20 | 1.80 | 0.41 | 146 | 73 | 43(25), 55(20), 85(5) |
| 18 | 4-allyloxy-2-methyl pentan-2-ol | 9.55 | 3.14 | 0.93 | 158 | 43 | 41(30), 45(20), 59(44) |
| 19 | 2,2,4,6,6-pentamethylheptane | 13.41 | 4.05 | 3.41 | 170 | 57 | 29(15), 41(25), 71(10) |
| 20 | 2,2,4,4-tetramethyloctane | 14.96 | 0.54 | 0.38 | 170 | 57 | 43(18), 70(4), 99(18) |
| 21 | Tridecane | 22.19 | 0.34 | 0.24 | 184 | 57 | 29(23), 43(90), 71(50) |
| 22 | 1-Dodecanol | 31.39 | 0.49 | 0.52 | 186 | 55 | 29(37),43(97), 69(87) |
| 23 | Octyl cyclohexane | 32.25 | 0.38 | 1.39 | 196 | 83 | 29(8), 41(21), 55(45) |
| 24 | 2-Methyltridecane | 31.67 | 0.23 | 0.21 | 198 | 57 | 29(18), 43(98), 71(55) |
| 25 | 1-Tridecanol | 33.99 | 0.78 | 0.79 | 200 | 55 | 29(20), 41(65), 69(82) |
| 26 | 2,5-bis(1,1-dimethylethyl) phenol | 33.01 | 0.66 | 0.89 | 206 | 191 | 29(5), 41(10), 57(17) |
| 27 | 2,6,10-Trimethyldodecane | 34.15 | 0.63 | 0.68 | 212 | 57 | 29(20), 43(48), 71(70) |
| 28 | Hexadecane | 34.10 | 2.93 | 2.21 | 226 | 57 | 29(17), 43(74), 71(61) |
| 29 | 1-Hexadecanol | 34.58 | 2.58 | 2.29 | 242 | 55 | 30(22), 41(77), 69(82) |
| 30 | 2-Ethyl-2-methyl tridecanol | 35.79 | 0.96 | 0.98 | 242 | 57 | 43(50), 71(80), 83(60) |
| 31 | 3-Methylheptadecane | 35.53 | 0.54 | 0.59 | 254 | 57 | 29(15), 43(50), 71(60) |
| 32 | n-Heptadecanol | 36.17 | 2.85 | 2.79 | 256 | 55 | 29(18), 43(57), 69(79) |
| 33 | 1-Nonadecene | 35.66 | 2.58 | 14.06 | 266 | 57 | 29(38), 43(92), 69(82) |
| 34 | Nonadecane | 35.73 | 3.39 | 4.78 | 268 | 57 | 29(23), 43(80), 71(65) |
| 35 | Methyl palmitate | 36.51 | 2.67 | — | 270 | 74 | 29(10), 43(35), 55(32) |
| 36 | 9-Octadecenamide | 41.30 | 42.81 | 44.49 | 281 | 59 | 29(10), 41(22), 72(50) |
| 37 | 2,6,10,14-Tetramethylhexadecane | 38.35 | 1.51 | 1.48 | 282 | 57 | 29(18), 43(61), 71(70) |
| 38 | 1-Eicosanol | 38.70 | 4.09 | 1.25 | 289 | 43 | 29(32), 57(85), 69(75) |
| 39 | 1-Docosene | 37.51 | 4.30 | 5.39 | 308 | 57 | 29(30), 43(95), 69(63) |
| 40 | Isooctyl laurate | 38.06 | 3.30 | 2.08 | 312 | 43 | 57(85), 71(62), 85(55) |
| 41 | Heptacosane | 37.15 | 2.77 | — | 380 | 57 | 29(19), 43(92), 71(61) |
| 42 | Butyl octadecyl sulfite | 36.91 | — | 0.75 | 390 | 57 | 43(55), 71(45), 85(35) |
| 43 | Octadecyl 2- pentanyl sulfite | 37.61 | — | 1.09 | 404 | 71 | 43(60), 57(55), 85(45) |
| 100 | 100 | ||||||
Rt= Retention time, M+= Molecular ion peak, B.P.= Base peak
Investigation of the Lipid Constituents
The GC/MS analysis of acetone insoluble fraction of leaves and stems indicated the presence of three n-hydrocarbons compounds with n-Pentacosane (n-C25H52) as major component in leaves while, in stems there are six compounds in which nonacosane (n-C29H60) was major component. Besides one fatty alcohol compound was present in acetone insoluble fraction of stems which identified as: 1-Pentacontanol (n-C25H50O). By reviewing the available literature, it was found that these data were reported for the first time for this plant. The results are summarized in table (2).
Table 2: GC/MS data for acetone insoluble fractions of leaves and stems
| No. | Compound | Rt (min) | Percentages | M.W. | |
| Leaves | Stems | ||||
| 1 | Pentacosane | 31.46 | 84.84 | — | 352 |
| 2 | Heptacosane | 30.63 | 6.25 | 2.390 | 380 |
| 3 | Nonacosane | 24.55 | — | 75.36 | 408 |
| 4 | Triacontane | 24.18 | — | 0.790 | 422 |
| 5 | Dotriacontane | 26.05 | — | 3.950 | 450 |
| 6 | Pentatriacontane | 25.43 | — | 9.790 | 492 |
| 7 | Hexatriacontane | 29.93 | 8.91 | — | 507 |
| 8 | Tetracontane | 23.00 | — | 5.440 | 563 |
| 9 | 1-Pentacontanol | 25.16 | — | 2.280 | 719 |
| 100 | 100 | ||||
Rt = Retention time, M.W.= Molecular weight
The obtained results from the GC/MS analysis of the unsaponifiable matter in leaves, stems and seeds present in table (3) unsaponifiable matter constituents were varying between hydrocarbons, alcohols, aldehydes and ketones. For leaves the data showed the presence of hydrocarbons as the majority (50.14 %). Also, the other (43.66 %) distributed among alcohols and fatty alcohols with 3,7,11,15-tetramethyl-2-hexadecenol in a high percentage (34.57%). Also, two ketone compounds (5.04 %). One aldehydic compound was present with small percentage (1.16 %) which is 15-heptadecenal. These data were in agreement with that reported by Abdelgawad et al., in 201519 where they investigated the unsaponifiable matter from the petroleum ether extract of W. somnifera leaves growing in Egypt by GC/MS. They proved the presence of 2-ethyl hexanol, hexadecane and heptacosane but disagree with our results in the percentages of the this compounds. Furthermore, The results of unsaponifiable matter of stems led to identification of fifteen compounds in which the hydrocarbons are in large quantities with the majority for (tetracontane 25.97%). Also, alcoholic compounds were present in percentage of 32.09 %. In addition, there was only one ketone compound in percentage 7.20 %. The data showed the presence of one aldehydic compound in small quantity (hexanal 1.17 %). Two steroidal compounds were present in the unsaponifiable matter of the seeds which were identified as ergost-5-en-3-ol ( 64.45%) and stigmasterol (12.06%) with one triterpenoidal compound a- amyrin (6.94 %) besides the presence of two hydrocarbon compounds (15.25 %). The data showed presence of one fatty alcohol compound 3,7,11,15-Tetramethyl-2-hexadecenol (1.30 %) which was also present in the unsaponifiable matter for leaves and stems. These findings were compatible with the recent study by Mishra and Patnaik in 202020 on methanolic extract of whole plant where the GC/MS results showed the presence of this compound also.
Table 3: GC/MS data of unsaponifiable matter of WS leaves, stems and seeds
| No. | Compounds | Rt (min) | Percentages | M.W. | ||
| Leaves | Stems | Seeds | ||||
| 1 | Heptane | 3.15 | 1.87 | — | — | 100 |
| 2 | Hexanal | 4.76 | — | 1.17 | — | 100 |
| 3 | 2-Methyl-2-pentanol | 3.75 | 2.02 | — | — | 102 |
| 4 | 3-Methyl-3-hexanol | 5.56 | 1.22 | — | — | 116 |
| 5 | 4-Methoxy-4-methyl-2-pentanone | 6.68 | 1.87 | — | — | 130 |
| 6 | 2-Ethyl-1-hexanol | 8.80 | 2.78 | 7.46 | — | 130 |
| 7 | 4-Isopropyl-3-(2-propenyl) cyclohexanol | 24.10 | — | 2.43 | — | 182 |
| 8 | 1-Dodecanol | 9.53 | — | 0.74 | — | 186 |
| 9 | 1-Pentadecene | 24.14 | 2.69 | — | — | 210 |
| 10 | Hexadecane | 24.75 | 34.27 | — | 0.80 | 226 |
| 11 | E-15-Heptadecenal | 20.38 | 1.16 | — | — | 252 |
| 12 | 6,10,14-Trimethyl-2-pentadecanone | 21.00 | 3.17 | 7.20 | — | 268 |
| 13 | 1-Octadecanol | 21.50 | — | 3.69 | — | 270 |
| 14 | 3,7,11,15-Tetramethylhexadecane | 25.81 | — | 0.56 | — | 282 |
| 15 | 1-Nonadecanol | 22.61 | 1.19 | — | — | 284 |
| 16 | 3,7,11,15-Tetramethyl-2-hexadecenol | 23.87 | 34.57 | 6.17 | 1.30 | 296 |
| 17 | 4,8,12,16-Tetramethylheptadecane | 26.06 | 2.65 | 2.16 | — | 296 |
| 18 | 1-Eicosanol | 27.10 | — | 7.84 | — | 298 |
| 19 | 1-Docosanol | 30.28 | 1.88 | — | — | 326 |
| 20 | Heptacosane | 27.16 | 2.28 | 11.67 | — | 380 |
| 21 | Ergost-5-en-3-ol | 27.44 | — | — | 64.45 | 400 |
| 22 | Stigmasterol | 28 | — | — | 12.06 | 412 |
| 23 | Triacontane | 26.33 | 2.68 | — | — | 422 |
| 24 | Alpha amyrin | 29.28 | — | — | 6.94 | 426 |
| 25 | Nonacosanol | 25.42 | — | 3.76 | — | 424 |
| 26 | Dotriacontane | 27.96 | 1.78 | 4.96 | 14.45 | 450 |
| 27 | Hexatriacontane | 28.75 | 1.92 | — | — | 507 |
| 28 | Tetracontane | 30.00 | — | 25.97 | — | 563 |
| 29 | Tetratetracontane | 31.18 | — | 14.22 | — | 619 |
| 100 | 100 | |||||
The GC/MS analysis after methylation of fatty acids of leaves, stems and seeds indicated the presence a variety of saturated and unsaturated fatty acids. The overall results are summarized in table (4). Fatty acids which exist in leaves were varying between saturated and unsaturated where, the unsaturated fatty acids were predominate (84.97 %) in which monounsaturated fatty acids with oleic acid as a major compound (76 % ). Polyunsaturated fatty acid (11,14,17-Eicosatrienoic acid 6.64 %) and diunsaturated fatty acids were present in a small quantity (linoleic acid 2.33 %). Moreover, the saturated fatty acids were represented by four fatty acids. Palmitic acid had the highest percentage (10.45 %). These data were in accordance with that reported by Chatterjee et al., in 20108 where, they found that, the hexane extract of leaves was rich with oleic, palmitic and linoleic acids. Furthermore, it was found that, the stems were rich with saturated fatty acids (63.77 %) and the majority for palmitic acid (29.95 %). Also, the unsaturated fatty acids were present in stems as (17.06 %), most of them were diunsaturated with linoleic acid forming (15.67 %). In addition, a small amount from monounsaturated was present in percentage (1.39 %) as one compound which was identified as elaidic acid. The results of stems were similar with that stated by Namdev and Gupta in 201521 where they studied methanolic extract of W. somnifera stems and the presence of palmitic, margaric, linoleic and arachidic acids but the percentages of these compounds were differ from our results. Finally, the seeds were found to contain a high percentage of monounsaturated fatty acids which constitutes about (64.59 %) with oleic acid as the main compound (63.78 %). Saturated fatty acids also present in seeds with (35.41%) and 10,13-dimethyltetradecanoic acid as predominant (33.85%). The lowest percentage was for palmitic acid (0.06 %). Some fatty acids which present in seeds were similar to that the study conducted by Arora et al., in 201822, whereas, the percentages of myristic acid and palmitic acid in our result were less than the percentages which described in their results. Oleic acid was present in our result as a major compound.
Table 4: GC/MS data of fatty acids methyl ester of WS leaves, stems and seeds
| No. | Compounds | Rt (min) | Percentages | M.W. | ||
| Leaves | Stems | Seeds | ||||
| 1 | Methyl Phthalate | 27.40 | — | 19.17 | — | 180 |
| 2 | Methyl 7- methyl nonanoate | 28.79 | — | 2.25 | — | 186 |
| 3 | Methyl 8-methyldecanoate | 26.50 | — | 1.69 | — | 200 |
| 4 | Methyl myristate | 19.56 | 2.07 | 4.34 | 0.16 | 242 |
| 5 | Methyl Palmitoleate | 14.78 | — | — | 0.81 | 268 |
| 6 | Methyl Palmitate | 21.90 | 10.45 | 29.95 | 0.06 | 270 |
| 7 | Methyl 10,13- dimethyl Tetradecanoate | 15.09 | — | — | 33.85 | 270 |
| 8 | Methyl Margarate | 22.93 | — | 1.35 | — | 284 |
| 9 | Methyl Linoleate | 23.61 | 2.33 | 15.67 | — | 294 |
| 10 | Methyl Oleate | 28.63 | 76 | — | 63.78 | 296 |
| 11 | Methyl Elaidate | 20.60 | — | 1.39 | — | 296 |
| 12 | Methyl Stearate | 23.90 | — | 10.07 | — | 298 |
| 13 | Methyl Isopropyl Palmitate | 22.87 | 0.96 | 1.59 | — | 312 |
| 14 | Methyl 11,14,17-Eicosatrienoate | 23.68 | 6.64 | — | — | 320 |
| 15 | Methyl Arachidate | 25.67 | 1.55 | 5.68 | 0.79 | 326 |
| 16 | Methyl Behenate | 27.30 | — | 4.23 | 0.31 | 354 |
| 17 | Methyl Lignocerate | 21.78 | — | — | 0.24 | 382 |
| 100 | 100 | |||||
Total Phenolics and Flavonoidal Content
The results of TPC and TFC for all the extracts are showed in figure (3). As is clear from the results, the butanol extract of stems (BE-2) exhibited the highest TPC as 215 mg of GAE/g powder weight followed by ethyl acetate extract of leaves (EE-1) with 213.4 mg of GAE /g of powder weight. On the other hand, the methanol extract of leaves (ME-1) exhibited the lowest TPC of 92.6 mg of GAE /g. These results disagree with that stated by Pal et al., in 201523 as they investigated the TPC of WS leaves extract. Their results revealed that the methanol extract had the highest TPC. The obtained results of TFC indicated that, the butanol extract of stems (BE-2) exhibited the highest TFC with 66.2 mg of QE/g powder weight which is the major contributor to scavenge the free radicals in oxidation pathways. Moreover, ethyl acetate extract of leaves (EE-1) showed a comparable TFC with 65.6 mg of QE/g powder weight when it was compared with BE-2. However, the methanol extract of leaves (ME-1) exhibited the lowest TFC.24
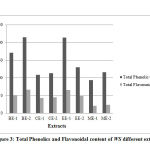 |
Figure 3: Total Phenolics and Flavonoidal content of WS different extract |
In Vitro Antimicrobial Activity of WS
About 20 extracts of different parts of WS were tested for their bioactivity against bacterial and fungal strains at concentration (1000) µg/ disc. From the re
sults which recorded in table (5) it was observed that, the gram positive bacteria are sensitive towards some extracts, but the gram negative bacteria showed resistance against the extracts. The fungus Candida albicans did not show any inhibition signs for all extracts in this concentration. The acetone insoluble fraction of leaves and stems showed high activity against gram positive bacteria. But the gram negative bacteria showed resistance. The methanol extracts of leaves showed inhibition zone against S. aureus and E. faecalis. Methanol extract of stems had some activity against S. aureus only. Methanol extract of seeds did not exhibited any activity against any type from the tested microbes. Chloroform extracts of leaves and stems showed marked activity against S. aureus and E. faecalis. However, chloroform extract of seeds did not showed any activity against any type from the tested microbes. These results are disagree with that reported by Singariya et al., in 201225 were they found that, the chloroform extract of WS fruits showed low activity against gram negative bacteria (P. aeruginosa). Moreover, ethyl acetate extract of leaves showed a weak activity against gram negative bacteria P. aeruginosa (inhibition zone , 8 mm) in addition to it’s moderate activity against gram positive bacteria S. aureus and E. faecalis. our results were disagree to the results of Sundaram et al., in 201126 where, they noticed that, the ethyl acetate extract of leaves possesses a great inhibition against gram positive bacteria and gram negative bacteria especially P. aeruginosa. Ethyl acetate extract of stems showed activity against S. aureus and E. faecalis while, ethyl acetate extract of seeds showed negative results for all tested microbes. The butanol extract of leaves showed some activity against S. aureus. Butanol extract of stems showed some activity against S. aureus only. Butanol extract of seeds did not show any activity against any type of tested microbes. Petroleum ether and mother liquor extracts of all subjected parts gave negative results for all tested microbes. All extracts showed positive results with inhibition zones less than that of the standard drugs Amoxicillin except ethyl acetate extract of leaves against P. aeruginosa.
Table 5: Antimicrobial activity of W S extracts
| Antibacterial Activity 1000 µg / disc (mm) | Antifungal Activity | ||||
| Extracts and standards | Gram Positive Bacteria | Gram Negative Bacteria | Yeast | ||
| S.a | E.f | E.c | p.a | C.a | |
| Amoxicillin | 38 | 34 | 26 | — | NT |
| Fluconazole | NT | NT | NT | NT | 15 |
| acetone insoluble fraction of leaves | 28 | 24 | — | — | — |
| Methanol extract of leaves | 24 | 14 | — | — | — |
| Chloroform extracts of leaves | 22 | 16 | — | — | — |
| Ethyl acetate extract of leaves | 19 | 14 | — | 8 | — |
| Butanol extracts of leaves | 16 | — | — | — | — |
| Petroleum ether extracts of leaves | — | — | — | — | — |
| Mother liquor extract of leaves | — | — | — | — | — |
| acetone insoluble fraction of stems | 25 | 23 | — | — | — |
| Methanol extracts of stems | 12 | — | — | — | — |
| Chloroform extract of stems | 17 | 12 | — | — | — |
| Ethyl acetate extract of stems | 11 | 8 | — | — | — |
| Butanol extract of stems | 13 | — | — | — | — |
| Petroleum ether extract of stems | — | — | — | — | — |
| Mother liquor extract of stems | — | — | — | — | — |
| Methanol extracts of seeds | — | — | — | — | — |
| Chloroform extracts of seeds | — | — | — | — | — |
| Ethyl acetate extracts of seeds | — | — | — | — | — |
| Butanol extracts of seeds | — | — | — | — | — |
| Petroleum ether extracts of seeds | — | — | — | — | — |
| Mother liquor extract of seeds | — | — | — | — | — |
S.a: Staphylococcus aureus; E.f :Enterococcus Faecalis; E.c: Escherichia Coli; P.a: Pseudomonas Aerugenosa ; C.a: Candia albican; NT: Not Tested; —: No Zone of inhibition
Conclusion
From the present study, it is possible to conclude that, 9-Octadecenamide is a major compound in volatile oils of leave and stems of WS , in addition the plant is consider as rich source of saturated and unsaturated fatty acids. As well as reasonable quantities from flavonoids and phenols. Furthermore, several compounds which were identified by GC/MS may be responsible for the activity of this plant as antimicrobial agent against gram positive bacteria specially leaves and stems extracts. These findings of our research could be beneficial in the future to complete a further study of biological activity and other possible therapeutic uses of the plant.
Acknowledgements
The authors are gratefully thanks Albaha University, faculty of science, Department of Chemistry for support and help us in completing this work. The authors would like to thank King Saud University, Riyadh, Saudi Arabia for GC/MS analyses.
References
- Dar R. A, Shahnawaz M and Qazi P. H. General overview of medicinal plants: A review. Phytopharm., 2017; 6(6): 349–351.
- Gupta G. L and Rana A. C. Withania somnifera ( Ashwagandha ): A Review. Rev., 2007; 1(1): 129–136.
- Meher S. K, Das B, Panda P, Bhuyan G.C and Rao M. M. Uses of Withania somnifera (Linn) Dunal (Ashwagandha) in Ayurveda and its pharmacological evidences. J. Pharmacol. Pharmacodyn., 2016; 8(1): 23–29.
- Mir B. A, Khazir J, Mir N. A , Tanvir-ul Hasan and Koul S. Botanical, chemical and pharmacological review of Withania somnifera ( Indian ginseng ): an ayurvedic medicinal plant. Indian Journal of Drugs and Diseases. 2012; 1(6): 147–160.
- Uddin Q, Samiulla L, Singh V. K and Jamil S. S. Phytochemical and pharmacological profile of Withania somnifera dunal: A review. Appl. Pharm. Sci., 2012; 2(1): 170–175.
- Bhasin S, Singh M and Singh D. Review on bioactive metabolites of Withania somnifera.(L.) Dunal and its pharmacological significance. Pharmacogn. Phytochem., 2019; 8(3): 3906–3909.
- Gavande K, Jain K and Mehta R. Few medicinal activities of Ashwagandha (Withania somnifera). J. Pharm. Life Sci., 2014; 5(6): 3603–3606.
- Chatterjee S, Srivastava S, Khalid A, Singh N, Sangwan R. S, Sidhu O, Roy R, Khetrapal C.L and Tuli R. Comprehensive metabolic fingerprinting of Withania somnifera leaf and root extracts. Phytochemistry, 2010; 71: 1085–1094.
- Alam N, Hossain M, Khalil M. I, Moniruzzaman M, Sulaiman S. A and Gan S. H. Recent advances in elucidating the biological properties of Withania somnifera and its potential role in health benefits. Rev., 2011; 11(1): 97–112.
- Singh N, Verma P, Pandey B. R, Gilca M. role of withania somnifera in prevention and treatment of cancer : an overview. J. Pharm. Sci. Drug Res., 2011; 3(4): 274–279.
- Velu S and Baskaran C. Phytochemical analysis and in-vitro antimicrobial activity of Withania somnifera ( Ashwagandha ) . Nat. Prod. Plant Resour., 2012; 2(6): 711–716.
- Jayaprakasam B, Padmanabhan K and Nair M. G. Withanamides in Withania somnifera fruit protect pc-12 cells from β-amyloid responsible for alzheimer’s disease. Res. 2010; 24: 859–863
- Ahmad M, Saleem S, Ahmad A. S, Ansari M. A, Yousuf S, Hoda M. N and Islam F. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Exp. Toxicol., 2005; 24:137–147.
- Zahiruddin S, Basist P, Parveen A, Parveen R, Khan W and Ahmad S. Ashwagandha in brain disorders: A review of recent developments. Ethnopharmacol., 2020; 257: 112876.
- Azmir J , Zaidul I.S.M, Rahman M.M, Sharif K.M, Mohamed A, Sahena F, Jahurul M.H.A, Ghafoor K, Norulaini N.A.N and Omar A.K.M . Techniques for extraction of bioactive compounds from plant materials: A review. Food Eng., 2013;117: 426–436.
- Slinkard K. and Singleton V. L. Total phenol analysis: automation and comparison with manual methods. J. Enol. Vitic., 1977; 28(1): 49–55.
- El Far M. M. M and Taie H. A. A. Antioxidant activities, total anthocyanins, phenolics and flavonoids contents of some sweetpotato genotypes under stress of different concentrations of sucrose and Aust. j. basic appl. sci., 2009; 3(4): 3609–3616.
- Bauer A. W. Antibiotic susceptibility testing by a standardized single disc method. J clin pathol., 1966; 45: 149–158.
- Abdelgawad S, Hetta M and Ross S. A. Chemical and biological study of Withania somnifera (L.) dunal leaves growing in upper Egypt : Beni-Suef region. Nat. Prod., 2015; 8: 64–74.
- Mishra S and Patnaik D. GC-MS Analysed phyto-chemicals and antibacterial activity of Withania somnifera (L.) dunal extract in the context of treatment to liver cirrhosis. Pharmacol. J.,2020; 13(1): 1–9.
- Namdev P and Gupta R. K. Herbal green tea formulation using Withania somnifera stems, Terminalia arjuna bark, Cinnamon bark and Tinospora cordifolia stems and nutritional & phytochemical analysis. Pharmacogn. Phytochem., 2015; 4(2): 282–291.
- Singhal S, Sonal N and Arora A. K. Withania somnifera seed oil : a potential source of unsaturated fatty acid from Western Rajasthan. Appl. Chem., 2018; 7(5): 1312–1318.
- Pal A, Kumar M, Saharan V and Bhushan B. Anti-oxidant and free radical scavenging activity of ashwagandha (Withania somnifera) leaves. Glob. Biosci., 2015; 4(1): 1127–1137.
- Alam N, Hossain M, Mottalib M. A, Sulaiman S. A, Gan S. H and Khalil M. I. Methanolic extracts of Withania somnifera leaves, fruits and roots possess antioxidant properties and antibacterial activities. BMC Complement. Altern. Med., 2012; 12(1): 175–183.
- Singariya P, Mourya K. K and Kumar P. Antimicrobial activity of the crude extracts of Withania somnifera and cenchrus setigerus in-vitro. J., 2012; 4(27): 60–65.
- Sundaram S, Dwivedi P and Purwar S. In vitro evaluation of antibacterial activities of crude extracts of Withania somnifera ( Ashwagandha ) to bacterial pathogens. Asian J. Biotechnol., 2011; 3(2): 194–199.








