Lapatrada Mungmai1* , Weeraya Preedalikit1, Nattapol Aunsri2
, Weeraya Preedalikit1, Nattapol Aunsri2 and Doungporn Amornlerdpison3
and Doungporn Amornlerdpison3
1Division of Cosmetic Science, School of Pharmaceutical Sciences, University of Phayao, Phayao, 56000, Thailand
2School of Information Technology and Brain Science and Engineering Innovation Research Group, Mae Fah Luang University, Chiang Rai, 57100, Thailand
3Center of Excellence in Agricultural Innovation for Graduate Entrepreneur, Maejo University, Chiang Mai, 50290, Thailand
Corresponding Author E-mail: Lapatrada.mu@up.ac.th
DOI : https://dx.doi.org/10.13005/bpj/1942
Abstract
P. frutescens leaves extract (PLE) has the potential to fight against free radical and oxidative stress. Antioxidants are known as important factors to protect damaged cell, stimulate collagen production, preserve hyaluronic acid levels in the skin, and also exert anti-inflammatory effect. This work presents the enhancing development of the cosmetic formulations containing PLE for skin aging prevention and its clinical evaluation. Clinical evaluation was conducted on thirty healthy volunteers, aged 40-60 years old for skin irritation, moisture content, skin texture and elasticity efficacy. The parameters were conducted by using Dermalab Combo® as noninvasive measuring methods. The volunteers filled a satisfaction form after using the serum for 8 weeks. The stability test suggested that serum containing PLE was stable at various storage conditions. From the clinical evaluation, there was no report of skin irritation or allergic reaction during the period of application. The PLES serum could increase skin hydration and skin elasticity after treatment. Additionally, after applying PLES serum on the skin surface it presented a sulcus cutis which is deep and linear in grooves at week 8 when compared to week 0. The volunteers were satisfied with the product especially with its color, odor and efficacy. This research suggests that PLE has the potential to be used as an active ingredient in cosmeceutical products developed for anti-inflammatory and anti-aging applications.
Keywords
Clinical Evaluation; Elasticity; Hydration; P. frutescens Leaves; Skin Surface; Wrinkles
Download this article as:| Copy the following to cite this article: Mungmai L, Preedalikit W, Aunsri N, Amornlerdpison D. Efficacy of Cosmetic Formulation Containing Perilla frutescens Leaves Extract for Irritation and Aging Skin. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Mungmai L, Preedalikit W, Aunsri N, Amornlerdpison D. Efficacy of Cosmetic Formulation Containing Perilla frutescens Leaves Extract for Irritation and Aging Skin. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/3dCyKRW |
Introduction
The normal skin contains approximately 80% of water content, whereas the stratum corneum has a lower water content of approximately 10-30% [1] which makes the skin to appear smooth, soft and glowing. Moisture capacitance changes depending on the water content in the stratum corneum. When the water content of the skin is lower than normal the lines are more visible, the skin feels tight, dry, and itching. Moreover, it presents redness and irritation.1, 2 In addition, it generally has red patches and can be characterized with less elasticity and a rough complexion. The loss of skin elasticity leads to the phenomenon of sagging skin and visible wrinkles.3 Transepidermal water loss (TEWL), followed by horny drying out precipitating condition, could be the result from environmental factors such as sunlight, low relative humidity, as well as repeated contact with detergents, soaps, surfactants, solvents including water. It should be noted that a study appeared in4 reporting that although the water can maintain the moisture and flexibility of the skin, it is one of the factors that also increases TEWL.
Dry skin tends to be more toward premature aging and likely on having more wrinkles. According to the theory, free radical and oxidative stress are important factors of skin aging while antioxidants protect the cell damage by neutralizing the free radical.1, 2 Therefore, applying the skin with antioxidants is beneficial in slowing down the aging of skin. Furthermore, antioxidant can stimulate collagen production, preserve hyaluronic acid levels in the skin and also exert anti-inflammatory effect.5 P. frutescens contains various important chemicals including phenolic acids, flavonoids, anthocyanins; these chemicals could be classified as hydrophilic compounds.6 On the other hand, essential oils, other volatile constituents, fatty acids and tocopherols could be classified as hydrophobic compounds.6 Phenolic compounds, flavonoids can be extracted from plant organs such as leaves, seeds and stems, while, anthocyanins can be found in leaves and stems accepted seeds. In addition, most of the volatile oil compounds are found in leaves and stems. The seeds of P. frutescens are a good source of fatty acid, especially tocopherols which have been found only in P. frutescens seed.6
The phytochemical of plants especially the phenolic acids, flavonoids, anthocyanins, and carotenoids are often attributed to the high antioxidant capacity. These chemicals show dermatological, pharmacological and therapeutic activities. The most important ones are antioxidant, anti-inflammatory, anti-allergic and antimicrobial effects.6-9 Previous studies reported that the extract of P. frutescens leaves could inhibit 2, 4-dinitrofluorobenzene (DNFB) – induced atopic inflammation by alleviating the expression of MMP-9 and IL-31.10
The utilizations of the extract from the leaves of P. frutescens are used as active compound in skin care product preparations for the moisturizing effect from its antioxidant, anti-inflammatory, anti-allergic and anti-aging activities which are the aims of this study. The purpose of applying moisturizer is to improve the skin quality, maintain the moisture content of the stratum corneum, as well as keep it smooth, maintain its flexibility and reduce formation of wrinkles.5 Even skin aging prevention by using the cosmetic formulations is one of the main goals of applying the product, but safety in using it must be evaluated before implementation. Therefore, the evaluation of the skin irritancy of each ingredient is indispensable in the safety assessment of the cosmetic ingredients before applying it to the skin.
This research presents the evaluation of the cosmetic properties of P. frutescens serum and efficacy of cosmetic formulations containing P. frutescens leaves extract on the skin hydration, skin surface photography, skin elasticity and skin irritation test in human skin to ensure its safety and efficacy.
Materials and Methods
Subjects
The research was conducted on thirty healthy volunteers (n=30), ages 40-60 years old. The volunteers received the information protocol and they were given the informed consent statement before taking part in the study.
The inclusion and exclusion criteria for subject selection are specified in Table 1. Each subject must meet all inclusion criteria and will be excluded if they meet any exclusion criteria.
Table 1: Subject inclusion and exclusion criteria for clinical test
| Inclusion criteria |
| 1. Males or females aged between 40-60 years old |
| 2. Not taking medication or under the care of doctor for a period of one month prior to, and throughout the entire test period |
| 3. Free of any dermatological or systemic disorder which, in the opinion of the investigator, that would interfere with test results or increase the risk of adverse reactions |
| 4. Available for the study duration -8 weeks |
| 5. Read, understood and signed an informed consent agreement |
| Exclusion criteria |
| 1. Visible skin disease at the study site which, in the opinion of the investigator, might have interfere the evaluation |
| 2. Using any systemic or topical drugs or taking medication which could mask or interfere with study results |
| 3. History of sensitivity to cosmetics in general and moisturizers in particular |
| 4. Any form of skin cancer or any other diseases that could interfere with test results |
| 5. Diagnosed with chronic skin allergies |
| 6. Having a known or suspected intolerance or hypersensitivity to the investigational products or any of its ingredients |
| 7. Females who were pregnant or are under lactation. |
Formulation of Topical Anti-Aging Serum Products And Stability Test
To evaluate the cosmetic properties of PLE, the test product applied was the PLES which is emulsion O/W (serum which contained PLE); this is referred to as the test moisturizer. PLES contains cetyl alcohol, cyclotetrasiloxane, PEG-7 glyceryl cocoate, sodium polyacrylate, propylene glycol, glycerin, caprylhydroxamic acid (and) 1, 2-hexanediol (and) butylene glycol, HDI/Trimethylol Hexyllactone Crosspolymer (and) Silica and PLE (0.2 %).
The serum base (SB) was prepared without the PLE. All samples had the pH values of 6.0 and freshly prepared.
The stability of all serum was tested in various conditions at room temperature, 4°C and 45°C for 3 months and 6 cycles of the heating/cooling conditions (45°C, 48 hours alteration with 4°C, 48 hours for 1 cycle). Moreover, their pH, viscosity (Pas), feel on skin and visually physical changing along with color, and smoothness were also investigated [11]. All tests were performed in triplicate.
Design and Methods of Study
This work was a double-blind study. The measurement methods had been approved by University of Phayao Human Ethic Committee, Thailand (The approval number was 3/019/58) which operates in accordance with the Guidelines for Good Clinical Practice and Thai Clinical Trial Registry (TCTR) Committee (TCTR identification number is TCTR20200404001). The measurements were performed in the room, under stable conditions: temperature 21±2°C and humidity level of 50% to 60 %. Prior to the measurements, the volunteers were subject to acclimatization to the room conditions without covering the measurement sites with clothes for a period of thirty minutes. The measurements were always performed by the same investigator and always measured at the same site. The subjects refrained from using cosmetic products for an interval of seven days before the beginning of the test. The subjects were not allowed to use other skin care products on each forearm during the trial period. On the day of examination, nothing was applied to the skin surface. All measured values were expressed as the median of three recordings.
Skin hydration and skin elasticity parameters were measured at 0, 4 and 8 weeks, while skin wrinkle surface topography parameters were measured at 0 and 8 weeks. Prior to the study, the volunteers were tested for any skin irritant in order to comply with the safety assessment.
Skin Irritation Test [12, 13]
The safety assessment was investigated by the individual conducting a skin irritation test. The skin irritation study was carried out by using Finn chamber®. Draize scoring system was used to calculate the primary dermal irritation index (PDII). The skin irritation was performed on the upper back of the volunteers. Finn chambers® which contained test substances of PLE, SB, PLES, 1 % w/v sodium lauryl sulfate (SLS) was used as a positive control, and deionized water was used as a negative control; the Finn chambers® was covered for 48 hours. After this process they were observed for any irritating reaction (erythema and edema formations) at 1, 24 and 48 hours after removal of the patch. For the results of this study, any volunteer who were suffering from a severe irritation were removed from the proceeding process of the study.
Efficiency of Skin Hydration, Elasticity, and Skin Surface Testing
The efficiency of skin hydration and elasticity test were adapted from Leelapornpisid et al. [11], the volunteers were asked to apply each test serum products (SB, PLES) of 0.2 g to the testing area on each site of their forearms. The volunteers were advised on how to apply the skin care products as follows; applying the test serum twice daily, morning-evening for 8 weeks. Dermalab Combo® Series (Cortex Technology ApS, Hadsund, Denmark) was used to measure the required data at 0, 4 and 8 weeks after applying the test products. Untreated area on one site of the volunteer’s forearm was used as the control group. The site that PLES serum was applied on aimed for the moisture values observation, and the SB serum was applied at the placebo area. Before the study, the volunteers were required to equilibrate at a constant temperature room (21±2°C, 50-60% relative humidity) for thirty minutes. Skin properties were measured by noninvasive methods.
Corneometry
Hydration or water content of the stratum corneum was determined via measuring electrical capacitance obtained from the hydration probe. Skin hydration was measured at three different closely adjoining points on the forearm skin of each volunteer.
Skin Elasticity
In vivo skin elasticity meter was used to measure skin elasticity and firmness of the study area based on the suction method, and stress/strain by the preset vacuum. The elasticity probe was used in this study. The resistance of skin was drawn into the probe by a negative pressure (firmness). The graphs showing skin ability to return to its original position (elasticity) are displayed as curves which can be used to calculate the elastic and viscoelastic properties of the skin. Skin elasticity was measured at three different closely adjoining points on the forearm skin.
Skin Surface Topography
The video scope probe allows optical and tridimensional imaging of selected skin areas to be acquired. The images were taken by using a sensitive digital camera with white LED lights that was diffused or polarized with light source. The images were taken in relaxed conditions and the digital image 1.3M pixels (SXGA) with 2 magnification ranges was used to evaluate mean wrinkle surface.
Self-Assessment Questionnaire
The volunteers were asked to fill out a questionnaire, presenting their satisfaction about the PLES serum products after using it for 8 weeks.
Statistical Analysis
The results were summarized from three independent experiments and presented as means±SD. The statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS), version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Significant differences were examined using the analysis of variance (two-way repeated and two-way ANOVA) followed by the Tukey’s test. Statistical significance was determined to be at p values lower than 0.05.
Results and Discussion
The Stability Test of the Topical Anti-Aging Serum Products
The stability test of the PLES serum product was evaluated under accelerated condition, the results showed that its physical properties including pH and appearance for all conditions did not change after storage. The viscosity values slightly changed but not significantly different at p<0.05. Moreover, results revealed that the heat slightly affected the viscosity of the formulation but not significantly different. The feeling and spreadability on the skin were good, while the separation and precipitation were not observed. The testing results are shown in Table 2.
Table 2: The stability testing results of PLES serum in various conditions; room temperature (RT), 4°C and 45°C for 3 months and the heating/cooling (H/C cycle) conditions for six cycles.
| Parameters | Initial | RT | 4 °C | 45 °C | H/C cycle |
| Appearance | Dark green color | Dark green color | Dark green color | Dark green color | Dark green color |
| Feel on application | Good absorptionability, Smooth | Good absorptionability, Smooth | Good absorptionability, Smooth | Good absorptionability, Smooth | Good absorptionability, Smooth |
| pH | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Separation and precipitation | No | No | No | No | No |
| Viscosity (Pas) | 970.67 + 5.13a | 979.67 + 7.57a | 976.33 + 19.86a | 965.67 + 11.68a | 960.33 + 17.79a |
Values are expressed as means ± standard deviation (SD). The viscosity values are significantly different at p<0.05. Letters a is a statistical comparison between groups in various conditions.
Clinical Evaluation
Skin Irritation Test
The skin irritation test aimed to determine the safety of the extract and the test serum products for this work. The dermal irritancy potential of the test substances is shown in Table 3 and the irritating reactions on the upper back after removal of the patch at 1 hour is shown in Fig. 1. We used the deionized water, 1 % w/v SLS, PLE, SB and PLES serum as the test substances. These substances were tested on the upper back of the thirty healthy volunteers. The position of the test site areas is shown in Fig. 1(a). The results shown that deionized water, PLE, and PLES serum were non-irritating with low Primary Dermal Irritation Index value (PDII < 0.5), whereas 1 % w/v SLS and SB serum were slightly irritating (PDII range from 0.5 to 2.0). The results revealed that the serum that contained PLE has low PDII value than SB due to the anti-inflammatory activity of the PLE that could reduce the irritant and inflammatory on the skin. According to previous reports, P. frutescens extract could be useful for inhibition of the inflammatory process [14-16]. Furthermore, the ethanol extract of P. frutescens was identified to display anti-inflammatory activity in LPS-induced Raw 264.7 macrophages [17, 18]. For the irritation reactions; erythema and edema formations on the upper back of the volunteer had slowly decreased after 24 hours of treatment and the irritation reaction were not observed after 48 hours of treatment.
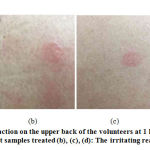 |
Figure 1: The irritating reaction on the upper back of the volunteers at 1 h after removal of the patch |
DW: Deionized water; PLE: P. frutescens leaves extract; SLS: 1 % w/v sodium lauryl sulfate; PLES: Serum containing P. frutescens leaves extract product; SB: Serum base product
Table 3: The Primary Dermal Irritation Index (PDII) and skin irritation reaction observed in thirty healthy volunteers
| Test substances | PDII value | Classification of skin reaction |
| DW (Negative control) | 0.00 | Non-irritating |
| PLE | 0.07 | Non-irritating |
| SLS (Positive control) | 0.86 | Slightly-irritating |
| SB serum | 0.50 | Slightly-irritating |
| PLES serum | 0.38 | Non-irritating |
Skin Hydration and Elasticity Test
The in vivo evaluations of moisturizing and elasticity of cosmetic formulations containing PLE in human skin are showed in Table 4. The results revealed that the percentages of moisturizing effect on the skin after applying SB and PLES serums for 4 weeks were not significantly different (p<0.05); the moisture content had been increased from 13.88 ± 7.26 at untreated area to 26.01 ± 6.51 and 18.96 ± 6.62, respectively. At week 4, SB serum showed the highest percentage of moisture content, whereas in long term use (8 weeks) the PLES serum presented the highest percentage of moisture content and the application of PLES serum indicated that there was no significantly increase in percentage of moisture content against untreated area and SB serum (placebo area). As a result, the serum products could improve the moisture of the skin after 4 weeks and it showed significantly different between week 4 and 8. This can be concluded that the serum containing PLE possessed a good skin moisturizing effect that is used in the long term.
Table 4: The percentage of moisture content and elasticity of the skin
| Test substances | % Moisture content | % Elasticity (RT) | ||||
| week 0 | week 4 | week 8 | week 0 | week 4 | week 8 | |
| Untreated area | 0 | 13.88 ± 7.26a | 34.58 ± 5.34A | 0 | 2.52 ± 2.70a | 11.14 ± 2.41A |
| SB serum | 0 | 26.01 ± 6.51a | 44.55 ± 9.71A | 0 | 5.86 ± 2.82a | 17.46 ± 3.33A |
| PLES serum | 0 | 18.96 ± 6.62a | 48.36 ± 7.64A | 0 | 17.93 ± 4.46b | 35.60 ± 4.51B |
The values are expressed as means ± standard error (SE), significantly different at p<0.05.
Letters a and b are statistical comparisons between groups in 4 weeks, A and B are statistical comparisons between groups in 8 weeks, a and A are statistical comparisons between application time. The same alphabet indicates that it is not statistically significant.
At week 4, the percentage of skin elasticity of the volunteer’s skin after applying SB and PLES serums had increased from 2.52 ± 2.70 at untreated area to 5.86 ± 2.82, and 17.93 ± 4.46, respectively. In addition, at week 8 after applying SB and PLES serums, the percentage of skin elasticity had increased from 11.14 ± 2.41 to 17.46 ± 3.33 and 35.60 ± 4.51, respectively (Table 4). It was found that the percentage of skin elasticity between applying the SB and PLES serums was significantly different (p<0.05) at weeks 4 and 8. The PLES serum presented the highest percentage of skin elasticity on weeks 4 and 8, which was significantly different (p<0.05) from the untreated area and SB serum. The result indicated that PLE could improve the elasticity of the skin. Dhyani et al. reported that P. frutescens contains potential antioxidant, anti-inflammatory, anti-allergic, and also anti-aging activities [19]. Furthermore, they also reported that flavonoids have been found to possess antioxidant, anti-allergic, anti-aging, and anti-inflammatory properties, and it can improve skin elasticity, skin hydration and collagen content [20].
The Skin Surface
Image analysis provides information on skin texture. Normally, skin texture is not smooth, based on the sulcus cutis and crista cutis. Sulci cutis is a multiple network of fine grooves. These can be deep or shallow. Cristae cutis is the slightly elevated areas that are surrounded by shallower areas of sulci cutis. When the skin is healthy, the skin texture is highly regular in appearance. On the other hand, it looks irregular when it is not healthy.21 Nitta et al. reported that when the amount of water in the stratum corneum is increased, the volume of crista cutis is also increased, and swelling and sulcus cutis between crista cutis are narrowed.22
The skin texture is adversely affected by maturation and aging, and skin appearance including roughness, dry surface of the skin and wrinkles formation, and the water content in the stratum corneum is decreased. These effects can be reduced by providing moisture to the skin surface.23-25 In this work, the skin surface images were compared between baseline (week 0) and after applying PLES serum for 8 weeks (Fig. 2). The results showed that the skin surface, after applying PLES serum presented finer skin texture and deep sulcus cutis, has linear grooves and is narrower before using PLES serum. It is a fact that narrower intervals between sulcus cutis imply finer textured skins. Nevertheless, finer skin texture is not only a phenomenon to justify the regularity of the skin texture, but also the equilateral triangles and their number of crista cutis are an index for considering regular evenness of texture.21 Fig. 2 shows the increased numbers of equilateral triangles after applying PLES serum as compared to the baseline. Sulcus cutis are found on the skin surface of the entire body, basically forming triangles, and the more uniform these shapes are, the finer and more regular the skin texture is. Consequently, the increased number of equilateral triangles within a testing area meant that the texture became much finer than it was before. This was a result of moisture effect from PLES serum, it can be concluded that this is proportional to the formation of P. frutescens leaves extract in PLES serum.
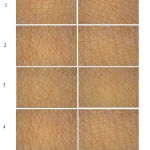 |
Figure 2: Appearance of the skin surface before and after using the PLES serum for 8 weeks. |
The Satisfaction of the Volunteers
The volunteers were asked to fill a satisfaction form after using the PLES serum for 8 weeks. Two relevant performance questions pertaining to serum texture, and skin moistures and smoothness. The range of satisfaction was a 5-point level from very well (level 5) to very poor (level 1). The results revealed that the volunteers’ satisfaction with the PLES serum was good to very well, exhibiting the satisfactory of more than 80% for all topics. Furthermore, the most satisfaction as a serum texture were color, odor, softness of serum, and overall satisfaction (approximately 67-77%). In addition, the proportions of volunteers who found an improvement of skin smoothness can be categorized as very well and good of 60 % and 40%, respectively. Besides, the volunteers observed an improvement in skin moisture or feel their skin being rehydrated very well with 40%, good with 53% and moderate with 7% of all volunteers (Fig. 3). The satisfaction results suggested that the volunteers liked the color of the serum extremely, although the color of the serum tends to be a dark green color. In literature, it was reported that the odor of the serum as aroma pleasant of the essential oil from the PLE can be used as a fragrance ingredient in cosmetic products.18Additionally, there was no report that the volunteers have skin irritation or allergic reaction during the period of application.
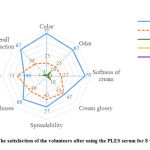 |
Figure 3: The satisfaction of the volunteers after using the PLES serum for 8 weeks (n=30) |
Conclusions
This study demonstrated that the serum containing PLE was stable at various storage conditions. The clinical evaluation indicated that the serum containing PLE could increase skin hydration and skin elasticity after treatment. In terms of skin texture, the sulcus cutis was narrower with the effect from the test serum, and the numbers of equilateral triangles were increased after applying PLES serum than the baseline. This means that the skin appears to have a finer texture. From the previous studies, P. frutescens leaves extract was presented as being anti-inflammatory, anti-aging activity, and identified as decreasing oxidative stress which caused skin disease such as an aging of the skin. This study further utilized the findings from the previous work to develop a serum containing PLES to promote skin moisturizing and skin elasticity efficiencies. Furthermore, the PLES can reduce the irritant and inflammatory on the skin. The volunteers were satisfied with the product, especially with its color, odor and efficacy. Therefore, P. frutescens leaves can used as a substitute for seed extract. In addition, it’s an added value for the products and has the potential to be used as an active ingredient in cosmeceutical products developed for anti-inflammatory and anti-aging applications.
Conflict of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Funding Source
University of Phayao, Phayao, Thailand. (Grant number: RD59066)
References
- Baki G, Alexander KS. Introduction to cosmetic formulation and technology – Skin care product. New Jersey: John Wiley & Sons, Inc., Hoboken; 2015.
- Hashizume H. Skin aging and dry skin. J. 2004; 31: 603-609.
- Ganceviciene R, Liakou A, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinl. 2012; 4(3): 308-319.
- Poucher W.A. Poucher’s Perfumes, Cosmetics and Soaps: Volume 3: Cosmetics 348. Springer science+Business Media Dordrecht. Hong Kong. 1993.
- Nusgens BV, Humbert P, Rougier A,et al. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J Invest Dermatol. 2001; 116(6): 853-9.
- Ahmed HM. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules. 2019; 24(1): 102: 1-23. doi:10.3390/molecules24010102.
- Jun HI, Kim BT, Song GS, Kim YS. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens acuta) leaves. Food Chem. 2014; 148: 367–372.
- Oh HA, Park CS, Ahn HJ, et al. Effect of Perilla frutescens acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Exp Biol Med (Maywood). 2011; 236: 99-106.
- Wang XF, Li H, Wang QQ, et al. Anti-inflammatory constituents from Perilla frutescens on lipopolysaccharide-stimulated RAW264.7 cells. 2018; 130: 61-65.
- Komatsu K, Takanari J, Maeda T, Kitadate K, Sato T, Mihara Y, Uehara K, Wakame K. Perilla leaf extract prevents atopic dermatitis induced by an extract of Dermatophagoides farinae in NC/Nga mice. Asian Pac J Allergy Immunol. 2016; 34: 272-277. doi: 10.12932/AP0717.
- Leelapornpisid P, Mungmai L, Sirithunyalug B, et al. A Novel Moisturizer Extracted from Freshwater Macroalga [Rhizoclonium hieroglyphicum(C.Agardh) Kützing] for Skin Care Cosmetic. Chiang Mai J Sci. 2014; 41(5.2): 1195-1207.
- Barel AO, Maibach HI. Handbook of Cosmetic Science and Technology, 3rd. In: Bashir SJ and Maibach HI, editors, In Vivo Irritation. New York: Informa healthcare; 2009.
- OECD Guideline for the Testing Chemicals. Acute Dermal Irritation/Corrosion. 404; 2002.
- Lee HA, Han Anti-inflammatory Effect of Perilla frutescens(L.) Britton var. frutescens Extract in LPS-stimulated RAW 264.7 Macrophages. Prev Nutr Food Sci. 2012; 17(2): 109–115.
- Jeon IH, Kim HS, Kang HJ, et al. Anti-Inflammatory and Antipruritic Effects of Luteolin from Perilla ( frutescens L.) Leaves. Molecules. 2014; 19: 6941-6951.
- Chen CY, Leu YL, Fang Y, et al. Anti-inflammatory effects of Perilla frutescens in activated human neutrophils through two independent pathways: Src family kinases and Calcium. Sci Rep. 2015; 5, 18204: 1-11.
- Huang BP, Lin CH, Chen YC, Kao SH. Anti-inflammatory effects of Perilla frutescens leaf extract on lipopolysaccharide-stimulated RAW264. 7 cells. Mol. Med. Rep. 2014; 10(2): 1077–1083. doi: 10.3892/mmr.2014.2298.
- Mungmai L, Preedalikit W, Aunsri N, Peerakam N. Bioactivity test and GC–MS analysis of different solvent extracts from Perilla frutescens (Linn.) Britton and cosmetic product application for sensitive skin. & Tech. RMUTTJ. 2019; 9(2): 78-93.
- DhyaniA, Chopra R, Garg M. A Review on Nutritional Value, Functional Properties and Pharmacological Application of Perilla (Perilla Frutescens ). Biomedical & Pharmacology Journal. 2019; 12(2): 649-660. doi: 10.13005/bpj/1685.
- Dixit D, Reddy CRK. Non-targeted secondary metabolite profile study for deciphering the cosmeceutical potential of red marine macro alga Jania rubens—an LCMS based approach. 2017; 4, 45; doi:10.3390/cosmetics4040045.
- Matsumoto M, Matsuo J, Dai M, et al. Influence of differences in washing methods on skin texture. Int J cosmetic sci. 2014; 36: 175–181.
- Nitta S, Matsumoto M, Sugama J, et al. New quantitive indicators of evaluating the skin care regimen for older adults with dry skin by using the digital image analysis. JNSE. 2016; 3(2): 93-100.
- Yuki K, Kawano S, Mori S, Murase T. Facial application of high-concentration carbon dioxide prevents epidermal impairment associated with environmental changes. Clin Cosmet Investig Dermatol. 2019; 12: 63-69.
- Choi JW, Kwon SH, Huh CH, et al. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol. 2013; 19(1):e349-55.
- Wiegand C, Raschke C, Elsner P. Skin Aging: A Brief Summary of Characteristic Changes. In: Textbook of Aging Skin. 2ed. Springer-Verlag Berlin Heidelberg; 2017.55-65p.








