I Made Jawi1 , I Wayan Putu Sutirta Yasa2
, I Wayan Putu Sutirta Yasa2 , Agung Nova Mahendra1*
, Agung Nova Mahendra1* and I Wayan Sumardika1
and I Wayan Sumardika1
1Department of Pharmacology and Therapy, Faculty of Medicine, Universitas Udayana, Bali, Indonesia
2Department of Clinical Pathology, Faculty of Medicine, Universitas Udayana, Bali, Indonesia
Corresponding Author E-mail : novamahendra@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1930
Abstract
Tablet is a solid preparation which is proven more stable and practical to be stored than the liguid counterpart, therefore the tablet form of purple sweet potato extract (PSPE) is expected to resolve the instability issues of liquid preparation. Single dose of PSPE tablet has been shown to exhibit weaker effect than the liquid preparation. The purpose of this study was to determine the effective dose of PSPE tablet in preventing oxidative stress and improvement of the lipid profile of the hyperlipidemic rats. In this study, we also investigated the safety of the tablet on rat’s livers and kidneys. This study was an experimental laboratoric study, with randomized pre- and post-test control group design. Subjects were assigned into 4 groups (7 rats in each group). Group 1 was control group, which were given high cholesterol diet for 4 weeks. Group 2, 3 and 4 were treatment groups, which were given high cholesterol diet and PSPE tablet of 100 mg/day, 2 x 100 mg/day, and 3 x 100mg/day, respectively, for 4 weeks. We found that the best dose was 2 x 100mg/rat/day in term of lipid profile, oxidative stress, and effect on liver and kidney. In conclusion, the relatively effective dose for hypercholesterolemia and oxidative stress mitigation, and safest dose of PSPE tablet for livers and kidneys of high cholesterol-fed rats is 2 x 100 mg/day.
Keywords
Hypercholesterolemia, Oxidative Stress, Liver and Kidney Function, Purple Sweet Potato Extract Tablet
Download this article as:| Copy the following to cite this article: Jawi I. M, Yasa I. W. P. S, Mahendra A. N, Sumardika I. W. Effective Dose and Safety Profile of Purple Sweet Potato Tablet Preparation in Rats With High Cholesterol Diet. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Jawi I. M, Yasa I. W. P. S, Mahendra A. N, Sumardika I. W. Effective Dose and Safety Profile of Purple Sweet Potato Tablet Preparation in Rats With High Cholesterol Diet. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/3e8tHcE |
Introduction
Purple sweet potato has been revealed to show antioxidant effect and has been extensively investigated for its potential therapeutic uses. The liquid preparation of purple sweet potato extract (PSPE) has been shown to exert beneficial effects in animals and humans (Jawi et al., 2008; Jawi et al., 2011; Jawi et al.,2015; Mahadita et al., 2016; Sutirta-Yasa et al.,2018). The liquid preparation of PSPE is unstable, therefore we need the solid preparation that is more stable than liquid preparation. Tablet is one of the solid preparations that is physically and chemically more stable than liquid preparation (Bajaj et al., 2012). Tablet preparation also has more advantages; dosage and volume of active ingredient can be managed precisely, can be packaged nicely, can be swallowed easilly, and practically easy to be transported and stored (Harbir, 2012; Kushwaha and Kori, 2014). Tablet preparation of purple sweet potato can maintain cholesterol concentration and prevent oxidative stress in rat that were given high cholesterol diet (Sutirta-Yasa et al., 2018), although the effective dose and chronic toxic effect in liver and kidney had not elucidated yet. Based on these findings, we conducted this current study to determine the effective dose for hypocholesterolemic and antioxidant effect, and effect on liver and kidney of PSPE tablet in rats that are given high cholesterol diet for 4 weeks.
Materials and Methods
Animal Model
This study was an experimental laboratoric study, with randomized pre- and post-test control group design. Male Wistar rats (170-200 g), 3-4 months old, were obtained from Animal House Facility, Department of Pharmacology and Therapy, Faculty of Medicine, Universitas Udayana, Denpasar, Bali, Indonesia. These rats were divided into 4 groups (7 rats per group). Group 1 was given high cholesterol diets as a control group. Group 2 was given high-cholesterol diet and 100mg/day tablet of sweet potato extract, namely as the treatment 1 (T1) group. Group 3 was the group of rats that were given high-cholesterol diet and 2 x 100 mg/day of PSPE tablet, namely as the treatment 2 (T2) group. Group 4 was were given high-cholesterol diet and 3 x 100mg/day tablet of purple sweet potato extract, namely as the treatment 3 (T3). The duration of treatment was 4 weeks and the tablet preparations were given orally via gastric intubation. All rats were maintained under standard laboratory conditions at 25 ± 2°C (temperature), 50 ± 15% relative humidity and normal photoperiod (12-hours light-dark cycle). Commercial high cholesterol pellet diets and water were provided ad libitum for those animals. The use of animal in this study was approved by Committee on Research Ethics, Faculty of Medicine, Universitas Udayana, Bali, Indonesia.
PSPE Tablets Preparation
Extract was made in the following manner: 1 kg of purple sweet potato powder was steamed for 15 minutes then macerated with 3 L of 70% ethanol which was acidified with 3% citric acid, for 24 hours. The macerate obtained was collected and then concentrated with a vacuum rotary evaporator at 40°C with a pressure of ± 70-80 mbar until a concentrated extract was obtained, with the volume of extract reaching 1/10 of the volume of the initial filtrate. Ten mL of thick extract was added by adsorbing 25 grams of purple sweet potato starch powder, then crushed until achieving homogeneous state. The extract mixture and the adsorbent were dried in an oven at 60°C for 5 hours, then made into tablets with a single puncher machine, to obtain 100 mg of anthocyanin per tablet.
Blood Examination and Analysis
Blood samples were taken via retro-orbital plexus of all rats, collected in plain tubes, and allowed to clot at room temperature. The blood samples were used for the quantification of lipid profile, MDA, and SOD level (pre- and post-test). The blood samples were centrifuged at 1500 rpm for 10 min. The supernatants (sera) were collected and stored at 20°C until further analysis. The plasma serum of blood samples at baseline and at the end of the study were used for the MDA, SOD, and lipid profile examination. The MDA examination was carried out by thiobarbituric acid reactive substances (TBARS) and total antioxidant status Randox kit for SOD. Both examinations were calculated in nmol/mL. Colorimetric kits from Azmun (Tehran, Iran) were used in assessing serum triglycerides, total cholesterol, and HDL-C in mg/d. All data were statistically analyzed using computer.
Results
This study was conducted to investigate the effective hypocholesterolemic and safe dose of PSPE tablet, specifically for liver and kidneys, in an animal model of hypercholesterolemia. We found that the blood cholesterol levels before the treatment (pre-test) were almost the same in all groups (p>0.05). The blood cholesterol levels increased in all groups after the rats were treated with high cholesterol diet for 4 weeks (p<0.05), but if we compare the control group (group of the rats that were given high cholesterol diet only) we found that the rats that were given tablet 100 mg/day had significantly lower blood cholesterol level (p<0.05). In this study, it has been shown that the most effective dose for significant cholesterol level reduction was 2 x 100 mg/day (p<0.05). The dose of 3 x 100mg/day did not exhibit greater hypocholesterolemic effect than the dose of 2 x 100 mg/day. Intriguingly, we revealed that the group with 3 x 100 mg/day had higher blood cholesterol level than the group that was treated with 2 x 100mg/day. The complete results are shown in figure 1.
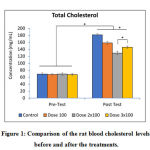 |
Figure 1: Comparison of the rat blood cholesterol levels before and after the treatments. |
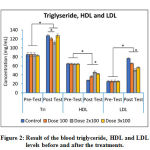 |
Figure 2: Result of the blood triglyceride, HDL and LDL levels before and after the treatments. |
As shown in figure 2, HDL, LDL and triglyceride (TG) levels were almost the same in all of the rat groups (p>0.05) before the commencement of treatment. The LDL and TG level significantly increased, and the HDL level decreased after the rats were treated for 4 weeks (p<0.05), but the rats that were given 100 mg/day had lower blood TG and LDL level than control group (rats that were given high cholesterol diet only) and these findings were statistically significant (p<0.05). Our result also revealed that HDL level of the group that was treated with 100 mg/day of PSPE tablet was lower than 2 group (p<0.05). We also found that the highest decrease of the blood TG and LDL levels were achieved by treatment with 2 x 100 mg/day and statistically significant (p<0.05), this dose also increased HDL level significantly compared to the control group. The dose 3 x 100 mg/day could not decrease the blood TG and LDL levels, but oppositely increased the triglyseride and LDL higher than the dose 2 x 100mg/day.
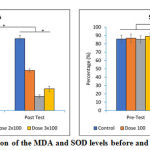 |
Figure 3: Comparison of the MDA and SOD levels before and after the treatments. |
The MDA and SOD levels were almost the same in all groups before the treatments. The levels of MDA and SOD were significantly different after the treatment (p<0.05). Interestingly, our result shown that the lowest MDA and the highest SOD levels were found in the group that was treated with dose 2 x 100mg/day.
 |
Figure 4: Comparison of SGOT and SGPT levels before and after the treatment. |
Figure 4 shows that the SGOT and SGPT levels before the treatment were almost the same in all rat groups (p>0.05). The SGOT and SGPT level increased after the rats were given with high cholesterol diet. Opposite results were found in the group that was treated with tablet of purple sweet potato. In these group, the SGOT and SGPT levels were lower than control group, the lowest levels were found in the group that was treated with dose 2 x 100 mg/day (p<0,05).
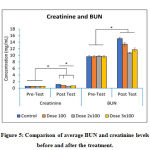 |
Figure 5: Comparison of average BUN and creatinine levels before and after the treatment. |
Figure 5 shows that the BUN and creatinine levels were almost the same in all groups before the treatment (p>0.05). The BUN and creatinine levels increased in the group that was given with high cholesterol diet. The BUN and creatinine levels in the group that was treated with tablet of purple sweet potato were lower than the control group and statistically significant. The lowest BUN and creatinine levels were found in the group that was given 2 x 100mg/day of PSPE tablet (p<0.05).
Discussion
The pre-test blood test results reveals homogenous finding in all parameters (p>0.05). The blood test parameters are altered after the treatment and coherent with the doses of the tablet of purple sweet potato that were given to the rats. The blood lipid profile of the rats that were given high cholesterol diet (control group) significantly increased in the total cholesterol after treatment for 4 weeks. The other parameters were also changed significantly, i.e decreased HDL level, increased LDL and TG level. The lipid profile of the group that were treated with 1 x 100mg/day of PSPE tablet showed increased cholesterol total level but still much lower than control group (p<0.05). This finding supported our hypothesis that PSPE tablet administration can prevent the elevation of blood cholesterol level. The normalization of cholesterol level after the PSPE tablet treatment is caused by flavonoid, a polyphenol that is well known as anthocyanin. Polyphenol prevents the elevation of blood cholesterol level through some known mechanisms, i.e. inhibition of cholesterol absorption in gastrointestinal system, attenuation of lipoprotein production in the liver, regulation of LDL receptor that will decrease plasma cholesterol level, and reduction of plasma TG level. These findings were supported by systematic review result that was done by Wallace (2016). Wallace evaluated 10 articles and found that administration of anthocyanin-containing extract could decrease cholesterol total and improve lipid profile. Our results suggest that increasing the dose of tablet of purple sweet potato could improve the lipid profile of the rats that were treated with high cholesterol diet, with the most effective dose of 2 x 100mg/day for 4 weeks.
In this study we also found that administration of PSPE tablet could decrease MDA and increase SOD level, which were consistent with our previous findings (Jawi et al., 2011; Jawi et al., 2015; Sutirta-Yasa et al., 2018). These observed findings can be attributed to the presence of active substance of purple sweet potato or by secondary metabolite of purple sweet potato, anthocyanins (pheonidin and cyanidin), which are antioxidants. Purple sweet potato also contains many phenolic acids, i.e., chlorogenic, isocholorogenic, caffeic, cinammic, and hydroxycinnamic acids, which are good antioxidants because they have the ability to scavenge free radicals in the cells (Ayeleso, 2016). The highest decrease of MDA level was achieved by treatment with the dose of 2 x 100 mg/day for 4 weeks. The group that was treated with 1 x 100 mg/day also showed decrease of MDA level but lower that of the group that was treated with 2 x 100 mg/day. Certain dose of extract that contain anthocyanin can protect the cell from oxidative stres. Anthocyanin in high dose can cause toxic effect on the cells (Sangkitikomol et al., 2010), therefore will elevate MDA level. Other study also found that high dose of anthocyanin (flavonoid) in the extract could increase MDA level. Flavonoids can protect the cell from oxidative stress through some mechanisms. Flavonoids are postulated to bind with lipid membrane of the cells, these binding will prevent free radicals entry into the cells (Sangkitikomol et al., 2010). The results of this study are supported by the study that was done by a group in Malang. They evaluated the effect of different doses of anthocyanin of purple sweet potato to MDA level in rat’s brain tissue. They found that the MDA level was lower in the group that was given low dose than high dose of anthocyanin, eventhough this finding was not statistically significant (Prabawati et al., 2019).
In our current study, we found that the administration of PSPE tablet can affect liver function, by evaluating SGOT and SGOT level before and after the treatment of the substance for 4 weeks. Anthocyanin-rich extract could exhibit hepatoprotective properties because of antioxidant and antiinflammatory effects of the anthocyanin and inhibit accumulation of fat in liver (Cardoso et al., 2015). The increasing and decreasing of SGPT and SGOT in this study were affected by the dose that was given to the rats. In the group that was given dose 2 x 100mg/day for 4 weeks, the SGOT and SGPT levels were almost normalized (p>0,05), therefore this finding can be used in further studies as a guidance to administer effective dose of PSPE tablet to normalize lipid profile. These findings were supported by a study, which found that anthocyanin of purple sweet potato had hepatoprotective effect in carbon tetrachloride-induced mice. In this study, Zhang’s group found that the levels of SGPT and SGOT were significantly decreased due to the administration of purple sweet potato extract (Zhang et al., 2016). In a clinical study on Caucasians with borderline hepatitis, PSP beverage had also been revealed to possess hepatoprotective effect, shown by its effect on altering serum level of hepatic biomarkers (Oki et al., 2017).
The administration 2 x 100 mg/day of PSPE tablet for 4 weeks showed the most optimal protection to kidney than the other doses. The BUN and creatinine levels in the group that was given the dose of 2 x 100mg/day were almost similar with the pre-test results (p>0.05). In certain doses, PSPE can protect the kidney via upregulation of some transporters (shown by increasing levels of mRNAs, i.e., mURAT1, mGLUT9, mOAT1 dan mOCTN2) which improve renal tubular function. These transporters also prevent renal inflammation, thus contributing to the maintenance of normal BUN and creatinine level. These findings were supported by study that was done by a group lead by Zi-Cheng. This group found that anthocyanin-rich PSPE could decrease BUN and creatinine serum level in hyperuricemic mices (Zi-Cheng et al., 2015).
Conclusion
Based on the findings in this study, we conclude that the most effective and safest dose of PSPE tablet for liver and kidneys in high cholesterol diet-treated rats is 2 x 100 mg/kg/ day, for 4 weeks.
Acknowledgment
We would like to acknowledge I Gede Wiranatha, S.Si., M.Si., for his tremendous efforts in nurturing the rats and administering the tablet preparations.
Conflict of Interest
None to be declared.
Source of Funding
This study was financially supported by a research grant conferred by UPPM, Faculty of Medicine, Universitas Udayana, Denpasar, Bali, Indonesia.
grant is: 61/UN14.2.2/UPPM/2018.
References
- Jawi I M, Suprapta D N, Dwi S U, Wiwiek I. 2008. Ubi Jalar Ungu Menurunkan Kadar MDA dalam Darah dan Hati Mencit setelah Aktivitas Fisik Maksimal. Jurnal Veteriner Jurnal Kedokteran Hewan Indonesia. 9(2):65-72.
- Jawi I M dan Budiasa K, 2011. Ekstrak air umbi ubi jalar ungu menurunkan total kolesterol serta meningkatkan total antioksidan pada darah kelinci. Jurnal Veteriner, Jurnal Kedokteran Hewan Indonesia. 12 (2); 120-125.
- Jawi I M, Indrayani W, I G K Arijana I G K, Subawa A A N, Suprapta D N. 2015.Aqueous Extract of Purple Sweet Potato Increaseed SOD-2 and SOD-3 on Human Umbilical Vein Endothelial Cell in Vitro. Journal of Biology, Agriculture and Healthcare. 6(2):103-110.
- Mahadita G W, Jawi M and Suastika K. 2016. Purple sweet potato tuber extract lowers mallondialdehyde and improves glycemic control in subjects with type 2 diabetes mellitus. Global Advanced Research Journal of Medicine and Medical Sciences 5(7):208-213.
- Sutirta Yasa I W P, Jawi I M, Astawa P. 2018. The Comparative Effect of Liquid and Tablet Preparation of Purple Sweet Potato (Ipomoea batatas L) Extract to Lipid Profile, MDA, and SOD Level in Male Wistar Rats After Given High Cholesterol Diet. Journal of Global Pharma Technology; 10(07):356-361.
- Bajaj S, Singla D, and Sakhuja N. 2012. Stability Testing of Pharmaceutical Products. Journal of Applied Pharmaceutical Science 02 (03); 2012: 129-138.
- Harbir K. 2012. Processing Technologies For Pharmaceutical Tablets: A Review. International Research Juornal of Pharmacy. 3(7):20-23.
- Kushwaha, S K; Kori M L. 2014. Development and Evaluation of Polyherbal Tablet from Some Hepatoprotective Herbs. Sch. Acad. J. Pharm. 2014; 3(3): 321-326
- Wallace T C, Slavin M, Frankenfeld C L. 2016. Systematic Review of Anthocyanins and Markers of Cardiovascular Disease. Nutrients. 8(1):32.
- Ayeleso T B, Ramachela K and Mukwevho E. 2016. A review of therapeutic potentials of sweet potato: Pharmacological activities and influence of the cultivar. Trop J Pharm Res, 15(12): 2751-2761.
- Sangkitikomol W, Tencomnao T, and Rocejanasaroj A. 2010. Antioxidant effects of anthocyanins-rich extract from black sticky rice on human erythrocytes and mononuclear leukocytes. African Journal of Biotechnology Vol. 9(48), pp. 8222-8229.
- Prabawati R K, Ratnawati R, Rahayu M, Prakosa A G. 2019. Effect anthocyanin of purple potato Gunung Kawi on MDA levels, expression of caspase-3 and spatial memory function on diabetic wistar rats. Malang Neurology Journal, 34-41.
- Cardoso L M, Novaes R D, Aparecida de Castro C, Alexandre Azevedo Novello A A, Gonçalves R V, Ricci-Silva M E, Ramos H J O, Peluzio M C G and Leite J P V. 2015. Chemical composition, characterization of anthocyanins and antioxidant potential of Euterpe edulis fruits: applicability on genetic dyslipidemia and hepatic steatosis in mice. Nutr Hosp. 2015;32(2):702-709.
- Zhang M, Li-jun P, Shao-Ting J and Yu-Wen M. 2016. Protective effects of anthocyanins from purple sweet potato on acute carbon tetrachloride-induced oxidative hepatotoxicity fibrosis in mice. Food and Agricultural Immunology, 27(2): 157–170.
- Oki T, Kano M, Ishikawa F, Goto K, Watanabe O, Suda I. 2017. Double-blind, placebo-controlled pilot trial of anthocyanin-rich purple sweet potato beverage on serum hepatic biomarker levels in healthy Caucasians with borderline hepatitis. European Journal of Clinical Nutrition. 71(2):290-292.
- Zi-Cheng Z, Guan-Hua S, Chun-Li L, Ya-Lu P, Wang L, Li X, Jia-Hao W and Jiu-Liang Z. 2015. Effects of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) on the serum uric acid level and xanthine oxidase activity in hyperuricemic mice. Food Funct. 6:3045–3055.







