Department of Clinical Laboratory Sciences, College of Pharmacy, Al-Nahrain University, Iraq
Corresponding Author E-mail : fermsc33@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1964
Abstract
Candesartan is one of the angiotensin II receptor blocker drugs that used for the treatment of hypertension. The plan of this prospective clinical study was to assess adequacy and acceptability of candesartan cilexetil (16 mg daily) in corpulent patients with essential hypertension. This research enrolled sixty-five corpulent patients (40 males and 25 females), aging (37 - 47 years) with stage 2 essential hypertension from Al yarmouk hospital in Iraq. Blood pressure (systolic and diastolic) ,body mass index (BMI) and clinical laboratory tests that included serum levels of lipid profile, leptin ,omentin-1 and chemerin were measured and studied at baseline (prior treatment) and after three months treatment with candesartan cilexetil (16 mg daily). The outcomes of this study declared that the treatment with candesartan cilexetil (16 mg daily) resulted in decreasing blood pressure (systolic and diastolic), P value < 0.001 and P value < 0.002 respectively, and in reducing serum levels of total cholesterol, triglyceride, leptin and chemerin as compared with their baseline levels. Contrarily, the treatment with candesartan cilexetil (16 mg daily) considerably elevated the serum level of omentin-1, P value < 0.01. Giddiness was the common adverse event presented in this study. The observation of this study on treatment of hypertensive patients with candesartan exhibited that this drug in addition to its adequacy in lowering blood pressure has a role in lessening blood levels of lipid profile and leptin and in amending blood level of olmentin-1.
Keywords
Adipocytokines; Candesartan; Corpulence; Hypertension
Download this article as:| Copy the following to cite this article: Rada F. H. Effect of Angiotensin II Receptor Blocker Treatment on Adipokine of Corpulence. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Rada F. H. Effect of Angiotensin II Receptor Blocker Treatment on Adipokine of Corpulence. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/3b4WMnu |
Introduction
Hypertension is a disease of cardiovascular system that associated with endothelial dysfunction, which thereafter through many pathways may lead to metabolic homeostasis disorders that include obesity and diabetes 1,2. Metabolic syndrome, an important risk factor for cardiovascular disease and mainly caused by obesity, is typified by hyperglycemia, hyperinsulinemia, dyslipidemia and hypertension 3,4 .
In the vascular wall, elevation of oxidative stress and then initiation of an inflammatory cascade were due to the stimulation of angiotensin II type-1 receptors. Therefore, treatment with angiotensin II type-1 receptor blockers (ARBs) drugs may lead to decrease these events in addition to decrease blood pressure 5,6 .
Many studies found that there is a relationship between obesity and hypertension because of over-expression of angiotensinogen (AGT) in adipose tissue. Actually, angiotensinogen formed mainly in the liver, expressed in adipose tissue, and is enzymatically converted to angiotensin I then angiotensin II by the action of renin-angiotensin system (RAS) 7.
Angiotensin II is biologically active and exerts its effect by binding to its receptors type -1 and type-2 . Angiotensin II type- 1 receptor (AT1R) antagonist drugs have been prioritized in the treatment of hypertension instead of angiotensin converting enzyme inhibitors (ACEIs) drug for many advantages such as high specificity of blocking AT1 receptor and then occluding renin-angiotensin system, non-elevating bradykinin level and not causing dry cough 8 .
The reduction of blood pressure by the using of Angiotensin II type- 1 receptor (AT1R) antagonists is dose dependent without increase incidents of adverse events. Besides high doses, these drugs may produce well protection of the organ than its ordinary doses 9 .
Candesartan cilexetil is one of the Angiotensin II type- 1 receptor antagonists that has a definite effect after additive dosing than other drugs such as losartan. Candesartan cilexetil is an oral drug that gave once-daily dose and is completely transformed into its active metabolite, during intestinal absorption, which has a longer duration of action and invincible effect 10 .
Omentin is a secretory protein that was superiorly and selectively express in visceral adipose tissue than in subcutaneous adipose tissue 11,12 .Additionally, omentin was inferiorly express in another tissues and called intelectin 13 .These tissues involved intestinal Paneth cells 14 , endothelial cells 15 , and stromal-vascular cells 11 . Omentin may has a role in adjusting insulin sensitivity by many ways such as elevated signal transduction of insulin by triggering the protein kinase Akt/ protein kinase B and improving the glucose transporter that stimulated by insulin and testified in isolated human adipocytes (In vitro study) 15 .
Chemerin is a protein that releases from adipocyte and hepatocyte. It has a role in controlling discrimination of adipocyte in an autocrine/paracrine mode of effect 16 . Chemerin has many splitting ways that produce different biological properties, one of them has a high attractive ability that responsible for inflammatory reaction and activation of dendritic cells and macrophages 17.Other has ability to deactivate macrophage and act as anti- inflammatory protein 18 .
The Purpose of this prospective clinical study is to appraise the therapeutic role of candesartan cilexetil (16 mg daily) in corpulent patients with essential hypertension.
Materials and Methods
Study Design
This study is a prospective clinical study, conducted at Al yarmouk hospital/in Iraq and is constituted of sixty five patients (40 males and 25 females), aged (37- 47) years of either sex, with essential hypertension at which the systolic blood pressure (SBP) ranged 140 -160 mmHg and diastolic blood pressure (DBP) ranged 90 -100 mmHg.
Patients’ Selection
All patients participated in this study were randomly selected and were put on wash-out period for two-weeks by stopping intake of all antihypertensive drugs or other drugs. Thereafter, they checked depending on their medical histories, physical examination, blood pressure measurement, and electrocardiogram. Preclusion criteria omitted the patients with secondary hypertension, liver function abnormality, kidney function abnormality, impaired cardiovascular conditions and patients with exceptional sensitivity to angiotensin-II receptor blocker or calcium antagonists. All eligible participants provided written informed consent to partake in this study. The study protocol accommodates to the ethical guidelines of the Helsinki declaration and endorsed by the institution’s ethics committee.
Data Collection and Laboratory Measurements
All parameters were evaluated at baseline (before treatment), and after three months of treatment with candesartan cilexetil (AstraZeneca) in a dose of 16 mg daily. All adverse events reported to evaluate adequacy and acceptability of the treatment.
All blood parameters were determined after 12-hrours overnight fasting. Venous blood samples took from all patients between 8 – 9 am. Body mass index calculated by measuring the weight of the patients in kilograms and divided by the height of the patients in squared meters.
Serum Leptin , Omentin-1, and Chemerin levels were estimated by using enzyme-linked immunosorbent assays (ELISA). While serum Total cholesterol, High-density lipoprotein-cholesterol and Triglyceride were assessed by using Photometric Colorimetric Tests.
Statistical Analyses
Results presented as mean ± SD (standard deviation) with 95% confidence interval (CI). Comparisons of continuous variables analyzed by using Student’s t-test. All tests for statistical significance were two-tailed and P values of <0.05 was chosen as cut-off point for statistical significance. All statistical analyses performed using series SPSS version 18 and Microsoft Excel.
Results
Clinical characteristics and statistical analyses of the data of the studied group stratified in Table 1. Adverse events assessment during the period of the treatment with candesartan cilexetil (16 mg daily) reported in Table 2. Giddiness was the most common neural adverse event observed during the period of the study. Most adverse events were mild or moderate in severity, therefore it does not intermitting the study. Obviously, a high significant decrease in blood pressure (systolic and diastolic) was noted after three months treatment with candesartan cilexetil (16 mg daily) when compared to baseline mean values (Table 1).
Table 1: Alterations of blood pressure and clinical bioassay before and after three months treatment with candesartan cilexetil (16 mg daily)
| Variables | Baseline (prior treatment) | After Candesartan treatment (three months) | P-value |
| Total number | 65 | 65 | 0.0 |
| Gender (Male / Female) | (40 , 25) | (40 , 25) | 0.0 |
| Age (years) | 42 ± 5 | 42 ± 5 | 0.0 |
| BMI (Kg/m2) | 30.6 ± 2.05 | 29.7 ± 2.04 | 0.0145 |
| SBP (mmHg) | 150 ± 13 | 143 ± 10 | 0.001 |
| DBP (mmHg) | 93 ± 10 | 88 ± 8 | 0.00245 |
| S.TC (mg/dL) | 195 ± 16 | 189 ± 15.2 | 0.032 |
| S.HDL-C (mg/dL) | 51.3 ± 5 | 51 ± 5 | 0.733 |
| S.TG (mg/dL) | 188 ± 20 | 181 ± 18 | 0.039 |
| S.Leptin (ng/mL) | 15.5 ± 4.5 | 13.4 ± 4.7 | 0.0113 |
| S.Omentin-1(ng/mL) | 248 ± 32 | 262 ± 28 | 0.00986 |
| S.Chemerin (ng/mL) | 217 ± 20 | 205 ± 17 | 0.00046 |
Data are presented as mean ± SD for continuous variable, P ˂0.05 significant difference vs. baseline, P ˂0.01 least significant difference (LSD) vs. baseline, P ˂0.001 high significant difference vs. baseline.
Table 2: The number (n) and the percent (%) of the patients who had adverse events during three months treatment with candesartan cilexetil (16 mg daily)
| Total number | N=65 | 100 % |
| 1. Nervous system disorder | 2 | 3.077 % |
| 2. Cardiac disorder | – | – |
| 3. Vascular disorder | – | – |
| 4. Skin and subcutaneous disorder | 1 | 1.54 % |
| 5. Musculoskeletal and connective tissue disorder | 1 | 1.54 % |
| 6. Gastrointestinal disorder | 2 | 3.077 % |
| 7. Renal and urinary disorder | – | – |
Analyses of lipid tests after three months treatment with candesartan cilexetil (16 mg daily) revealed a significant decrease (P ˂ 0.05) in mean serum levels of total cholesterol and triglyceride as compared with their baseline mean. Conversely, insignificant alteration noted in mean serum level of high-density lipoprotein cholesterol as compared with its baseline means, Table 1.
In contrast to the prior treatment, the mean serum levels of leptin and chemerin were appreciably lower in patients with candesartan treatment (Table-1).There were 0.86 folded decreased in mean serum level of leptin and 0.94 folded decreased in mean serum level of chemerin as compared to their baseline mean (Figure 1). Contrarily, the mean serum level of omentin-1 was considerably higher in those patients after the treatment with candesartan as compared to their baseline level and it was appear to be increased by 1.06 folded as noted in Figure 1.
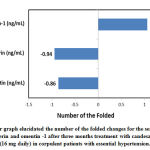 |
Figure 1: Bar graph elucidated the number of the folded changes for the serum levels |
Power analysis for the minimum detectable effect of candesartan cilexetil treatment 16 mg daily on serum levels of total cholesterol, triglyceride, leptin, chemerin , and omentin-1 after three months treatment are illustrated in Figure 2. There were 80% probability that the treatment with candesartan cilexetil 16 mg daily caused the detection of 6.83 mg/dL(3.5%) decrease in mean serum level of total cholesterol, 8.33 mg/dL (4.43%) decrease in mean serum level of triglyceride, 2.01 ng/ml (13%) decrease in mean serum level of leptin , 8.12 ng/ml (3.74%) decrease in mean serum level of chemerin and 13.16 ng/ml (5.31%) increase in mean serum level of omentin-1 .
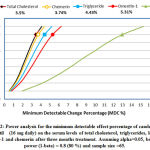 |
Figure 2: Power analysis for the minimum detectable effect percentage of candesartan |
Discussion
Hypertension is the most popular diseases that if untreated, may lead to high-risk complication associated with cardiovascular function and renal function. This study evaluated the effect of candesartan treatment on reducing blood pressure in patients with high body weight. The most common adverse effect reported in this study was Giddiness, which may be occurs because of reducing blood pressure.
Many studies quested the hypotensive effect of angiotensin-I receptor blocker drug, candesartan, either alone or in combination with other hypotensive drugs. They found that candesartan was a potent hypotensive drug and its ameliorative effect seems to be at higher doses rather than usual doses 9,19 .
Other studies inferred that there is alliances between elevation of leptin and origination and development of hypertension, coronary disease , and left ventricular hypertrophy as a result of leptin effects on increased levels of the inflammatory mediators of the blood vessel, excessive growth of vascular smooth muscle, and lipid peroxidation 20, 21.
Moreover, Candesartan can suppress the elevated level of leptin that enhanced by Angiotensin II 22 .Along with the above studies, the results of the present study ascertained that treatment with candesartan suppress the elevated blood level of leptin and lipid profile.
As predicted, the serum level of omentin-1 in this study ameliorated after three months treatment with candesartan in those corpulent patients.Various studies found that the blood level of omentin-1 was indirectly proportional to the blood level of leptin and corpulence and was directly proportional to the blood level of adiponectin. Likewise, the effect of omentin-1 and adiponectin have shared in modulating insulin sensitivity and protecting coronary arteries 23,24.
The blood level of chemerin in this study suppressed with candesartan treatment. This result is consistent with numerous studies that showed a positive effect between serum chemerin levels and corpulence risk factors like decrease in insulin sensitivity and metabolic syndrome 25,26 . Corpulence risk factors also associated with increased incidence of hypertension and blood vessels injuries. Therefore, the elevated level of chemerin is associated with hypertension as observed in this study and consistent with other studies 27,28.
Kaur et al. revealed that chemerin has specific receptor at the endothelial cells of blood vessels and the activation of this receptor was enhanced by the inflammatory cytokines such as tumor necrosis factor-alpha (TNF-a), interleukin-one (IL-1b), or interleukin –six 29 . Likewise, in this study, the decreased blood pressure with candesartan treatment may be associated with the lessening of blood vessels injuries and thereafter lowering chemein level.
Limitation of this study comprised the number of the enrolled patients, which was nearly small, and the follow-up period, which was approximately short. Therefore, advanced studies with larger sample size and longer follow- up period are suggested to confirm the effect of candesartan on adipocytokines of corpulence.
Conclusion
The treatment with candesartan cilexetil (16 mg daily) exhibited suppression of blood pressure (P value < 0.001) , serum levels of leptin (0.86 folded), chemerin (0.94 folded) and lipid profile (P value < 0.05). As well, amelioration of serum level of omentin-1 (1.06 folded) was achieved. Therefore, candesartan treatment may be more favorable for corpulent patients with hypertension.
Acknowledgements
The author would like to express her genuine thanks to the president of Al-Nahrain University/ Iraq, for the reinforcement to perform this research.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Han SH, Quon MJ, Koh KK. Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction.Curr Opin Lipidol. 2007;18 :58–65.
- Rada FH. Peroxisome proliferator- activated receptors family overview. Eur J Pharm Med Res. 2019;6(1) :167-170.
- Maury E, and Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010 ; 314(1) : 1-16.
- Rada FH. Effect of lipid fractions levels with cardiovascular disease. Asian J Pharm Clin Res. 2017;10 (3) :180-182.
- Dohi Y, Ohashi M, Sugiyama M, Takase H, Sato K, Ueda R. Candesartan reduces oxidative stress and inflammation in patients with essential hypertension. Hypertens Res. 2003; 26 :691–697.
- Rada FH. Oxidative stress and some inflammatory biomarkers in patients with coronary heart disease. Eur J Pharm Med Res. 2018;5(1):9 -12.
- Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004 ;30(1):13-19.
- Siegl PKS, Kivlighn SD, Broten TP.Pharmacology of angiotensin II receptor antagonists: Comparison with renin inhibitors and angiotensin converting enzyme inhibitors. Expert Opin Invest Drugs .1994; 3: 925-944.
- Burgess E, Muirhead N, Rene de Cotret P, Chiu A, Pichette V, Tobe S. Supramaximal dose of candesartan in proteinuric renal disease .J Am Soc Nephrol. 2009; 20(4) : 893–900.
- Belz GG, Breithaupt-Grogler K, Butzer R, Fuchs W, Hausdorf C, Mang C. The pharmacological potency of various AT-1 antagonists assessed by Schild regression technique in man. J Renin Angiotensin Aldosterone Syst (JRAAS). 2000; 1:336–341.
- Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depotspecific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006 ; 290 (6) : E1253–E1261.
- Kralisch S, Klein J, Bluher M, Paschke R, Stumvoll M, Fasshauer Therapeutic perspectives of adipocytokines. Expert Opin Pharmaco ther. 2005; 6: 863–872.
- Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, Toyoshima Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem. 2001;276 :23456 –23463.
- Komiya T, Tanigawa Y, Hirohashi Cloning of the novel gene intelectin, which is expressed in intestinal Paneth cells in mice. Biochem Biophys Res Commun. 1998;251:759 –762.
- Lee JK, Schnee J, Pang M, Wolfert M, Baum LG, Moremen KW, Pierce Human homologs of the Xenopus oocyte cortical granule lectin XL35. Glycobiology. 2001; 11: 65–73.
- Ernst MC, Haidl ID, Zuniga LA, Dranse HJ, Rourke JL, Zabel BA, et al. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 2012;153(2):672e82.
- Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, et al. Specific recruitment of antigenpresenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198 (7): 977–985.
- Du XY, Leung Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochim Biophys Sin (Shanghai). 2009;41:973e9.
- Kloner RA, Weinberger M, Pool JL, Chrysant S, Prasad R, et al. For the Comparison of Candesartan and Amlodipine for Safety, Tolerability and Efficacy (CASTLE) Study Investigators. Comparative effects of candesartan cilexetil and amlodipine in patients with mild systemic hypertension. Am J Cardiol. 2001;87: 727-731.
- Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation 2008;117:3238–3249.
- Correia ML, Haynes WG. Leptin, obesity and cardiovascular disease. Curr Opin Nephrol Hypertens. 2004; 13 : 215–223.
- Skurk T, van Harmelen V, Blum WF, Hauner Angiotensin II promotes leptin production in cultured human fat cells by an ERK1/2-dependent pathway. Obes Res. 2005;13 : 969–973.
- Tschritter O,Fritsche A,Thamer C, Haap M, Shirkavand F, Rahe S, et al .Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 2003; 52: 239 –243.
- De Souza Batista CM, Yang R, Lee MJ, Glynn NM. Omentin Plasma Levels and Gene Expression Are Decreased in Obesity. Diabetes 2007; 56:1655-1661.
- Hart R, Greaves DR. Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J Immunol. 2010;185: 3728e39.
- Okpechi IJ and Rayner Update on the role of candesartan in the optimal management of hypertension and cardiovascular risk reduction. Integr Blood Press Control 2010 ; 3: 45-55.
- Meric M, Soylu K, Avci B, Yuksel S, Gulel O, Yenercag M, Coksevim M, Uzun Evaluation of plasma chemerin levels in patients with non-dipper blood pressure patterns. Med Sci Monit. 2014; 20: 698–705.
- Bozaoglu K, Segal D, Shields K, Cummings N, Curran J,Comuzzie A, et al. Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab. 2009; 94(8) :3085e8.
- Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010 ; 391:1762e8.
Abbreviations
SBP: Systolic blood pressure, DBP: Diastolic blood pressure, S.TC: Serum Total cholesterol, S.HDL-C: Serum High density lipoprotein cholesterol, S.TG: Serum Triglyceride.









