Marselina Irasonia Tan* , Assifa Nur Hisana
, Assifa Nur Hisana , Ahmad Ridwan
, Ahmad Ridwan , Lulu L. Fitri
, Lulu L. Fitri , Putri Ayu Fajar
, Putri Ayu Fajar
School of Life Sciences and Technology, Institut Teknologi Bandung, Bandung, Indonesia
Corresponding Author E-mail : marsel@sith.itb.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1934
Abstract
Diabetes is the most prevalent chronic disease worldwide. In type 2 diabetes, target tissues decrease their sensitivity to insulin. Before the onset of type 2 diabetes, insulin resistance begins to grow, and beta cells produce more insulin for a longer time until beta cells become burned out and finally develop into type 2 diabetes. In prediabetes and diabetes patients, the retinol-binding protein (RBP4) concentration is high in the blood plasma. In this study, we sought to observe free RBP4 and exosomal RBP4 in the blood plasma as well as the urine of healthy, prediabetes, and diabetes patients. Three participants with type 2 diabetes mellitus, two participants with prediabetes, and five healthy participants were involved in this research. Blood and urine were collected from each participant, and exosomes from blood plasma and urine were isolated. RBP4 was analyzed using ELISA. The results showed that RBP4 was not only found as free RBP4 but also as exosomal RBP4 in the blood plasma as well as the urine. No previous research reported urinary exosomal RBP4. Interestingly, the concentration of exosomal RBP4 in the blood plasma of diabetes, prediabetes, and healthy participants was inversely proportional to the free RBP4 concentration in diabetes, prediabetes, and healthy participants. The exosomal RBP4 concentration of healthy participants was higher than that of prediabetes and diabetes participants. In conclusion, exosomal RBP4 can be found in urine and blood plasma. The concentration of exosomal RBP4 in diabetic participants is lower than prediabetes and healthy participants.
Keywords
Exosome; Prediabetes; Plasma; RBP4; Type 2 Diabetes; Urine
Download this article as:| Copy the following to cite this article: Tan M. I, Hisana A. N, Ridwan A, Fitri L. L, Fajar P. A. Diabetes and Retinol-Binding Protein (RBP4): The Study of Free and Exosomal RBP4 in Blood Plasma and Urine of Patients . Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Tan M. I, Hisana A. N, Ridwan A, Fitri L. L, Fajar P. A. Diabetes and Retinol-Binding Protein (RBP4): The Study of Free and Exosomal RBP4 in Blood Plasma and Urine of Patients . Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/3crB6n5 |
Introduction
Diabetes mellitus is one of the most frequently occurring chronic diseases in almost all countries and continues to increase in numbers, mainly due to changes in lifestyles in many countries recently [1]. There are two types of diabetes mellitus. In type-1 diabetes (T1D), patients suffer from absolute insulin deficiency because of the autoimmune destruction of pancreatic beta cells. In contrast, in type-2 diabetes (T2D), there is a heterogeneous metabolic disorder due to the decreased sensitivity of target tissues to insulin [2]. Prediabetes is a high-risk state of T2D. When insulin resistance worsens in prediabetes, the pancreatic beta cells will try to become more insulin-insensitive. This condition can occur for a long time without any symptoms until finally, the beta cells will be exhausted and will no longer be able to synthesize enough insulin; moreover, blood sugar will start to rise to above-average levels.
Prediabetic people have higher-than-normal blood sugar. When prediabetes is not treated early, prediabetes will develop to T2D. If there are many damaged beta cells, diabetes becomes an irreversible condition [3]. In order to detect either insulin resistance or beta-cell dysfunction, glycemic parameters (fasting and two-hour postprandial blood glucose and HbA1c) will be measured. However, glycemic parameters alone cannot show the pathophysiology of the disease.
In the last decade, it was found that RBP4 in blood plasma (Retinol Binding Protein 4) correlated with Diabetes Mellitus and has been described as a link between impaired glucose uptake in adipocytes and systemic insulin sensitivity. [4,5,6]. Plasma retinol-binding protein (also termed as RBP or RBP4) belongs to the lipocalin family of proteins, which transport vitamin A (all-trans-retinol) from the liver to the peripheral tissues. In the blood, RBP4 circulates as a complex with thyroxine-binding protein, the transthyretin (TTR) [7,8,9].
Yang et al. [4] reported that RBP4 was increased in insulin-resistant mice with adipose tissue-specific GLUT4 knockout and in humans with obesity and type 2 Diabetes Mellitus. In this GLUT4 null mice, RBP4 was high. Overexpression of RBP4 in normal mice causes insulin resistance and glucose intolerance. On the other hand, genetic deletion of RBP4 increased insulin sensitivity [10]. The authors also stated that RBP4 seems to decrease glucose uptake in the muscle tissue by inhibiting phosphorylation of the insulin receptor substrate-1 (IRS-1) and phosphoinositide-3-kinase (PI-3-kinase) activity, which are essential components of the insulin signaling pathway. While in the liver, RBP4 upregulated the expression of the gluconeogenic enzyme phosphoenol-pyruvate carboxykinase (PEPCK). This condition can subsequently increase hepatic glucose output.
Until now, it was known that RBP4s circulate in the blood as free RBP4s; however, there is no information about whether there is another way by which RBP4 is delivered to the target cells or non-target cells. Nowadays, it is known that exosomes serve as one of the essential intracellular communications. It can contain protein, micro RNA, and mRNA [11]. Circulating exosomes in diabetic blood might correlate with the regulation of lipid and glucose metabolism [12]. Therefore, in this research, we aim to examine free RBP4 and exosomal RBP4 in the blood plasma as well as in the urine of healthy participants, prediabetes patients, and diabetes patients.
Material and Methods
Participants of the Research
This research was approved by the ethics committee of Hasan Sadikin Hospital, Bandung, Indonesia, with ethics approval agreement no. 118/UN6.C1.3.2/KEPK/PN/2017.
The participants were recruited from the staffs of Institut Teknologi Bandung in Indonesia. This study only included adult participant (>30 years old) both men and women who are willing to participate through informed consent.
These participants are then categorized into three categories (normal, prediabetes, and diabetes) based on the level of fasting glucose, postprandial (2 hour) glucose, and HbA1C (glycosylated hemoglobin). The categorization based on these parameters are summarized in Table 1.
Table 1: Categories of participants in the study
| Category of participants | Fasting glucose (mg/dL) | Postprandial (2 hour) glucose (mg/dL) | HbA1C (percentage) | Number of participants (n) |
| Normal | <100 | <140 | <6% | 5 |
| Prediabetes | 100-126 | 140-199 | 6-6.4% | 2 |
| Diabetes | >126 | ≥200 | >6.4% | 3 |
Clinical Characteristics of Blood
Ten mL of venous blood was collected after a 14 hour overnight fast. Two hours postprandial, blood was collected again. Glycosylated hemoglobin, glucose, urea, creatinine, uric acid, and lipid profile were analyzed by Pramita clinical laboratory service (Bandung, Indonesia)
Exosome Isolation and ELISA of RBP4
For RBP4 quantification in blood plasma, another five mL of blood was collected. Two mL of it was used to isolate exosomes using Total Exosome Precipitation reagent kit for plasma (Invitrogen). The rest (3 mL) was allowed to clot to collect the blood plasma for quantification of free RBP4. Both exosomes and blood plasma were stored at -20oC for subsequent RBP4 quantification.
For RBP4 quantification in urine, exosomes were isolated from two mL of urine using Total Exosome Isolation kit for urine (Invitrogen). Free RBP4 is quantified directly using untreated urine samples.
Both in blood and urine analysis, exosomal and free RBP4 were quantified using RBP4 ELISA kit (Abcam). In this study, free RBP4 in blood plasma is only found in one participant in each diabetic (subject #1) and normal (subject #2) category.
Statistical Analysis
All statistical analysis in this study was done using GraphPad Prism 8.4.2. Comparison of free RBP4 in urine and blood samples of subject #1 and subject #2 utilized unpaired and paired T-test (*P < 0.05, **P<0.01). Meanwhile, comparison of exosomal RBP4 between normal, prediabetes, and diabetes categories employed two-tail ANOVA (P<0.05).
Results and Discussion
In this research, we found that in blood plasma and urine, there is not only free RBP4 but also exosomal RBP4 (Table 2). The concentration of free RBP4 in the blood plasma of diabetes participant is significantly higher than the healthy participant (Figure 1). This result is consistent with the previous researches, such as in Yang et al. and Cho et al. [5, 6]. Free RBP4 in blood plasma is synthesized and secreted from adipocyte and majorly from the liver [13]. This high concentration of RBP4 in the blood plasma of diabetic participants is probably correlated with its function for maintaining metabolic control. Ma et al. [14] stated that the important role of hepatic RBP4 is to maintain metabolic control.
Table 2: Free RBP4 and exosomal RBP4 concentration in plasma and urine of diabetes and normal participant (subject #1 and #2)
| Normal (Subject #2) | Diabetes (Subject #1) | |||
| Plasma | Urine | Plasma | Urine | |
| Free RBP4 (mg/mL) | 35.125 | 0.124 | 42.189 | 0.126 |
| Exosomal RBP4 (ng/mL) | 99.132 | 7.704 | 97.876 | 7.000 |
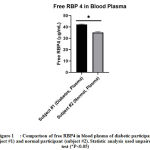 |
Figure 1: Comparison of free RBP4 in blood plasma of diabetic participant |
The concentration of free RBP4 in urine is much lower than the concentration of free RBP4 in blood plasma. However, the concentration of RBP4 in urine is nearly similar between diabetic and healthy participants (Figure 2). In contrast, Park et al. [15] showed that free urinary RBP4 from prediabetic and diabetic patients is higher than the healthy persons. Park et al. [15] also showed that urinary RBP4 concentrations were correlated with several cardiometabolic parameters, such as insulin resistance, inflammation, and arterial stiffness. The inconsistency in the results between ours and Park et al. [15] might be due to the very small number of samples in this study; therefore, further research should be performed.
 |
Figure 2: Comparison of free RBP4 in plasma and urine of diabetes and healthy participants. |
Table 2 showed exosomal RBP4 in blood plasma and urine. The presence of RBP4 in exosome from blood plasma has been listed in ExoCarta [16, 17, 18]. However, there was no previous information that RBP4 can also be found in the urinary exosome. The concentration of exosomal RBP4 in urine is lower than that of blood plasma. Exosomal RBP4 will probably support free RBP4 by transporting functional RBP4 to other cells that do not express RBP4 and helping vitamin A uptake by the cells. This phenomenon was identified by Street et al. [19], who found that exosomes transported functional aquaporin to cells that did not express the protein, thus causing a significant increase in water flow.
Exosomal RBP4 in the blood plasma of health and diabetic participants might be produced by adipocytes. Deng et al. [20] and Guay and Regazzi [21] showed that in the mice model for obesity and diabetes (ob/ob mice), exosomes containing RBP4 were secreted by adipocytes and could activate the TLR4/NFκB pathway and stimulate macrophages (M1) to secrete of IL-6 and TNFα, which are important for inflammation. In this research, it is also found that exosomal RBP4 is produced in healthy participants. In this research, exosomal RBP4 in diabetes plasma blood is probably correlated with the stimulation of the M1 macrophage to secrete IL-6 and TNFα. This condition could reduce the insulin sensitivity of the liver and muscles. In this research, we also found exosomal RBP4 in urine; however, we still do not know the function of exosomal RBP4 in urine.
The concentration of exosomal RBP4 in healthy, prediabetes, and diabetes participants is shown in Table 3. In this study, only exosomal RBP4 in blood plasma has been observed. Interestingly, we observed an opposing trend in which exosomal blood RBP4 in healthy participants is lower compared to those in prediabetes as well as diabetes patient (Figure 3), while free RBP4 in the blood of healthy participant is significantly higher compared to diabetes participant (Figure 1). It is still unclear why the concentration of exosomal blood RBP4 is higher in healthy participants. The low concentration of exosomal RBP4 in prediabetes and diabetes patients might be associated with a high concentration of free RBP4 in blood plasma. Xiao et al. [22] stated that exosomes in T2D can participate in regulating various physiological and pathological processes in vivo by being transported between cells.
Table 3: Clinical characteristics of participants
| Healthy (n=5) | Prediabetes (n=2) | Diabetes (n=3) | |
| Age | 46±15.7 | 55.5±2.12 | 57±10.15 |
| Gender M/F | 5/0 | 1/1 | 3/0 |
| Urea (mg/dL) | 29.28±10.62 | 18.6±3.68 | 24.33±6.03 |
| Creatinine (mg/dL) | 1.02±0.22 | 0.8±0 | 0.93±0.11 |
| Uric acid (mg/dL) | 6.78±0.96 | 6.55±0.64 | 4.2±0.89 |
| Lipid profile: | |||
| · Cholesterol (mg/dL) | 190.2±44.60 | 192±16.97 | 183±39.28 |
| · Triglyceride (mg/dL) | 125.4±72.73 | 120±11.31 | 116.33±36.20 |
| · HDL cholesterol (mg/dL) | 41.6±7.70 | 43.5±6.36 | 46.67±9.02 |
| · LDL cholesterol (mg/dL) | 123.6±37.65 | 125±25.46 | 113±39.95 |
| Glucose fasting (mg/dL) | 89.6±15.95 | 105.5±4.95 | 160±42.79 |
| Glucose 2hours (mg/dL) | 125.8±29.48 | 186±12.73 | 328.33±117.49 |
| HbA1C (%) | 5.42±0.29 | 6.2±0 | 8.17±1.69 |
| Exosomal RBP4 in blood plasma (ng/mL) | 279.2±114.74 | 226.5±53.03 | 199.67±104.61 |
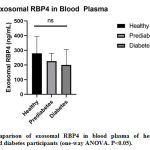 |
Figure 3: Comparison of exosomal RBP4 in blood plasma of healthy (normal) |
Ying et al. [23] stated that adipocytes from healthy animals produce exosomes containing certain miRNAs that regulate the sensitivity of the liver and muscles to insulin. However, in obese mice, exosomes containing miRNA 155 from adipocytes decrease the sensitivity of muscles and the liver to insulin [23]. Moreover, Lippai and Szabo [24] and Mahesh and Biswas [25] showed that miRNA 155 is the major inflammatory regulator. As we know, T2D is accompanied by chronic low-grade inflammation in several tissues, such as adipose tissue, liver tissue, and muscle tissue [26, 27]. Therefore, together with RBP4 in exosome of prediabetes and diabetes, probably miRNA 155 in exosome supports inflammation in peripheral organs. Further observation to investigate the mechanism underlying exosomal and free RBP4 is required.
Conclusion
This study attempted to examine the level of free and exosomal RBP4 in the blood plasma and urine of prediabetic, diabetic, and healthy participants. We found that RBP4 is secreted as free RBP4 and exosomal RBP4 in blood plasma as well as in urine of healthy and diabetes patients. We also found that the concentration of exosomal RBP4 in healthy participants is higher than it is in prediabetic and diabetic patients, although not significant. Interestingly, we observed an inverse trend in the level of free and exosomal RBP4 in the healthy and diabetes patient. However, the reason behind this finding is still unclear. Further observation might have to be done in order to elucidate the mechanism causing this result.
Conflict of Interest
The authors declare no conflict of interest with other parties
Funding Source
This research is funded through P3MI Institut Teknologi Bandung No. 1111-I1.C02.2-KU-2020 grant year 2017
Acknowledgment
Thanks to Institut Teknologi Bandung who has funded this research through P3MI funding project 2017
References
- Shaw J.E., Sicree R.A., and Zimmet P.Z. Diabetes Atlas. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract., 2010; 87: 4-14.
- Pullakhandam, Palika R., Ghosh S., and Reddy G.B. Contrasting Effects of Type 2 and Type 1 Diabetes on Plasma RBP4 Levels: The Significance of Transthyretin. IUBMB Life, 2012; 64(12): 975-982.
- DiaTribe Foundation. Type 2 diabetes. Available online: https://diatribe.org/type-2-diabetes, (accessed on 23 March 2018).
- Luft V.C., Pereira M., Pankow J.S., Ballantyne C., Couper D., Heiss G., Duncan B. B., and ARIC Investigators. Retinol binding protein 4 and incident diabetes–the Atherosclerosis Risk in Communities Study (ARIC Study). Brazilian journal of epidemiology, 2013; 16(2): 388–397.
- Yang Q., Graham T.E., Mody N., Preitner F., Peroni O.D., Zabolotny J.M., Kotani K., Quadro L., Kahn B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature, 2005; 436: 356–362.
- Cho Y.M., Youn B.S., Lee H., Lee N., Min S.S., Kwak S.H., and Park K.S. Plasma Retinol-Binding Protein-4 Concentrations Are Elevated in Human Subjects With Impaired Glucose Tolerance and Type 2 Diabetes. Diabetes Care; 2006: 29: 2457–2461.
- Raghu P. and Sivakumar, B. Interactions amongst plasma retinol-binding protein, transthyretin and their ligands: implications in vitamin A homeostasis and transthyretin amyloidosis. Biophys. Acta, 2004; 1703: 1–9.
- D’Ambrosio D. N., Clugston R. D., and Blaner W. S. Vitamin A metabolism: an update. Nutrients, 2011; 3: 63–103.
- Pullakhandam R., Palika R., Ghosh S., and Reddy G. Contrasting effects of type 2 and type 1 diabetes on plasma RBP4 levels: the significance of transthyretin. IUBMB Life, 2012; 64(12): 975-982.
- Yan, Chang X., Xia M., Bian H., Zhang L., Lin H., Chen G., Zeng M. and Gao X. Serum retinol binding protein 4 is negatively related to beta cell function in Chinese women with non-alcoholic fatty liver disease: a cross-sectional study. Lipids Health Dis., 2013; 12: 157.
- Corrado C., Raimondo S., Chiesi A., Ciccia F., De Leo G. and Alessandro R. Exosomes as Intercellular Signaling Organelles Involved in Health and Disease: Basic Science and Clinical Applications. Int. J. Mol. Sci., 2013; 14: 5338-5366.
- Mueller Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab. Syndr. Obes., 2012; 5: 247–282.
- Noy N., Li L., Abola,M.V., and Berger, N.A. Is retinol binding protein 4 a link between adiposity and cancer?. Hormone molecular biology and clinical investigation, 2015; 23(2), 39–46.
- Ma, Zhou Z., Chen Y., Wu Y and Liu Y. RBP4 functions as a hepatokine in the regulation of glucose metabolism by the circadian clock in mice. Diabetologia, 2016; 59: 354–362.
- Park S.E., Lee N.S., Park J.W., Rhee E.J., Lee W.Y., Oh K.W., Park S.W., Park C.Y. and Youn B.S. Association of urinary RBP4 with insulin resistance, inflammation, and microalbuminuria. Eur. J. Endocrinol., 2014; 171: 443–449.
- Keerthikumar S., Chisanga D., Ariyaratne D., Al Saffar H., Anand S., Zhao K., Samuel M., Pathan M., Jois M., Chilamkurti N., Gangoda L. and Mathivanan S. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol., 2016; 428(4): 688-692.
- Mathivanan S. Fahner C.J., Reid G.E., and Simpson R.J. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res, 2012; 40: D1241–D1244.
- Mathivanan S. and Simpson R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics, 2009; 9(21): 4997-5000.
- Street J., Birkhoff W., Menzies R., Webb D., Bailey M., and Dear J. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. Physiol., 2011; 589: 6119–6127
- DengB., Poliakov A., Hardy R.W., Clements R., Liu C., Liu Y., Wang J., Xiang X., Zhang S., Zhuang X, Shah S.V., Sun D., Michalek S., Grizzle W.E., Garvey T., Mobley J. and Zhang H.G. Adipose Tissue Exosome-Like Vesicles Mediate Activation of Macrophage-Induced Insulin Resistance. Diabetes, 2009; 58(11): 2498-2505.
- Guay and Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab., 2017; 19(Suppl. 1): 137–146.
- Xiao Y., Zheng L., Zou X., Wang J., Zhong and Zhong T. Extracellular vesicles in type 2 diabetes mellitus: key roles in pathogenesis, complications, and therapy. J. Extracell. Vesicles, 2019; 8: 1625677.
- Ying, Riopel M., Andyopadhyay G., Dong Y., Birmingham A., Seo J.B., Ofrecio J.M., Wollam J., Hernandez-Carretero A., Fu W., Li P., and Olefsky J.M. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell, 2017; 171: 372–384.
- Lippai and Szabo G. Prevention of Alcohol-Induced Inflammation of Murine Small Intestine by MicroRNA-155 Deficiency. In Insights to Neuroimmune Biology (Second Edition). Elsevier, 2016: 243-256.
- Mahesh G. and Biswas R. MicroRNA-155: A Master Regulator of Inflammation. J Interferon Cytokine Res., 2019; 39(6): 321-330, 2019
- Burhans M.S., Hagman D.K., Kuzma J.N., Schmidt K.A., and Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Comprehensive Physiology, 2018; 9(1)” 1–58.
- Zhong, Gong Q. and Mima A. Inflammatory Regulation in Diabetes and Metabolic Dysfunction. J. Diabetes Res., 2017; 2017: 5165268.







