Nagham Mahmood Aljamali1* and Imad Kareem Alwan Alsabri2
and Imad Kareem Alwan Alsabri2
1Synthetic Chemistry Field, Department of Chemistry, Iraq.
2Master in Oncology, College of Medicine, Kufa University, Iraq.
Corresponding Author E-mail : dr.nagham_mj@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1925
Abstract
Cancer tumors cause not few deaths among the total deaths in the world, despite the great progress in the treatment of cancer, so it has become necessary to understand the molecular explanations that contribute to the development and progress of cancer and to search for more effective and less toxic treatments to treat this disease.The work included the innovation and development of the drug trimethoprim by linking it with the innovative compound sulfazane and creation of several derivatives of sulfazane –trimethoprim for the first time then studying the behavior and impact of innovative compounds on vital activities as anti-cancer tumors by following an innovative procedures of preparation for sulfazane compounds ,then linking them with several chemical reactions with the drug trimethoprim to develop its effectiveness and efficiency. Several spectral techniques were used to diagnose and demonstrate the formation of innovative derivatives (sulfazane- trimethoprem) thereof (FT.IR - Spectra ,H.NMR – Spectra , HMBC- Spectrum ,Mass Spectra), chemical properties, Flowing through TLC ,in addition to laboratory study of cancerous tumors. The results indicated to formation of these drug derivatives by appearance of new bands and disappearance of bands in starting compounds., besides to conclusions from our paper that gave good data for inhibition efficiency for these drug derivatives against cancer cells.
Keywords
Azo; Coupling; Cancer; Innovation; Invented Compounds; New Reaction of Diazonium; Sulfide-Azo; Sulfazane; Tumor; Toxicity; Trimethoprim; (-S-N=N-)
Download this article as:| Copy the following to cite this article: Aljamali N. M, Alsabri I. K. A. Development of Trimethoprim Drug and Innovation of Sulfazane-Trimethoprim Derivatives as Anticancer Agents. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Aljamali N. M, Alsabri I. K. A. Development of Trimethoprim Drug and Innovation of Sulfazane-Trimethoprim Derivatives as Anticancer Agents. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/2UJpCEB |
Introduction
The newly innovative sulfazane compounds that were invented and prepared for the first time by the researcher (Dr. Nagham Aljamali in year 2019 in the first research work(1)) The researcher laid the first foundations for the innovative sulfazane compounds in terms of (methods of preparation , their properties , chemical and physical characteristics, their colors, stability, some of their vital applications and behavior towards some types of bacteria and fungi).(1,2) Now in this research work continued to prepare other drug derivatives by linking sulfazane to the drug trimethoprim via (-S-N=N-) sulfazane group to study its effect on cancerous tumors.Trimethoprim contains the active ingredient trimethoprim, an antibiotic used to treat infections with bacteria.Bacterial cells in order to grow and multiply need a genetic material (DNA) to produce DNA and need folic acid (folate).(3-10) Methoprim works by preventing bacteria from producing folic acid and without it bacteria cannot produce DNA, and thus become unable to reproduce(11-22) and increase numbers, so methoprim stops the spread of infection, and kills the remaining bacteria by the immune system.(23-34)
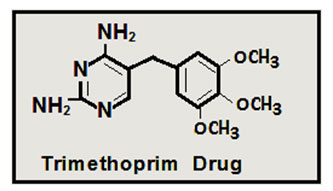
Trimethoprim (C14H18N4O3) : 5-(3,4,5-Trimethoxybenzyl)pyrimidine-2,4-diamine., its names (Proloprim, Monotrim, Triprim, others) ,M.Wt : 290.32 g/mol .,It is used to treat bacterial infections in the urinary tract and also to prevent frequent bacterial infections in the urinary tract,(35-43) as well as to treat bacterial infections in the lungs and bronchi (respiratory system), such as acute bronchitis, chronic bronchitis or pneumonia.(44-56) The cancer is a type of non-infectious disease that is characterized by uncontrolled growth of cells, there are more than (100) different types of cancer, which were classified according to the type of cell that was affected at the beginning, and cancer causes a death rate of more than 20% of the total deaths in the world on according to the World Health Organization, where lung cancer is the most prevalent,(57-59) followed by breast, colon and prostate cancer, despite significant progress in cancer treatment, the death rate has not decreased, so it has become necessary to understand the molecular explanations that contribute to the development and progress of cancer and search for more treatments. Efficacy and least toxic for a processor with this disease,(60-63) there are many methods for treating this disease, including tumor lift, multiple chemical treatments, and radiological treatments
Experimental Part
All chemicals compounds supplied from Sigma –chemicals company and fluka –chemicals company.
An innovative method(1, 2) was used to develop the trimethoprim drug by linking it to an innovative sulfazane compounds through several steps, chemical reactions and reaction conditions that differ for each of the innovative derivatives prepared in this research. The chemical composition of the innovative derivatives prepared in this research was proven by several investigation techniques (FT-IR spectra (FT-IR 8300 Shimadzu) with the range (400-4000)cm-1 using discs of KBr., 1H.NMR–Spectra in solvent (d-DMSO) Fourier transformation broker spectrometer ,operating at (400MHz)., HMBC- Spectrum ,Mass spectra for some of them) in Kashan university, and the preparation was followed by (TLC), then a laboratory study of cancer cells line to know the effectiveness and efficiency of the innovative compounds.
Paths of Synthesis(1, 2)
Innovation and Creation of Sulfazane- Trimethoprim {1}
P-thiol aniline (0.01 mole) with (0.01 mole) ammonium thiocyanate in bromine with glacial acetic acid ,after rotation for (2 hrs) at (10 C°) via three reactions ,then precipitation filtered, washed, dried, purification by recrystallization, then (0.01 mole) from precipitation dissolved in basic alcoholic solution, and added to acidic diazo- trimethoprim salt in three steps according to invented procedure in papers(1, 2) ,after (3 days), filtered ,washed ,dried ,recrystallized to yield sulfazane- trimethoprim {1}.
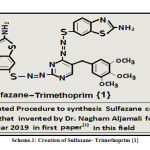 |
Scheme 1: Creation of Sulfazane- Trimethoprim {1} |
Innovation and Creation of Sulfazane- Trimethoprim {2}
Cystein(0.01 mole) in basic alcoholic solution by two steps, then added to acidic diazo- trimethoprim salt in three steps according to invented procedure in papers(1, 2) ,after (3 days), filtered ,washed ,dried ,recrystallized to yield sulfazane- trimethoprim {2}.
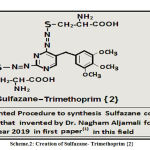 |
Scheme 2: Creation of Sulfazane- Trimethoprim {2} |
Innovation and Creation of Sulfazane- Trimethoprim {3}
Thiol benzoic acid (0.01 mole) in basic alcoholic solution by two steps, then added to acidic diazo- trimethoprim salt in three steps according to invented procedure in papers(1, 2) , after (3 days), filtered ,washed ,dried ,recrystallized to yield sulfazane- trimethoprim {3}.
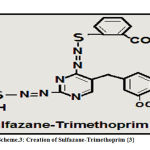 |
Scheme 3: Creation of Sulfazane-Trimethoprim {3} |
Innovation and Creation of Sulfazane- Trimethoprim {4}
2-Thiol imidazole in previously steps was prepared ,then (0.01 mole) dissolved in basic alcoholic solution by two steps, then added to acidic diazo-trimethoprim salt in three steps according to invented procedure in papers(1, 2) , after (3 days), filtered ,washed ,dried ,recrystallized to yield sulfazane- trimethoprim {4}.
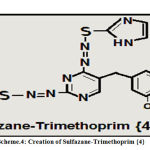 |
Scheme 4: Creation of Sulfazane-Trimethoprim {4} |
Results
Our research described invented procedure(1, 2) via original process to creation and preparation of Sulfazane compounds linked with trimethoprim drug via designation of reaction conditions, type of used catalyst, mechanism of this reaction, we called it (Name–Sulfazane) as a first and Original preparation to this novel type of trimethoprim- derivatives., then all these drug derivatives investigated via numerous spectral techniques and other chemical with physical studies :
Spectral Evidences
FT.IR- Spectra
The infrared spectrum is the first technique that has demonstrated, with certain evidence, the creation of innovative, advanced derivatives in this study through the emergence of effective and functional grouping frequencies in derivatives indicating the reason for the accuracy of their preparation and the correctness of the innovative procedure to prepare them :
Sulfazane- Trimethoprim {1}
Bands at (-OCH3) methoxy group: 1183 ,(-N=N-S-) Azo-Sulfide: (1398, 1489, 1500) , (S-CH-) Sulfide : 1226 ,(C-S) endocyle of benzothiazole : 756 ,(C=N) endocycle of pyrimidine: 1652 ,(-NH2) amine group: (3358 ,3409).
Sulfazane- Trimethoprim {2}
Bands at (-OCH3) methoxy group: 1118 ,(-N=N-S-) Azo-Sulfide: (1363, 1454, 1496) , (S-CH2-) Sulfide : 1224 , (C=N) endocycle of pyrimidine: 1664 ,(-NH2) amine group: (3314 ,3379), (CO-O-)carbonyl of carboxyl : 1729 .
Sulfazane- Trimethoprim {3}
Bands at (-OCH3) methoxy group: 1134 ,(-N=N-S-) Azo-Sulfide: (1377, 1462, 1499) , (S-CH-) Sulfide : 1231 , (C=N) endocycle of pyrimidine: 1651 ,(CO-O-)carbonyl of carboxyl : 1721 .
Sulfazane- Trimethoprim {4}
Bands at (-OCH3) methoxy group: 1130 ,(-N=N-S-) Azo-Sulfide: (1382, 1460, 1502) , , (C=N) endocycle of pyrimidine: 1657 ,(NH) in imidazole : 3210 ,(C=N) endocycle of imidazole: 1632. Other active groups are shown in some selected spectra (1 , 2) .
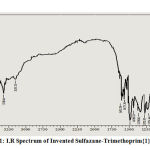 |
Figure 1: I.R Spectrum of Invented Sulfazane-Trimethoprim{1} |
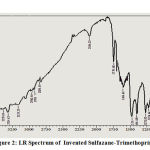 |
Figure 2: I.R Spectrum of Invented Sulfazane-Trimethoprim {2} |
1H.NMR- Spectra
The nuclear resonance spectrum is the second technique that has demonstrated, with certain evidence, the creation of innovative, advanced derivatives in this study through the emergence of effective and functional grouping frequencies in derivatives indicating the reason for the accuracy of their preparation and the correctness of the innovative procedure to prepare them by disappearance of amine signal of (-NH2) as a result of formation of (sulfazane)- group(1, 2) in derivatives ,all spectra showed signals at (2. 50) for solvent (d-DMSO) ,besides to other signals like:
Sulfazane- Trimethoprem{1}
It gave signals at (5. 40) due to proton of amine group (NH2) .,(6. 78–7. 74) to protons of aromatic ring ,(2. 98) for (O-CH3) protons of methoxy groups.
Sulfazane- Trimethoprem{2}
It gave signals at (5. 11) due to proton of amine group (NH2) .,(6. 36–7. 42) to protons of aromatic ring ,(2. 94) for (O-CH3) protons of methoxy groups ,(COOH) proton of carboxyl group : (13. 26) , (S-CH2-CH-N-): (3. 56 , 3. 76 ).
Sulfazane- Trimethoprem{3}
It gave signals at (6. 77–7. 57) to protons of aromatic ring ,(2. 86) for (O-CH3) protons of methoxy groups ,(COOH) proton of carboxyl group : (13. 08) .
Sulfazane- Trimethoprem{4}
It gave signals at (6. 69–7. 52) to protons of aromatic ring ,(2. 92) for (O-CH3) protons of methoxy groups ,(NH) proton of amine in imidazole ring: (8. 14) . Other protons of functional groups appeared in some spectra (3 , 4) .
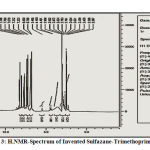 |
Figure 3: H.NMR-Spectrum of Invented Sulfazane-Trimethoprim {1} |
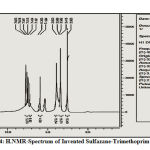 |
Figure 4: H.NMR-Spectrum of Invented Sulfazane-Trimethoprim { 2} |
HMBC- Spectrum
The HMBC- spectrum is the third technique that has demonstrated, with certain evidence, the creation of innovative, advanced derivatives in this study through the emergence of effective and functional grouping frequencies in derivatives indicating the reason for the accuracy of their preparation and the correctness of the innovative procedure to prepare them via appearance of functional groups for invented derivative, Figures (5).
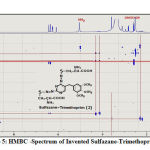 |
Figure 5: HMBC -Spectrum of Invented Sulfazane-Trimethoprim { 2} |
Mass Spectra of Sulfazane –Trimethoprim Derivatives
The mass spectrum is the fourth technique that has demonstrated, with certain evidence, the creation of innovative, advanced derivatives in this study through the emergence of effective and functional grouping frequencies in derivatives indicating the reason for the accuracy of their preparation and the correctness of the innovative procedure to prepare them via appearance of fragments of some of invented derivatives, Figures (6, 7):
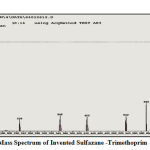 |
Figure 6: Mass Spectrum of Invented Sulfazane -Trimethoprim {1} |
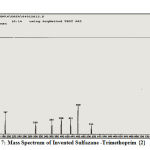 |
Figure 7: Mass Spectrum of Invented Sulfazane -Trimethoprim {2} |
All chemical with physical properties and information about TLC for invented derivatives in Table(1)
Table 1: All chemical with physical properties and information of TLC
| Invented Derivatives | Product % | Color | M .P
(C °) |
Rf | Solvents (TLC) |
| Sulfazane-Trimethoprim {1} | 74 | Deep Yellow | 202 | 0.68 | Ethanol : Benzene |
| Sulfazane-Trimethoprim {2} | 80 | Yellowish Orange | 168 | 0.60 | Ethanol : Benzene |
| Sulfazane-Trimethoprim {3} | 78 | Yellowish Orange | 182 | 0.62 | Ethanol : Benzene |
| Sulfazane-Trimethoprim {4} | 82 | Pale Orange | 194 | 0.70 | Ethanol : Benzene |
Discussion Innovative Derivatives Test Against Breast CancerInitialization of Cancer Cell Line(13)
Line processing and implantation of breast cancer cells and live cell line were carried out at Biotechnology Center – the Nahrain (MCF-7 cell line) and (WRL cell line grew in 95% of RPMI–1640) supplemented with (10% FBS), cell suspension and incubation(13, 53) at (37 °C) in incubator {(CO2) % 5}. The suspended cells were centrifuged at (250 g) for (10 minutes) and the supernatant was removed, the cells were re-suspended in a freezing medium, then placed at (-70 °C) in beaker for (1-3) days, the beaker was transferred from the standard freezer boxes to the liquid (N2) container.
Processing Method
MTT was used to determine cell viability by chromatic examination(64-70) of two (MCF-7 and WRL cell lines):
Cell suspension (100 µL) was added to the wells of a small flat plate bottom.
The solution was prepared by dissolving the crystals of 5 mg MTT in 1 ml of PBS solution (phosphate buffer solution).
The concentrations of each innovative derivative of the prepared derivatives were used in this research (500, 250, 125, 62.5 , 31.5 , 15.6 (µg/ml of methanol, which were added to each well (three replicates per concentration).
A 10 ml MTT solution was added to each well of a plate containing 96 wells and then incubated for 4 hours with a test sample at 37 °C (the solution became yellow).
DMSO was added (200 µL (to each hole and stirred for 5 minutes (to become a purple DMSO solution).
After the complete dissolution of the dye, the absorption of the colored solution from the living cells was read at (575 nm) using the ELISA reader.7- The mean absorption was calculated for each group of iterations and the validity ratio of the cells exposed to different treatments was obtained as follows(13):
Cell Vitality% = [(Absorption from the treated sample /Absorption from the untreated sample) X 100
Table 2: Mean Percentage (%) for each cell line (Respond to Treatment) for Derivative{1}
| Sulfazane- Trimethoprim{1} | IC50 (μg/ml) : ( 190. 1654 ) | ||
| Mean Percentage (%) for each cell line (Respond to treatment ) | |||
| Conc (μg/ml) | Killing and inhibition of Carcinoma Cells % | Toxic Effect on Normal Cell Line | |
| 500 | 66 % | 30 % | |
| 250 | 58 % | 25 % | |
| 125 | 50 % | 25 % | |
| 62.5 | 46% | 20 % | |
| 31.5 | 46 % | 20 % | |
| 15.6 | 44 % | 20 % | |
Table 3: Mean Percentage (%) for each cell line (Respond to Treatment) for Derivative{2}
| Sulfazane- Trimethoprim{2} | IC50 (μg/ml) : (228. 1930) | ||
| Mean Percentage (%) for each cell line (Respond to treatment ) | |||
| Conc (μg/ml) | Killing and inhibition of Carcinoma Cells % | Toxic Effect on Normal Cell Line | |
| 500 | 56 % | 20 % | |
| 250 | 52 % | 14 % | |
| 125 | 46 % | 14 % | |
| 62.5 | 40 % | 14 % | |
| 31.5 | 32 % | 10 % | |
| 15.6 | 30 % | 10 % | |
Table 4: Mean Percentage (%) for each cell line (Respond to Treatment) for Derivative{3}
| Sulfazane- Trimethoprim{3} | IC50 (μg/ml) : (258. 5202 ) | ||
| Mean Percentage (%) for each cell line (Respond to treatment ) | |||
| Conc (μg/ml) | Killing and inhibition of Carcinoma Cells % | Toxic Effect on Normal Cell Line | |
| 500 | 50 % | 12 % | |
| 250 | 38 % | 10 % | |
| 125 | 36 % | 10 % | |
| 62.5 | 34 % | 8 % | |
| 31.5 | 30 % | 8 % | |
| 15.6 | 30 % | 4 % | |
Table 5: Mean Percentage (%) for each cell line (Respond to Treatment) for Derivative{4}
| Sulfazane- Trimethoprim{4} | IC50 (μg/ml) : (210. 3871 ) | ||
| Mean Percentage (%) for each cell line (Respond to treatment ) | |||
| Conc (μg/ml) | Killing and inhibition of Carcinoma Cells % | Toxic Effect on Normal Cell Line | |
| 500 | 60 % | 22 % | |
| 250 | 50 % | 18 % | |
| 125 | 44 % | 18 % | |
| 62.5 | 40 % | 16 % | |
| 31.5 | 30 % | 16 % | |
| 15.6 | 30 % | 16 % | |
Conclusions
The results indicated to formation of these drug derivatives by appearance of new bands and disappearance of bands in starting compounds., besides to conclusions from our paper that gave good data for inhibition efficiency for these drug derivatives against cancer cells. Our results appeared good inhibition for carcinoma cells line for all invented derivatives , and gave high response for derivatives {1 and 4} more than other invented derivatives ,(it was = 66 % response percentage of derivative {1} for inhibition and killing of cancer cells due to sulfazane(1, 2) group(-S-N=N- Drug ) that linked with drug which gave it more response against cancer cells also due to thiazole core in this derivative{1}.
Acknowledgments
We would like to express our heartfelt thanks to Biotechnology Center-the Nahrain for providing assistance samples of cells and bioinformatics analysis.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Source
None
References
- Afaq Jaber Kadhium, Ahmed Adnan Abdul Hussein, Nagham Mahmood Aljamali, Afaq J K, Ahmed A A H, Invention of Imidazole & Thiazole-Sulfazane Ligands(Synthesis, Spectral Investigation, Microbial Behavior) for the First Time.,International Journal of Pharmaceutical Research ,2020 ,Vol 12 ,Issue 2, 989-998 .,org/10.31838/ijpr/2020.12.02.0151 .
- Jelal H M, Alia Jawd Kadhim, Nagham M A, Nagham Mahmood Aljamali.,2020.,Thiophene-Cyclic and Sulfazane Derivatives (Preparation, Spectral Analysis, The Behavior in Organic Solvents ,Microbial Testing).,Indian Journal of Forensic Medicine & Toxicology, 14, 2, 2020.
- Ch, J. (1992). Advanced Organic Chemistry (5th ed.). New York: J. Wiley and Sons. ISBN978-0-471-60180-7.
- Klaus Hunger, Peter Mischke, Wolfgang Rieper, Roderich Raue, Klaus Kunde, Aloys Engel: “Azo Dyes” in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim.doi:1002/143560 07.a03_245.
- Ohme, R.; Preuschhof, H.; Heyne, H.-U. (1988). “Azoethane”. Organic Syntheses.; Collective Volume, 6, p. 78
- Jean-Pierre Schirmann, Paul Bourdauducq: “Hydrazine” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:1002/14356007.a13_177.
- Alaa J K, Jalal H M, Nagham M A, Alaa Jawad Kadhim, Jalal Hasan Mohammed, Nagham Mahmood Aljamali ., Thiazole Amide Derivatives (Synthesis, Spectral Investigation, Chemical Properties, Antifungal Assay)., NeuroQuantology , 2020, 18, 1 ,Page 16-25 ., doi: 10.14704/nq.2020.18.1.NQ20102
- Hagen T J. (2007). “Prilezhaev reaction”. In Li, Jie Jack; Corey, E. J.(eds.). Name Reactions of Functional Group Transformations. John Wiley & Sons. pp. 274–281. ISBN9780470176504.
- Anslyn, Eric V.; Dougherty, Dennis A.(2006). ” Miscellaneous Experiments for Studying Mechanism” . Modern Physical Organic Chemistry. University Science Books. pp. 471–482. ISBN 9781891389313.
- Mansuy, D., Valadon, P., Erdelmeier, I., López García, P., Amar, C., Girault, J. P., and Dansette, P. M. (1991). “Thiophene S-oxides as new reactive metabolites: Formation by cytochrome-P450 dependent oxidation and reaction with nucleophiles”. J. Am. Chem. Soc. 113(20): 7825–7826. doi:1021/ja0 0020a089.
- Rademacher P. M., Woods C. M., Huang Q., Szklarz G. D., Nelson S. D.; Woods; Huang; Szklarz; Nelson (2012). “Differential Oxidation of Two Thiophene-Containing Regioisomers to Reactive Metabolites by Cytochrome P450 2C9”. Chem. Res. Toxicol. 25(4): 895–903. doi: 1021/tx200519d. PMC3339269. PMID 22329513.
- Mansuy D., Dansette P. M.; Dansette (2011). “Sulfenic acids as reactive intermediates in xenobiotic metabolism”. Archives of Biochemistry and Biophysics. 507(1): 174–185. doi :1016/j.abb.2010.09.015. PMID 20869346.
- Aseel Mahmood Jawad, Nagham Mahmood Aljamali, Aseel M J .,”Innovation, Preparation of Cephalexin Drug Derivatives and Studying of (Toxicity & Resistance of Infection)”., International Journal of Psychosocial Rehabilitation, Vol. 24, Issue 04, 2020 , 3754-3767 .
- Dansette, PM, Rosi, J, Debernardi, J, Bertho G, Mansuy D; Rosi; Debernardi; Bertho; Mansuy (2012). “Metabolic Activation of Prasugrel: Nature of the Two Competitive Pathways Resulting in the Opening of Its Thiophene Ring”. Res. Toxicol.25 (5): 1058–1065. doi:10.1021 /tx3000279.
- Henry Y. Lew and C. R. Noller (1963). “2-Iodolthiophene”. Organic Syntheses.; Collective Volume, 4, p. 545
- S. Emerson and T. M. Patrick, Jr. (1963). “2-Vinylthiophene”. Organic Syntheses .;Collective Volume, 4, p. 980
- B. Wiberg and H. F. McShane (1955). “2-Chloromethylthiophene”. Organic Syntheses .; Collective Volume, 3, p. 1
- Roncali (1992). “Conjugated poly(thiophenes): synthesis, functionalization, and applications” . Chem. Rev.92 (4): 711–738. doi:10.1021/cr00012a009.
- Rauchfuss, T. B., “The Coordination Chemistry of Thiophenes”, Progress in Inorganic Chemistry 1991, volume 39, pp. 259-311. ISBN978-0-471-54489-0
- Jones and I. M. Moodie (1988). “2-Thiophenethiol”. Organic Syntheses.; Collective Volume, 6, p. 979.
- Nagham Mahmood Aljamali., Synthesis of Antifungal Chemical Compounds from Fluconazole with (Pharma-Chemical) Studying, Research journal of Pharmaceutical, biological and chemical sciences, 2017, 8 (3), 564 -573.
- Lipshutz, Bruce H.; Moretti, Robert; Crow, Robert (1990). “Mixed Higher-order Cyanocuprate-induced Epoxide Openings: 1-Benzyloxy-4-penten-2-ol”. Org. Synth. 69: 80. doi:15227/orgsyn.069.0080.
- Daniel Lednicer (1999). The Organic Chemistry of Drug Synthesis. 6. New York: Wiley Interscience. p. 187. ISBN0-471-24510-0.
- Asiri AM, Khan SA. Synthesis and anti-bacterial activities of a bis-chalconederived from thiophene and its bis-cyclized products. Molecules. 2011 Jan 12;16(1):523-31. PubMed PMID: 21228758.
- Bondock S, Fadaly W, Metwally MA. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur J Med Chem. 2010 ;45(9):3692-701. Epub 2010 May 15. PubMed PMID: 20605657.
- Maloy Kumar Parai, Gautam Panda, Vinita Chaturvedi, Y.K. Manju, Sudhir Sinha, Thiophene containing triarylmethanes as antitubercular agents, Bioorganic & Medicinal Chemistry Letters,18, 1, 2008, Pages 289-292.
- Junbo Wang, Chunhao Yang, Huasheng Ding, Xueming Yan, Xihan Wu, Yuyuan Xie. Synthesis of novel bone-targeted agents for treatment of osteoporosis. Ind. J. Chem. 2006; 45B; 318-321.
- Neeru Vasudeva, Pankaj Gupta, Surendra Sharma K. A new bithienyl constituent from Tagetes Erect Linn. Roots. Ind. J. Heterocycic Chem. 2007; 16; 303-304.
- Nagham Mahmood Aljamali .,”Synthesis and Chemical Identification of Macro Compounds of (Thiazol and Imidazol)”.,Research J. Pharm. and Tech, 2015, 8,1,78-84., DOI: 10.5958/0974-360X.2015.00016.5 .
- Hoshang E Master, Shabana I khan and Krishna A Poojari . Design and synthesis of nontoxic tetrahydrothieno [3,2-c] pyridine derivatives exhibiting compliment inhibition activity. Ind. J. Chem. 2008; 47B; 97-105.
- Takemoto, Y. Org; Enantioselective Organocatalysis: Reactions and Experimental Procedures Biomol. Chem. 2005, 3, 4299.
- Marja Äikiä, Leena Jutila, Tuuli Salmenperä, Esa Mervaala, Reetta Kälviäinen, Long-term effects of tiagabine monotherapy on cognition and mood in adult patients with chronic partial epilepsy, Epilepsy & Behavior, 8, 4, 2006, Pages 750-755.
- William J Giardina, Anticonvulsant action of tiagabine, a new GABA-uptake inhibitor, Journal of Epilepsy, 7, 3, 1994, Pages 161-166.
- Christine D. Waugh, Tioconazole, In xPharm: The Comprehensive Pharmacology Reference, edited by S.J. Enna and David B. Bylund, Elsevier, New York, 2007, Pages 1-4.
- Cuyun-Lira, M. Kaneko, T. Takafuta, K. Satoh, M. Ohnishi, Y. Yatomi, Y. Ozaki, Inhibitory effects of ticlopidine on platelet function as assessed by three different methods, European Journal of Pharmaceutical Sciences, 30, 1, 2007, P.21- 25 .
- Maria I. Aguilar, Ruth S. Kuo, William D. Freeman, New Anticoagulants (Dabigatran, Apixaban, Rivaroxaban) for Stroke Prevention in Atrial Fibrillation, Neurologic Clinics, 31, 3, 2013, Pages 659-675.
- Nagham M A ,Alaa Jawad Kadhim, Jalal Hasan Mohammed, Rajaa Abdul Ameer Ghafil, Nagham Mahmood Aljamali, Rajaa A A G . Review on Preparation and Applications of Formazan Ligands. International Journal of Thermodynamics and Chemical Kinetics. 2019; 5(2): 23–33p.
- Saulnier M, Velaparthi U, Zimmermann K (2003) Chapter 5.5: Five-membered ring systems: with N and S(Se). In: Gribble GW, Joule JA (eds) Progress in heterocyclic chemistry, vol 15. Elsevier, OxfordGoogle Scholar.
- Wu Y-J, Velaparthi U, Yang BV (2004) Chapter 5: Five-membered ring systems: with N and S(Se). In: Gribble GW, Joule JA (eds) Progress in heterocyclic chemistry, vol 16. Elsevier, OxfordGoogle Scholar.
- Nagham M A ., “The Various Preparation Methods in Synthetic Chemistry”.,1 Edt. ,Evincepub Publishing house, 2019., ISBN :978-93-88277-82-2 .
- Nagham Mahmood Aljamali, Rasha Neama H, Aafaq Jaber Alnajem, Ali Jassim Alzuhairi , Afaq Jaber Kadhium , Afaq J K., Studying of (Chemical ,Physical ,Biological)–Applications of Oxo- Sulfur Derivatives., Journal of Natural Sciences Research., 6, 7, 2016.
- Nagham Mahmood Aljamali. “Reactions and Mechanisms”.,1 Edt., IJMRA Publication ,2018 .,ISBN : 978-93-87176-25-6 .
- Aseel M J ,Mostafa N. Mohamed Salih ,Nagham M A, Nadia H O, Aseel Mahmood Jawad, Thanaa A. Helal ,Nadia Hussein Obaid, Nagham Mahmood Aljamali., 2019., Review on Chalcone (Preparation ,Reactions, Medical and Bio Applications). International Journal of Chemical Synthesis and Chemical Reactions.; 5,(1):16–27p.
- Wu Y-J, Yang BV (2009) Chapter 5.5: Five-membered ring systems: with N and S(Se). In: Gribble GW, Joule JA (eds) Progress in heterocyclic chemistry, vol 21. Elsevier, OxfordGoogle Scholar.
- Nawfel M B ,Hayder H K, Noor H D, Nawfel Muhammed Baqer ,Nagham Mahmood Aljamali., “Preparation of Chemical Inhibitors to Treat the Corrosion and Erosion of Machines”, International Journal of Engineering, Applied and Management Sciences Paradigms., 2019, 54, 3,89-93p.
- Wu Y-J, Yang BV (2011) Chapter 5.5: Five-membered ring systems: with N and S(Se). In: Gribble GW, Joule JA (eds) Progress in heterocyclic chemistry, vol 23. Elsevier, OxfordGoogle Scholar.
- Farmer LJ, Bemis G, Britt SD, Cochran J, Connors M, Harrington EM, Hoock T, Markland W, Nanthakumar S, Taslimi P, Ter Haar E, Wang J, Zhaveri D, Salituro FG (2008) Bioorg Med Chem Lett 18:6231–6235CrossRefGoogle Scholar.
- Nagham Mahmood Aljamali. “Synthesis and Biological Study of Hetero (Atoms and Cycles) Compounds”, Der Pharma Chemica, 2016, 8,6, 40-48.
- Zhou C, Garcia-Calvo M, Pinto S, Lombardo M, Feng Z, Bender K, Pryor KD, Bhatt UR, Chabin RM, Geissler WM, Shen Z, Tong X, Zhang Z, Wong KK, Roy RS, Chapman KT, Yang L, Xiong Y (2010) J Med Chem 53:7251–7263CrossRefGoogle Scholar.
- Zoltewicz, J. A.; Deady, L. W. (1978). Quaternization of Heteroaromatic Ligands. Quantitative Advances in Heterocyclic Chemistry. 22. pp. 71–121. doi:10.1016/S0065-2725(08)60103-8 . ISBN 9780120206223.
- Eicher, T.; Hauptmann, S. (2003). The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications. ISBN 978-3-527-30720-3.
- Mak, Jeffrey Y.W.; Xu, Weijun; Fairlie, David P. (2015). Peptidomimetics I Topics in Heterocyclic Chemistry. 48. Springer Berlin Heidelberg. pp. 235–266. doi:10.1007/7081_2015_176. ISBN 978-3-319-49117-2.
- Imad Kareem Alwan Alsabri, Hasaneen Kudhair Abdullabass ,Nagham Mahmood Aljamali, Imad K A A, Hasaneen K A., Invention of (Gluta.Sulfazane-Cefixime) Compounds as Inhibitors of Cancerous Tumors., Journal of Cardiovascular Disease Research, 2020,11, 2.
- Kriek, M.; Martins, F.; Leonardi, R.; Fairhurst, S. A.; Lowe, D. J.; Roach, P. L. (2007). “Thiazole Synthase from Escherichia coli: An Investigation of the Substrates and Purified Proteins Required for Activity in vitro” (PDF). J. Biol. Chem. 282 (24): 17413–17423 . doi:10.1074/jbc.M700782200 . PMID 17403671.
- Dondoni, A.; Merino, P. (1995). “Diastereoselective Homologation of D-(R)-Glyceraldehyde Acetonide using 2-(Trimethylsilyl)thiazole”. Organic Syntheses. 72: 21.; Collective Volume, 9, p. 952
- Nagham Mahmood Aljamali.; Intisar Obaid Alfatlawi., “Synthesis of Sulfur Heterocyclic Ligands and Study of Expected Biological Activity”,Research J. Pharm. and Tech., 2015, 8,9 ,1225-1242 , DOI: 10.5958/0974-360X.2015.00224.3.
- Gitai, Z . “The new bacterial cell biology: moving parts and sub cellular architecture”. Cell, 2005 , 120 ,5, 577–86.
- Vanita S .; Supriya, M. “Development of furfuraldehyde formazans as potential antitubercular agents “., Der Pharma Chemica, 2016,8,18, 144-148.
- Park, S.; Park, B.; Yun, S.; Kang, H. and Yun, “Antimicrobial activities of honey bee venom against pathogens isolated from clinical bovine mastitis in Korea” , Planta Med., 2013, 79, , PL16.
- Habibe,T .;Mehmet, L. A. “Electrochemical properties of 1-(o−, m−, pnitrophenyl)-3-(m−nitrophenyl)-5-phenylformazans and their nickel complexes”.,Turk J Chem ,2010,34, 465 – 479.
- Angela, K.; Lucica, V.; Nicoleta, C . “Synthesis and structural studies of complexes of Cu, Co, Ni and Zn with isonicotinic acid hydrazide and isonicotinic acid (1-naphthyl methylene) hydrazide”, J. Serb. Chem. Soc, 2010, 75 ,2, 229-242.
- Wright, G.D. ” The antibiotic resistome: the nexus of chemical and genetic diversity”., Nature Reviews Microbiology, 2007, 5, 175-186.
- Nagham Mahmood Aljamali., “(Synthesis, Investigation, Chromatography, Thermal)-Behavior of (Five, Seven)- Membered Ring with Azo and Anil Compounds”, Pak. J. Biotechnol. 2018; 15(1): 219-239.
- Hegazi, A.G.; Abdou, A.M. and Abd, A. F. “Evaluation of the antibacterial activity of bee venom from different sources”, World Applied Sciences Journal, 2014, 30 ,3, 266-270.
- Aseel Mahmood Jawad, Nagham Mahmood Aljamali, Saher Mahmood Jwad, Aseel M J, Saher M J., Development and Preparation of ciprofloxacin Drug Derivatives for Treatment of Microbial Contamination in Hospitals and Environment, Indian Journal of Forensic Medicine & Toxicology, 2020,14, 2, p:1115-1122.
- Intisar Obaid Alfatlawi ,Nuha Salman S, Zainab Mahmood Jawad , Nagham Mahmood Aljamali., Synthesis of New Organic Ligands Via Three Components Reaction with Studying of (Identification ,Thermal Behavior, Bioactivity on Bacteria of Teeth)., Journal of Global Pharma Technology. 2017; 11, 9 ,157-164.
- Mieaad Mohamd, Nagham Mahmood Aljamali ,Wassan Ala Shubber ,Sabreen Ali Abdalrahman .,”New Azomethine- Azo Heterocyclic Ligands Via Cyclization of Ester”., Research J. Pharm. and Tech. 11, 6 , 2018 .
- Al-Shammari, A.M.; Salman, M.I.; Saihood, Y.D.; Yaseen, N.Y.; Raed, K.; Shaker, H.K.; Ahmed, A.; Khalid, A.; Duiach, A. (2016) In vitro synergistic enhancement of Newcastle Disease Virus to 5-fluorouracil cytotoxicity against tumor cells. Biomedicines, 4(3): 1-10.
- Nagham Mahmood Aljamali., “(Synthesis, Investigation, Chromatography, Thermal)- Behavior of (Five, Seven)- Membered Ring with Azo and Anil Compounds”, Pak. J. Biotechnol. ,2018; 15(1): 219-239.
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. (2014) Isoxazoline containing natural products as anticancer agents: a review. European journal of medicinal chemistry, 77(2014): 121-133.








