Suhair Abdul-Kareem Al-Rammahi , Jinan Mohammed Hussein
, Jinan Mohammed Hussein , Elham Jwad Kadhem
, Elham Jwad Kadhem and Aseel Said Ismail Al-Hadad
and Aseel Said Ismail Al-Hadad
Department of Biology, Girl of Education College ,University of Kufa.
DOI : https://dx.doi.org/10.13005/bpj/1968
Abstract
The study involved the isolation of enterococcus bacteria and diagnosis of different types of diseases, where (190) samples of cows suffering from different diseases were obtain (34) (18.1%) isolation of Enterococcus were divided into 21 (15.6% ) Of the blood, 8 (52.4%) and 3 (17.6%) of the vagina, but not isolated from the spinal cord samples. The study was conducted on 2 isolates from the blood samples to become the number of isolates (36 isolates). And the study showed that type E. Faecalis was the most common species, with 75% followed by E. faecium (20.2%) and E.galinarium (4.8%), which also revealed the virulence factors of these bacteria, which play an important role in their disease. Bacteria have the ability to produce Haemolysin, Protease, Lipase, β-Lactamase, and the formation of capsules and their ability of Haemagglutination and of the effect of mannos on this process.
Keywords
Capsule Presence; Enzymatic Properties; Enterococcus Intestinal Bacteria; Hemagglutination; Virulance Factors
Download this article as:| Copy the following to cite this article: Al-Rammahi S. A. K , Hussein J. M, Kadhem E. J, Al-Hadad A. S. I. Bacteriological Study of Enterococcus Bacteria Isolated from Different Diseases in the Female Cows. Biomed Pharmacol J 2020;13(2). |
| Copy the following to cite this URL: Al-Rammahi S. A. K , Hussein J. M, Kadhem E. J, Al-Hadad A. S. I. Bacteriological Study of Enterococcus Bacteria Isolated from Different Diseases in the Female Cows. Biomed Pharmacol J 2020;13(2). Available from: https://bit.ly/37Jn2Ut |
Introduction
Enterococcus faecium belongs to the Enterococcaceae family and the Firmicutes division, which is part of the LAB (Lactic Acid Bacteria,). These bacteria can cause a wide range of diseases if they affect the urinary tract. (Gilmore et al., 2002) . Nowadays, enterococci have become the second most common bacterial cause of acquired infections, causing many therapeutic difficulties, also thier resistance to new antibiotics due to widespread use of these antibiotics leading to true resistance by enterococci. In addition, the ability of these bacteria to rapidly acquire chromosomal elements that produce virulence quickly lead to increased infection with enterococcus infections since many scientific research has proven the ability of these bacteria in the infection of natural hosts and inducing serious cases of inflammation such as infections and urinary tract infections Bacteria have the ability to adhesion, and invade some areas of the body, giving these bacteria a good chance of 92% of infections caused by enterococcal bacteria in animals caused by strains of Enterococcus faecalis and others caused by strains faecium Enterococcus and other infections caused by other species (Baldassar, et al. 2005). Several experiments on laboratory animals have demonstrated the ability of these cocci to cause many infections as they have multiple virulence factors, which can be divided into secretory virulence factors such as Haemolysin, Protease, Hyaluronidase, Bacteriocin, Pheromones and Lipase, as well as aggregate proteins (Agents). Polysaccharides wall cell, and some studies have shown that they have the ability to produce a capsule (Sava et al., 2010). As a pathogen acquired community, the bacterium enterococci became involved in increasing the number of poultry deaths (alchowdhury, 2009, Leavis at el., 2006). Despite the medical development in the field of microorganisms, the incidence of opportunistic pathogens and acquired infections, including enterococcus bacteria, is still causing many medical problems. Thus the goal of this study Conducting a local study of these bacteria in cattle females.
Isolation and diagnosis of enterococci from different pathogenic models in cows and their diagnosis at the species level.
Studying virulence factors which include:
Study of characteristic enzymes .
Investigating the existence of the capsule
Study of the ability of bacteria to cause blood clotting and the effect of mannose sugar in the process of clotting.
Materials and Methods
The Media Used in the Diagnostic Tests for Enterococci Bacteria
Alkaline tolerance test medium Prepare from the brain broth infusion medium (Himedia), then adjust the pH at 9.5 by adding 6.5 NaOH and pour the medium into test tubes and sterilize the autoclave at 121 ° C. For 15 minutes Collee et al., 1996).
Salinity tolerance medium test Salt tolerance was prepared by adding 1% sodium chloride salt to the broth-heart broth medium, pouring the medium into test tubes and then sterilizing with autoclave at 121 ° C for 15 minutes (Collee et al., 1996).
Motility test medium Prepare the medium according to Himedia instructions and pour it into test tubes and sterilize the autoclave at 121 ° C for 15 minutes. The medium was used to detect the ability of gastrointestinal for motility (Langston., et al.. 1960).
Bile salt esculin agar, which has been synthesized from its base materials according to Finegold and Baron, 1990.
Medium fermentation of sugar xylos and arabenose prepared this medium by adding 1% of arabinose and xylose to the medium of the brain heart infusion broth each individually and also added 1 ml of 0.006% g of the phenol red reagent and adjust the pH at 7 and distributed to test tubes and sterilized autoclave device at 121 ° C for 5 minutes (Hanson and Cartwright, 1999)
The carbohydrate fermentation test, red phenol agar was prepared according to Himedia’s instructions, and sterilized at 121 ° C for 15 minutes and then left at room temperature to cool to a temperature of 50-45 ° C then 10% sugars were added individually to each of the sterile sugar solutions, with a concentration of 10% (Collee., et al., 1996).
Potassium Tellurate Salinity Tolerance Test (0.04%) Prepare 1 liter of brain-heart infusion medium, adjust pH to 6.1 by adding HC l (0.1 N), sterilize medium with autoclave and let cool to a temperature of 50-45 مC. 50 ml of healthy human blood and sterile potassium tellurite solution filtered (0.5 g salt to 150 ml distilled water) and kept in the refrigerator after pouring into sterile dishes until use ((Facklam, 1972)
Pigment test medium Use Trypticase soya agar medium to detect the ability of enterococci to produce pigments.
The three-axis modification medium. This medium was used for the rapid initial diagnosis of enterococci bacteria and includes three biochemical tests in one medium: a bacterial isolation test for hydrolyzed insulin, a salinity tolerance test, a motion test, and according to the basic components as described in (HannaNo, 2002).
Isolation and Diagnosis
Bacterial isolates were diagnosed, based on MacFaddin (2000) and Collee al et., (1996). Cultural, microbiological and biochemical characteristics were determined.
Detection of Virulence Factors Possessed by Bacterial Isolates
Investigation Lipase Production: This medium used to investigate Lipase production was used for this purpose as it was prepared from its basic materials as stated in (Starr, 1941). The appearance of a transparent halo around the colonies is an indication of positive screening
Screening for Protease Production: Three methods were used to prepare the screening medium for protease production:-
Trypticase soya agar medium was prepared according to the instructions of the company Himedia and added 1.5% fat-free milk and sterilized at 121 ° C for 5 minutes and poured into the dishes and left to solidify and then kept in the refrigerator until use (Coque at. el, 1995).
Prepared this medium in the same way as the previous except for the use of 3% g gelatin instead of milk and this medium used to detect the ability of enterococci to produce gelatinase enzyme (Coque at. el, 1995).
The milk skim agar medium was used according to the instructions of the Himedia Company, then sterilized with an autoclave at 121 ° C for 5 minutes and kept in the refrigerator after pouring into sterile dishes until use (Elsner al et., 2000). The appearance of a transparent halo around the growing colonies in the prepared medium indicates a positive test.
Detection of Production of Haemolysin
Brain heart infusion agar was prepared according to the instructions of the company (Himedia) and added 5% cattle blood, For this purpose as the emergence of haemolysis of blood around colonies indicates a positive assay (Elsner al et., 2000)
Detection of Beta-Lactamase Production
The direct capillary tube method was used for this purpose as reported in (Koneman et al., 1992). The coloration of the capillary tubes in yellow indicates positive test (Elsner et.al., 2000).
Capsule Production Test
The Indian ink pigmentation method was used to detect the presence of the capsule A portion of the colony was taken and placed on the glass slide and a drop of Indian ink was added to it and blended, Then spread the mixture on the surface of the slide and left to dry and examined by microscope( Bottone al et., 1998), The appearance of strains under the microscope in the form of luminous regions indicates that they have this structure.
Agglutination Test in The Presence And Absence Of Mannose
This method was used to detect the ability of bacteria to cause blood agglutination according to (Elsner et.al, 2000). 2.5% of mannose was added to the phosphate solution, which was used to prepare the bacterial suspension of the strains of isolates studied in this test to determine the effect of mannose on agglutination process.
Results and Discussion
Isolation and Diagnosis
The number of isolates obtained from a total of 190 different pathological samples reached (34) isolates, distributed between 21 (15.6%) from 135 urine samples and 8.4% (8) isolates from 16 stool samples and 3 (17.6%) isolates from 18 vaginal swabs. but not obtain Enterococci From (25) sample of spinal fluid samples, and two isolates were obtained from cases of bloody bacteremia obtaine from the veterinary hospital and veterinary health centers in the city of Najaf, as this study showed that the total incidence of intestinal bacteria was (17.3) %.
Cultural Characteristics and Microscopic Test
All pathological specimens as well as hematological bacterial isolates were cultured on selective media, and intestinal isolates were diagnosed initially by studying the phenotypic characteristics of the growing colonies, as they appeared in the form of small circular colonies with a grayish-white color on the blood agar, and the blood with Azide agar, Also some of them showed complete or partial haemolysis, and some were not haemolyse, while the pink color appeared on the Macconkey agar free from crystals violite for fermenting lactose sugar in the medium, and the medium of blood and Azide is considered selective medium to isolate the intestinal bacterium from pathogenic samples because it contains Sodium Azide substance which inhibiting the growth of Gram- negative bacteria, Black colonies also appeared on asculin and bile salts agar due to their ability to analyze ascoline to ascolitine, as well as gradually turning the color of the medium from yellowish green to black. And the results of the microscopic examination, the colonies of the bacteria showed in the form of single and double cocci, some of which were in the form of short chains positive for Gram-stain dye (2).
Biochemical Tests
The results showed that all strains were negative for the tests of catase ,the production of dyes and movement and positive tests for salinity and base tolerance and the hydrolyse of ascolin and the tolerance of bile salts, in addition to its ability to tolerate 0.04% of potassium tellurite, as all strains were able to grow in temperatures of 10 ° C and 45 ° C as well as their ability Withstand 60° C fo 30 minutes. The results also showed that the strains of type E. faecalis were ferment for glycerol, glucose, mannos and ribose and lactose by 100%, while the rest of the sugars were fermented in different proportions, and strains E. faecium were ferment for glycerol, sugar of glucose, mannitol arabinose, glucose, mannos, ribose, and lactose in 100% of the sugar. While E .gallinarium were ferment to glycerol and all sugars except for sorbitol sugar as in Table No. (1).
Table 1: Shows the biochmical diagnostic tests for enterococcus bacteria.
| Initial tests | E. faecalis | E. faecium | E.gallinarium | |||
| Catalase | – | – | – | |||
| salinity tolerance | + | + | + | |||
| Base tolerance | + | + | + | |||
| 0.04% potassium tellurite tolerance | + | + | + | |||
| Ascolin lysis and bile salts tolerance | + | + | + | |||
| Growth at 60 ° C for 30 minutes | + | + | + | |||
| Growth at 10 ° C and 45 ° C | + | + | + | |||
| Carbohydrates ferement | No. | % | No. | % | No. | % |
| glucose | 29 | 100 | 9 | 100 | 4 | 100 |
| manitol | 20 | 74.3 | 9 | 100 | 4 | 100 |
| arabinose | – | – | 9 | 100 | 4 | 100 |
| sorbitol | 24 | 83.5 | – | – | – | – |
| raminose | – | – | – | 100 | 4 | 100 |
| sucrose | 19 | 70.8 | 9 | 100 | 4 | 100 |
| glycerol | 29 | 100 | 9 | 100 | 4 | 100 |
| xylose | – | – | – | 100 | 4 | 100 |
| manose | 29 | 100 | 9 | 100 | 4 | 100 |
| ribose | 29 | 100 | 9 | 100 | 4 | 100 |
| lactose | 29 | 100 | 9 | 100 | 4 | 100 |
| Pigment production | – | – | – | – | – | – |
| motility | – | – | – | – | – | – |
API 20 Strep Diagnostic Kit
Types of isolated strains of enterococci were diagnosed using diagnostic Api20 kit figure (1), where 27 (75%) isolates were of type E. faecalis are as follows 16 (64%) isolation from urine samples, 6 (24%) isolation from stool samples, and 3 (12%) isolation from vaginal swabs as well as two isolates from bacteriamia), as for isolates E. faecium, it was 7 (19.6%) isolation distributed between 5 (57.8%) isolation from urine and 2 (42.2%) isolation from faeces. Also, 2 (5.4%) were belonged to E.gallinarium type. Where the percentages of isolating the bacteria of the enterococcus bacteria showed that the type E. Faecalis is most frequently found in isolates compared to other species, This results was consistent with the researcher Dupre et al., (2003), who indicated that the percentage of isolation of type E. faecalis strains is higher than type E. faecium strains ,The isolation ratios differed in this study with the study of Al-Azzawi researcher 2008)) which indicated that the strains of type E. faecium are the most isolated followed by strains of type E. Faecalis then strains of type E.gallinarium. The reason for the different high isolation ratios may be due to the different isolation conditions and the type of samples.
 |
Figure 1: API 20 Strep Diagnostic Kit. |
Detection of Virulence Factors
Detection of the Hematolytic Enzymes ( Haemolysin)
The results showed a difference in the proportions of hemolysin-producing isolates, where 9 (25%) was a fully hemolysis (β-hemolysis), 8 (22.2%) was a partially hemolysis (α haemolysis), and 19 (52.8)% was not a haemolysis ( y-hemolysis). As in Table (2), this study is agree with many studies that showed that most of the intestinal bacterium are not hemolysis for blood, and some of them partially hemolysis and others completely (Blanch and Manero, 1999) as they were compatible with Gilmore and Hancock study (2002), Which showed that the percentage of hemolytic enterococci with total and partial blood quality ranges between (45-60%) and also agreed with the Al-Azzawi study (2008), which showed that the percentage of these bacteria producing hemolysin from the gamma type constitutes the majority followed by those that produce beta and then alpha type The results also showed that the strains of type E. faecalis and E.faecium Fully or partially blood-analyzed ratios accounted for 55.5% and 28.6%, respectively, while E. gallinarium isolates were unable to analyze blood and this is consistent with many studies that confirmed the prevalence of the E.faecalis type over the rest of the species in their production of this enzyme as a study ( Ike et. al, 1987 and (Coque et. al, 2005). As for the local level, it was also agreed with Al-Azzawi study (2008). but didn’t agree with the study (Desai et. al, (2001) which indicated that the most of intestinal bacteria were producing hemolysin of the beta type, and the researcher attributed the high rate of production of hemolysin to the continuous presence For those isolates with other pathogenic bacteria producing this enzyme, which led to the acquisition of the characteristic of hemolysin production.
Table 2: It shows the factors of virulence in the bacteria of enterococci.
|
Virulence factors |
E. faecalis | E. faecium | E.gallinarium | total | % | ||||
| No. | % | No. | % | No. | % | ||||
| Β- haemolysis | Blood Haemolysis | 9 | 33.3 | 0 | 0 | 0 | 0 | 9 | 25 |
| α- haemolysis | 6 | 22.2 | 2 | 28.6 | 0 | 0 | 8 | 22.2 | |
| y-haemolysis | 12 | 44.4 | 5 | 71.4 | 2 | 100 | 19 | 53 | |
| Gelatinase production | 21 | 77.8 | 3 | 56.1 | 0 | 0 | 24 | 66.5 | |
| Lipsase production | 10 | 37 | 2 | 26.6 | 0 | 0 | 12 | 33.3 | |
| β-Lactamase production | 3 | 11.3 | 0 | 0 | 0 | 0 | 3 | 6 | |
| Capsule formation | 10 | 37 | 2 | 28.5 | 0 | 0 | 12 | 33.3 | |
|
absence mannose |
haemaglutination |
25 | 92.8 | 3 | 40.6 | 0 | 0 | 28 | 78.8 |
| mannose | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
0 |
|
Detection of Gelatinase Enzyme Production
The results of this test showed that there is a difference in the rates of production of this enzyme, as the results showed that 24 (66.5%) isolation of the intestinal bacterium was produced for this enzyme. The result was read as clear areas around the colonies on the Trypticase soya agar medium containing milk, as well as the Skim milk agar medium, which also showed white coagulation on the surface of the medium with a late incubation period, and the results were distributed as follows and according to the table (2), It was 21 (77.8)% isolation and 3 (56.1)% isolation of the two species E. faecalis and E. faecium respectively were produced for this enzyme whereas E. gallinarium isolates were not able to produce it as in Table 2, and these results were consistent with Gilmore and Hancock (2002) and Gulhan et. al, 2006). Which confirmed the ability of enterococcus bacteria to produce this enzyme. This study also agreed locally with Al-Azzawi study (2008), as these studies confirmed that the isolates of type E.faecalis are the most capable of producing this enzyme, followed by isolates of type E.faecium. also indicated some of type E gallinarium isolates a lack thier ability to produce this enzyme, and many studies have not indicated the production of Gelatinase in type E. gallinarium Perhaps this is due to the fact that most studies focus on the two species E. faecalis and E. faecium as the two most common pathogens in pathological infections.
Detection of Lipase Enzyme Production
The results showed that 12 (33.3%) isolates from the bacteria of enterococci were producing this enzyme, as in Table (2), they were distributed differently on the types of strains of these bacteria, where 10 (37)% isolates of E. faecalis and 2 (26.6)% isolate of E. faecium, and strains produced for this enzyme that appear blue colony on the lipolysis medium. The results of this study were consistent with (Elsner et . al, 2000, and Majid study ,2006). Which confirmed the prevalence of type E. faecalis over other types in its ability to produce this enzyme, followed by type E .faecium, while isolates of type E.gallinarium were negative for this test. This study did not agree with what Majid (2006) indicated in that 1 (14.28%) isolates of type E. gallinarium isolates was able to produce this enzyme and this may be due to the difference in the nature of single specie strains.
Detection of the Production of β-Lactamase
The results showed that 3 (11.3)% of type E. faecalis isolates was positive for this test and as in Table (2), the color of the solution in the capillary tubes changed to yellow while the strains of the two types were E. facium and E.gallinrium are non-beta-lactamase production. These results were consistent with the Patterson et. al, (1988), Wells et. al, (1992) study, which confirmed the ability of some strains of E.faecalis able to produce this enzyme, while no isolates of either E. faecium and E. gallinarium were recorded to produce β-Lactamase. Several studies indicated that this enzyme was only produce by strains of E. faecalis, which was referred to by the name HH22, as it was distinguished by its high resistance to gentamicin antibiotic, and other studies indicated the acquisition of this characteristic of this bacterium by the staphylococcus (Blanco and Lopardo, 2007).
Hemagglutination Test with the Presence and Absence of Mannos
Different blood group of cows were used to detect the ability of bacteria to cause of hemagglutination, as this test is one of the effective tests for detection the ability of bacteria to adhere on the surface of red blood cells, which play important role of infections (Elsner et. al, 2000).The results showed that, as in Table (2), there is a difference in the ratios of Hemagglutination between the three species, where 25 (92.8%) isolates of the E.faecalis had the ability to adhere on the red blood cell outside the body of the organism (invitro). and E. Faecium was 3 (40.6%) was binding to different blood groups, while E. gallinarium were not shown positive for this examination. These results are consistent with the study of Elsner et. al, (2000) and Gulhan et. al, (2006), which showed that the E. faecalis was agglutinate (97%) for erythrocytes of an animals. As for the local level, Al-Azzawi study (2008) agreed that E. faecalis and E. faecium was positive for this test, and it differed with Hanna Hanoo (2002) in that E–Galinarium were also positive for this test and this may be due to the difference in the nature of the one-type strains). Also, this study did not agree with the results of the Teixeira and Carvalho (1995) study, which indicated that the intestinal bacterium was unable to agglutinate the red blood cells of an animals. This is due to increase this characteristic of these bacteria in the past few years, as the researchers pointed out that the reason is due to the differences in Ratios of receptors present on the surface of bacteria. also explained that all enterococci bacteria that are able to agglutinate the red blood cells of an animal lose their ability hemagglutination when treating these bacteria with mannose in concentration of 2.5%, as in the picture (3), and this indicates that these substances compete for surface adhesives spread over the entire surface of these bacteria. Thus, it is prevented from adhesion and agglutinate of red blood cells when treated with bacteria treated with mannose, and therefore it is indicated by the sensitive adhesives of mannose, and these results came in agreement with many studies that showed that the concentration of 2.5% of mannose inhibits the process of adhesion of red blood cells by many bacterial types and thus affects Adhesion to host tissue (Chick., Etal1981). It is also possible to inhibit the hemagglutinate process when treating these bacteria with trypsin, as Hanna Hanoo (2002) indicate that the inhibition of hemagglutination process when treating intestinal bacteria with it, and this attributed to to the fact that some surface receptors of this bacterium are of a protein nature.
 |
Picture 3A: Shows negative blood hemagglutination in the intestinal bacterium, shows when treated with 2.5% of mannos. |
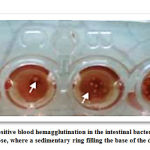 |
Picture 3B: Shows positive blood hemagglutination in the intestinal bacterium, without treating 2.5% of mannose |
Detect Capsule Formation
The results showed that if the strains appear under the microscope in the form of illuminated areas, this indicates that they possess this structure. The colonies that contain the capsule are distinguished by their viscous strength on the of the blood agars, while this character did not appear on the other media (Bottone et.al, 1998). The results of this test showed in this study the ability of enterococcus bacteria in the formation of this structure and in different rates between three types, where 10 (37)% was isolated E. faecalis and 2 (28.5)% isolates of E .faecium have the capsule while E. gallinarium. were not form capsule as in Table (2), and these results agreed with the study (Huebner et.al,1999), which indicated the capacity of E. faecalis and E .faecium the formation of capsule antigens as the results of this study agree with local studies such as Al-Azzawi study (2002) where it indicated possession of E. faecalis and E. faecium on this structure was lack in E. gallinarium
Conclusions
The bacteriological study included isolation of Enterococcus and species diagnosis from (190) clinical specimens of patients which suffered from various infections at. A (34) isolates of Enterococcus were obtained at ratio (18.1%)and distributed into 21 (15.6%) urine , 8(52.4%) feces , and 3 (17.6%) vaginal swab , but no specimen were isolated from cerebral spinal fluid , two additional isolates from blood specimens were also isolated, so the total number were (36) isolate .The study revealed that the species E. faecalis was the most abundant species with (75%) , followed by E. faecium with (20.2%) and E. gallinarium with (4.8%) .The investigation of virulence factors of this bacterium which play a majorrole in its pathogenicity , it was approved that these bacteria had the ability to produce haemolysin, protease , lipase , β-lactamase , capsules formation , enterosin production , and their ability of haemagglutination , the study also showed mannose effect on haemagglutination test .
Acknowledgments
We would like to express our heartfelt thanks to the veterinary hospital and veterinary health centers in the city of Najaf, for providing assistance samples
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding source
There is not funding source
References
- Baldassarri, L.; Creti, R.; Montanaro, L.; Orefici, G. and Arciola, C.R. (2005). Pathogenesis of implant infections by enterococci. Int. J. Artif. Organs., 28: 1101-1109.
- Baron, E.J. and Finegold, S.M. (1990). Diagnostic Microbiology. Baily and Scotts. 8 th ed. C.V. Mosby Company, St. Louis, pp: 223-236.
- Bottone, E.J.; Patel, L.; Patel, P. and Robin, T. (1998). Mucoid encapsulated Enterococcus faecalis an emerging morphotype isolated from patients with urinary tract infections. Diagn. Microbiol. Infect. Dis., 31: 429-430.
- Carvalho, M.G.S. and Teixeira, L.M. (1995). Haemagglutination properties of Enterococcus. Curr. Microbiol., 30: 265-268.
- Hick, S.; Harber, M.J.; Mackenzie, R. and Asscher, A.W. (1981). Modified method for studing bacterial adhesion to isolated uroepithelial cells and uromucoid . Infect. Immun., 34(1): 256-261.
- Chowdhury, S.A.; Arias, C.A.; Nallapar eddy, S.R.; Reyes, J.; Willems, R.J.L. and Murray, B.E. (2009). A Trilocus Sequence Typing Scheme for Hospital Epidemiology and Subspecies Differentiation of an Important Nosocomial Pathogen, Enterococcus faecalis. J. Clin. Microbiol., 47: 2713-2719.
- Colle e, J.G.; Marmion, B.P.; Fraser, A.G. and Simmons, A. (1996). Mackie and McCartney Practical Medical Microbiology. 14 th ed. Churchill Livingstone, New York, pp: 263-298.
- Coque, T.M.; Willems, R.L.; Fortun, J.; Diz, S.; Loza, E.; Caton, R. and Baquero, F. (2005). Population structure of Enterococusfaecium causing bacterimia in a Spanish university hospital: setting the scene for future increase in vancomycin resistance . Antimicrob. Agents Chemother., 49(7):2693-2700.
- Coque, T.M.; Patterson, J.E.; Steckel berg, J.M. and Murray, B.E. (1995). Incidence of hemolysin, gelatinase and aggregation substance among Enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community based persons. J. Infect. Dis., 171: 1223-1229.
- Desai, P.T.; Pandit, D.; Mathur, M. and Gogate, A. (2001). Prevalence , and disterbution of varous species of Enterococci isolated from clinical specimens with special reference to urinary tract infection in catheterzed patients. Indian. J. M ed. Microbiol., 19:132-137.
- Dupre, I.; Zanetti, S.; Schito, A.M.; Fadda, G. and Sechi, L.A. (2003). Incidence of virulence determinants in clinical Enterococu s faecium and Enterococcus faecalis isolated collected in Sardinia (Italy). J Med.Microbiol., 52: 491-498.
- Elsner, H.A.; Sobottka, I.; Mack, D.; Claussen, M.; Laufs, R. and Wirth, R. (2000). Virulence factors of Enterococcus faecalis and Enterococcus faecium blood culture isolates. Eur. J. Clin. Microbiol. Infect. Dis., 19: 39-42.
- Facklam, R.R. (1972). Recognition of group D streptococcal species of human origin y biochemical and physiological tests. Appl. Microbiol., 23(6):1131 b-1139.
- Facklam, R.R. and Teixeira, L.M. (1997). Enterococcus. In: Collier, A., Balow, S. and Sussman, M., (Eds.), Microbiolog y and microbial infections. Topiey and Wilson, 9 th ed., Edward Arnold, London, pp. 669-682 .
- Gilmore, M.S.; Coburn, P.S.; Nallapareddy, S.R. and Murray, B.E. (2002). EnterococcalVirulence, pp: 301-354. In Gilmore, M.S.; Clewell, D.B.; Courvalin, P.M.; Dunny, G.M.; Murray, B.E. and Rice, L.B. (2002). The enterococci: pathogenesis, molecular biology, antibiotic resistance, and infection control. ASM Press, Washington, D.C.
- Guzman, C.A.; Pruzzo, C.; Lipira, G. and Calegari, L. (1989). Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infection and endocarditis. Infect. Immun., 57(6): 1834-1838.
- Hanson, K.L. and Cartwright, C.P. (1999). Comparison of simple and rapid methods for identifying Enterococci intrinsically resistant to vancomycin. J. Clin. Microbiol., 37(3): 815-817.
- Hancock, L.E. and Gilmore, M.S. (2002). The capsular polysaccharide of Enterococcus faecalis and it’s relationship to Polysaccharides in cell well. Proc Nati. Acad. Science. Microbiol. USA, 99(3):1574-1579.
- Huebner, J.; Wang, Y.; Krueger, W.A.; Madoff, L.C.; Martirosian, G.; Boisot, S.; Goldmann, D.A.; Kasper, D.L.; Tzianabos, A.O. and Pier, G.B. (1999). Isolation and chemical characterization of a capsular polysaccharide an tigen shared by clinical isolates of Enterococcus faecalis and vancomycin resistant Enterococcus faecium. Infect. Immun., 67(3): 1213-1219.
- Ike, Y.; Hashimoto, H. and Clewell, D.E. (1987). High incidence of hemolysin production by Enterococci(Streptococcus) faecalis stains associated with human parental infection. J. Clin. Miocrobiol., 25(8):1524-1528.
- Kau, A.L.; Martin, S.M.; Lyon, W.; Hayes, E.; Caparin, M.G. and Hultgren, S.J. (2005). Enterococcufaecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun., 73(4):2461-2468.
- Koneman, E.W.; Allen, S.D.; Janda, W.M.; Scheckenber, P.C. and Winn, J.W. (1992). Color Plate and Text Book of Diagnostic Microbiology. 4 th ed. J.B. Lippincott company. Washingtion, pp:429
- Langston, C. W.; Gutierre, Z.J. and Bouma, C. (1960). Motile Enterococci(Streptococcus faecium var. Mobilis var. N.) isolated from grass silage. J. Bacteriol., 80: 714-718.
- Leavis, H.L.; Bonten, M.J. and Willems, R.J. (2006). Identification of high- risk enterococcal c lonal complexes: global dispersion and antibiotic resistance. Curr. Opin. Microbiol., 9:454-460.
- Libertin, C.R.; Dumitru, R. and Stein, D.S. (1992). The hemolysin/bacteriocin produced by enterococci is a marker of pathogenicity. Diagn. Microbiol. Infect. C ontrol 15:115-120.
- Lomberg, H.; Cedergren , B.; Leffer,H.; Nelsson, B.; Carlstrom, A. and Eden, C. (1986). Influence of blood group on the availabl ability of receptors for attachment of uropathogenic Eschrichia coli. Infect. Immun., 51(3): 9190-9206.
- Lopardo, H. and Blanco, A. (2007). Methods for the detection of beta lactamase producing enterococci. Rev. Argent. Microbiol., 39(2):105.cx
- MacFaddin, J.E. (2000). Individual biochemical tests for identification of medical bacteria. 3 th ed. Lippincott Williams Wilkins, London. pp:57-424.
- Manero, A. and Blanch, A. (1999). Identification of Enterococci spp with a biochemical key. Appl. Envrion. Microbiol., 65(10):4425-4430.
- Patterson, J.E.; Colodny, S.M. and Zervos, M.J. (1988). Serious infection due to β- lactamase- producing Streptococcus faecalis with high- level resistance to gentamycin. J. Infect. Dis., 158:1144-1145.
- Pompel, R.L.; Lampis, G.; Berlutti, F. and Thatter, M. (1991). Characterization of yellow- pigmented Enterococci from several human infections. J. Clin. Microbiol., 29(12):2884-2886.
- Qi, X.; Bai, D.; Zhao, X.; Wang, A.; Li, Y. and Jin, Y. (2011). Probiotic activities of lab in endometritis: probiotic activities of three lactic acid bacteria strains isolated from healthy bovine cervix: In Vitro adherence to immortalized endometrial epithelialcells and antimicrobial properties. J. Animal and Veterinary Advances., 10(4): 484-488.
- Rozdzinski, E.; Marre, R.; Susa, M.; Wirth, R. and Muscholl- Silberhorn, A. (2001). Aggregation substance- mediated adherence of Enterococcus faecalis to immobilized extracellular matrix proteins. Microb. Pathog., 30:211-220.
- Sava, I.G.; Heikens, E.; Kropec, A.; Theilacker, C.; Willems, R. and Huebner, J. (2010). Enterococcal surface protein contributes to persistence in the host but is not a target of opsonic and protective antibodies in Enterococcus faecium infection. J. Med. Microbiol., 59: 1001-1004.
- Starr, M.P. (1941). Spirit blue agar: a medium for the detection of lipolytic microorganisms. Science, 39: 333-334.
- Styriak, I.; Laukova, A.; Fallgren, C. and Wadstrom, T. (1999). Binding of selected extracellular matrix proteins to Enterococci and Streptococcus bovis of Animal origin. Curr. Microbiol., 39: 327-335.
- Wells, V.D.; Wong, E.S.; Murray, B.E.; Coudron, P.E.; Williams, D.S. and Markowitz, D.M. (1992). Infections due to beta- lactamase- producing, high- level gentamycin- resistant Enterococcus faecalis. Ann. Intern. Med., 116:285-292.
- Zhanel, G.G.; Hoban, D.J. and Karlowsky, J.A. (2001). Nitrofurantion is active against vancomycin- re sistant Enterococci. Antimicrob. Agents. Chemother., 45(1):324-426.
- Hana Hano,Gorget nysan Shmon(2002). Study of diagnosi
- Hanna Hanoo, Georgette Nissan Shimon (2002). A diagnostic and pathological study of isolated enterococci from patients with urinary tract infection and its ability to cause experimental endocarditis. PhD thesis. College of Science. University of Al Mosul.
- Al-Azzawi, Zainbhsin Mahdi (2008) Study of virulence factors and response to bacterial antibodies in enterococci isolated from patients. Master Thesis . Faculty of Education . Diyala University.
- Majeed, Rasheed guitar (2006). Study of antibiotic resistance patterns of enterococcus bacteria isolated from different sources. PhD thesis. College of Science . Albasrah university







