Astrid Feinisa Khairani1* , Umar Islami2
, Umar Islami2 , Mas Rizky Anggun Syamsunarno3
, Mas Rizky Anggun Syamsunarno3 and Uci Ary Lantika4
and Uci Ary Lantika4
1Division of Cell Biology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia
2Graduate School of Biomedical Sciences Master Program, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia
3Division of Biochemistry and Molecular Biology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia
4Department of Histology and Medical Biology, Faculty of Medicine, Universitas Islam Bandung, Bandung, West Java, Indonesia
Corresponding Author E-mail : astrid.khairani@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/1874
Abstract
Dyslipidemia has high prevalence worldwide. Unhealthy diet such as high calories and high fat contribute to dyslipidemia. Purple sweet potato yogurt is a synbiotic product containing combination of probiotic fermentation and natural ingredient (prebiotic). Probiotics give beneficial effect to intestinal function. Purple sweet potato serves as prebiotic, also contains anthocyanin as antioxidant. This experimental study aimed to identify the effect of purple sweet potato yogurt consumption towards lactic acid bacteria colony in the intestine, lipid profile (total cholesterol, triglyceride, LDL), body weight, visceral fats, and liver weight of male mice model (Balb-c) with high fat diet. Total of 30 male mice were divided into six groups with different treatment on its diet. Lipid profiles were investigated using enzymatic colorimetric assay. Statistical data was analyzed using one-way ANOVA and post hoc tukey multiple comparison test. Result shown that in the group with administration of 0.3 mL of purple sweet potato yogurt had significantly increase the growth of lactic acid bacteria in the intestine (p<0.0001). The same group also shown favorable outcome in total cholesterol, triglyceride, LDL level, visceral fat and liver weight. Purple sweet potato yogurt has potential protective effect in lipid metabolism under high fat diet condition. Thus, further study is needed to explore more detail on its mechanism.
Keywords
Anthocyanin; Dyslipidemia; High Fat Diet; Lipid Profile; Lactic Acid Bacteria; Synbiotic
Download this article as:| Copy the following to cite this article: Khairani A. F, Islami U, Syamsunarno M. R. A, Lantika U. A. Synbiotic Purple Sweet Potato Yogurt Ameliorate Lipid Metabolism in High Fat Diet Mice Model. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Khairani A. F, Islami U, Syamsunarno M. R. A, Lantika U. A. Synbiotic Purple Sweet Potato Yogurt Ameliorate Lipid Metabolism in High Fat Diet Mice Model. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2u3cdwU |
Introduction
Dyslipidemia has a high prevalence worldwide. This condition happened as a result of unhealthy diet and lifestyle. High calories and high fat intake along with lack of routine physical activities will leads to obesity.1 Complication of obesity with other conditions such as hypercholesterolemia, hyperglyceridemia, hypertension, and disruption of glucose metabolism are considered as the risk factors of the rising LDL oxidation level. Furthermore, this condition will also contribute in increasing the risk of atherosclerosis plaque formation by which leads to development of coronary heart disease and stroke.2
Lipid from food will be broken down into cholesterols, triglycerides, phospholipids, and free fatty acids during the digestion process in small intestines with triglyceride as the major form of digested fats. The absorption process of lipid upon digestion occur in the mucosal enterocytes particularly in the proximal part.3 One of dyslipidemia prevention strategy that can be done by inhibiting lipid absorption using probiotic and prebiotic lining the small intestine, which are closely related to the function of gut microbiome in the body.
There are many factors that could influence gut microbiome in the human body, including dietary, micronutrient intake, genetic, specific region of country where a person lives and antibiotic consumption.4 The amount of bacteria in human intestine is also closely related with interaction between host, environment, and antigen inside the human body which represent intestines protection with wide area (250-400 m2).5 With these factors, the modification of gut microbiome for health benefit is highly possible and one of the potential method is by the consumption of functional foods such as yogurt.
Recently, there are a lot of modification on the yogurt production process available. The source of probiotic and prebiotic can be from natural ingredients based processed food in the form of yogurt.6 Sweet potato (Ipomoea batatas L.) can be used as the prebiotic element in yogurt production. There are several types of sweet potato available which can be differentiated based on its color due to antioxidant content. The purple color in purple sweet potato represents the high content of anthocyanin.7
The presence of anthocyanin in purple sweet potato as natural antioxidant is quite intriguing to be explored more due to its abundance of benefits. In vitro studies had been done to discover the effect of anthocyanin extract from purple sweet potato. Several studies revealed antioxidant effect of anthocyanin through several physiological mechanism including (1) free radical-scavenging activity, (2) increasing radical oxygen absorbance capacity, (3) inhibition of Angiotensin-I Converting Enzyme, (4) inhibition of α-glucosidase, and (5) anti-mutagenic activity.7–9 Thus, it is fascinating to do further study on anthocyanin due to its great potential in preventing numerous diseases related to unhealthy lifestyle along with its complication condition such as dyslipidemia, obesity, diabetes mellitus (DM) type 2, and other metabolic diseases.
Several approaches such as diet program, physical activity, and medication has been done to overcome dyslipidemia condition. However, many patients with this condition could not keep their persistency in continuing the treatment program. Therefore, another approach such as utilizing functional food to help in dyslipidemia treatment is needed. Functional food is a food product that has an improved nutritional values beyond its traditional nutrients, sensory profile, physiochemical and rheological characteristics as well as to provide therapeutic properties. Yogurt is one of functional food with combination of probiotic fermentation and natural ingredient (prebiotic) which is called synbiotic. Probiotic that commonly used in yogurt production is lactic acid bacteria (LAB), in which known to have positive effect in preventing hypercholesterolemia and obesity.10,11
There are many modifications in yogurt production process. The high content of antioxidant particularly anthocyanin make it’s quite intriguing to explore more on the effect of purple sweet potato as prebiotic element of yogurt. With the fortification of purple sweet potato into the yogurt, it is expected to show more positive effect on treating dyslipidemia. Thus, this experimental study aims to identify the effect of purple sweet potato yogurt (PSPY) consumption towards visceral fat weight and lipid profile on mice with high fat diet condition.
Material and Methods
Purple Sweet Potato Yogurt (PSPY) Making Process
Lactic acid bacteria (LAB) strains used in development of starter PSPY including: Lactobacillus bulgaricus ATCC 11842, Lactobacillus acidophilus ATCC 4356, and Bifidobacterium longum. These probiotics were provided and confirmed to be used in these study of PSPY by Advanced Biomedical Laboratory, Division of Microbiology and Parasitology Laboratory, Faculty of Medicine, Universitas Padjadjaran, Indonesia. Two streaks of inoculating loop of each bacteria strain was inoculated into 100 mL of commercial low fat pasteurized milk from PT UltraJaya Milk Industry, and then incubated in 5% CO2 incubator at 37oC for 24 hours. After incubation, starter culture from all bacteria strains was combined along with milk (10% v/v) and incubated at 37oC for 24 hours. The fermented product became mother culture of purple sweet potato yogurt (PSPY).
Purple sweet potatoes were obtained from agricultural area in Tanjung Sari, Sumedang, West Java, Indonesia. Five grams of purple sweet potato was washed and then steamed until tender. Following that, 3 grams of steamed purple sweet potato was peeled and cut into small pieces before it was mashed into puree using blender. During the blending process, warm water was added into the purple sweet potato with ratio 1:1. After that, Purple sweet potato puree was filtered using cotton fabric until purplish liquid was separated from the puree. The filtrate was then combined with low fat pasteurize milk (60% v/v) and 10% v/v of starter culture of combined bacteria. Lastly, the fortified milk was incubated at 37oC for 24 hours until it was fermented into yogurt product (Figure 1).
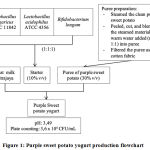 |
Figure 1: Purple sweet potato yogurt production flowchart |
Animal Preparation
This experimental study used male mice (Mus musculus) Balb/c from Laboratory of PT. Bio Farma, West Java, Indonesia. The mice were acclimated in a cage size 41,5 x 29,5 x 20 cm with all its condition during night and day as well as its food and drink have been approved by Research Ethical Committee of Universitas Padjadjaran No. 234/UN6.KEP/EC/2019. Adaptation of the animal by providing standardized diet was done for 7 days before intervention experiment.
Purple Sweet Potato Yogurt (PSPY) Intervention
Total of 30 male mice (4-6 months old) with each weight around 20-35 gram were divided into 6 groups with different treatment on its diet: [1] standard diet (SD), [2] high fat diet (HFD), [3] HFD with 0.3 mL of PSPY [4] HFD with 0.5 mL PSPY, [5] HFD with 1 mL PSPY, [6] HFD with simvastatin. In the first 3 weeks, group 2 until 6 were given HFD treatment to induce dyslipidemia condition. Subsequently, each group was treated by respective arranged diet in the next 9 weeks. The standard diet and high fat diet used in this experimental study was obtained from Animal Model Development Division of Pharmacy Study Program, Institut Teknologi Bandung (ITB), Indonesia. The formula was modified from previous research12 with detail composition as shown in table 1.
Table 1: Standard Diet (SD) and High Fat Diet (HFD) Composition
| No. | Analysis Parameters | Analysis result | Result Unit | Testing Method | ||
| SD | HFD | |||||
| 1. | Moisture | 9.23 | 4.74 | % | SNI-01-2891-1992 | |
| 2. | Ash content | 16.53 | 9.19 | % | ||
| 3. | Protein content | 13.63 | 13.96 | % | ||
| 4. | Fat content | 6.84 | 35.25 | % | ||
| 5. | Carbohydrate content | 53.77 | 36.88 | % | By Difference | |
| 6. | Total Calories | 331.16 | 520.61 | Kkal | – | |
Body Weight Measurement
During the experiment, body weight of the mice was measured every week using digital precision balance, brand ACS, AD-300i. The result was recorded in gram unit.
Evaluation of Cholesterol, Triglyceride, and LDL Level.
Blood were harvested from retro-orbital venous sinus for investigation of total cholesterol level, triglycerides level, and LDL level. The experiment was done by using enzymatic colorimetric assay.
Cholesterol level was determined by using Cholesterol Kit Assay (LABKIT, LKBSDTT48, Chemelex SA, Barcelona). Total of 10µL of plasma was mixed with reagent (PIPES pH 6.9, phenol, cholesterol esterase, cholesterol oxidase, 4-aminoantipyrine) and incubated at room temperature for 10 minutes. The result was read by using spectrophotometer at wavelength 500-550 nm for 60 minutes.
Triglycerides were examined by using TG Kit (DUMOLAB, D95380). Total of 10µL of plasma was mixed with reagent (Good’s buffer pH 7.2, 4-chlorophenol, Mg2+, ATP, glycerol kinase, peroxidase, lipoprotein lipase, 7-aminoantipyrine, glycerol 3-phosphate-oxidase) and incubated at 37oC for 10 minutes. The result was read using spectrophotometer at wavelength 500-546 nm for 60 minutes.
LDL level was examined using LDL Cholesterol Kit Assay (LABKIT, LKDSDTT51, Chemelex SA, Barcelona). Total of 4µL plasma was mixed with reagent 1 (PIPES pH 7.0, cholesterol esterase, cholesterol oxidase, catalase, N-Ethyl-N-(2-hydroxy-3-sulfopropyl)-3-methylanine) and incubated at 37oC. After 5 minutes of incubation, 100 mL of reagen 2 (PIPES pH 7.0, 4-Aminoantipyrine, peroxidase) was added into the mixture and incubation was continued for another 5 minutes. Result was read using spectrophotometer at wavelength 600nm.
Measurement of Visceral fat and Liver Weight
In the end of the experiment, visceral fats and liver were harvested to undergo weight measurement. Visceral fats were harvested from abdominal and epididymis area. The weight measurement was performed by using digital precision balance brand ACS, AD-300i.
Intestinal Lactic Acid Bacteria Identification and Measurement
Proximal part of jejunum was harvested from mice of each group. Around 4-6 cm of jejunum was cut and put into physiological NaCl 0.9% for identification of lactic acid bacteria characteristic in mice’s intestine. After that, the sample was chopped into little pieces and put into sterile vial filled with deMan, Rogosa, Sharpe (MRS) broth (OXOID, CM0359). Then the sample was stored at 37oC for 24 hours. Following that, total plate count (TPC) was done on bacteria culture from the sample. Dilution of 1 mL sample was done 6 times (10-1, 10-2, 10-3, 10-4, 10-5, 10-6). Each dilution was then cultured on MRS agar (OXOID, CM0361) and incubated for 24-48 hours. After that, colony number (CFU/mL) of each sample was calculated.
The presence of lactic acid bacteria was identified by using catalase test. Bacteria was taken using inoculation loop and then mixed into 3 mL of physiological NaCl 0.9%. After that, one drop of H2O2 was put on the sample. The formation of bubble on the sample upon addition of H2O2 will determines the presence of bacteria other that lactic acid bacteria.13
Gram staining (ST-REAGENSIA, 055-0746, Indonesia) was done on bacteria culture for morphological identification of the intestinal bacteria of mice models. Bacterial colony was taken using inoculation loop and mixed with 1 drop of physiological NaCl 0.9% on object glass and then let it air dry followed by gram staining with sequence: Crystal violet as primary stain, lugol, alcohol, and lastly safranin as counter stain. Morphology of the stained bacteria was observed using microscope Olympus CX23LEDRF51 with serial number 5M8758120152.
Statistical Analysis
Presented data is Standard Error Mean ( SEM). Results were analyzed using one-way ANOVA and post hoc tukey multiple comparison test using GraphPad-Prism 7. p values < 0.05 considered significant.
Results
Intestinal Lactic Acid Bacteria Colony Profile
After purple sweet potato yogurt (PSPY) intervention, microbiota from intestine were cultured on MRS agar to identify and calculate the amount of lactic acid bacteria. The result revealed that total amount of lactic acid bacteria colony from HFD group relatively lower compare to SD group. From the figure 2A, in can be determined that administration of 0.3 mL of PSPY could significantly increase the growth of lactic acid bacteria in the intestine. In addition, catalase test and gram staining experiment of the bacterial culture showed bacillus form of gram negative bacteria (Figure 2B and 2C).
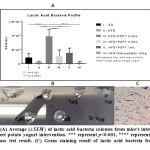 |
Figure 2: (A) Average ( of lactic acid bacteria colonies from mice’s intestine upon purple sweet potato yogurt intervention. |
Purple Sweet Potato Yogurt (PSPY) Effect on Inhibiting the Weight Gain
Elevation of body weight on mice group with High Fat Diet significantly higher compared to mice group with standard diet. High content of fats and calories from the diet contributed in body weight elevation as it increased fat deposit. This study showed that administration of PSPY on high fat diet mice group could inhibit the elevation of body weight (Figure 3.).
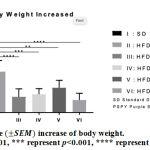 |
Figure 3: Average increase of body weight. |
Purple Sweet Potato Yogurt (PSPY) Effect on Improvement of Lipid Profiles
Group with high fat diet intake demonstrate the elevation of circulating lipid profile including cholesterol, triglycerides, and LDL level compared to group which took standard diet. However, consumption of PSPY by group which took HFD showed the improvement of its lipid profiles. The 3rd group showed the most favorable result in lipid profile improvement even compare to positive control group (treated with simvastatin). The result also showed indication of increasing of lipid profiles along with the increase of the dosage of yogurt administered. Nevertheless, the improvement of lipid profile in the group treated by in general can be seen (Figure 4.).
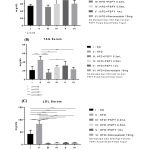 |
Figure 4: (A) Cholesterol, (B) triglycerides, and (C) LDL levels of each experimental group. |
Purple Sweet Potato Yogurt (PSPY) Effects on Visceral Fats and Liver Weight
The weight of visceral fats and liver of HFD group were significantly higher compared to the SD group. The 3rd experiment group showed the most favorable outcome in term of reducing liver and visceral fat weight even compared to control positive group treated by simvastatin However, this experiment also revealed the increasing of visceral fat and liver organ following the increase dose of yogurt treated to group 4 and 5 (Figure 5).
 |
Figure 5: Comparison of visceral fat weight and liver fat weight between experimental groups. |
Discussion
Probiotic used in fermentation process had been reported to contribute in formation of nutritional component that is beneficial for health, such as protein, vitamin, and antioxidant.14 In addition, fortification of yogurt using various natural ingredients had been done a lot.15-18 This kind of fortification was expected to help improve the health benefit of yogurt for its consumer. Nutrition fact screening has been done on purple sweet potato yogurt (PSPY) used in this experimental study. The result of the screening revealed some amount of anthocyanin content, a unique characteristic of purple sweet potato, which was a type of flavonoid that had antioxidant activity.8,15,18 The amount of anthocyanin content in the PSPY stated in nutritional fact screening was 143.06mg/dL. Further, acidity level as well as total colony of bacteria in the yogurt product had been evaluated and both results were within normal range.
The finding of this study showed significant result on the improvement on lipid profile including total cholesterol, triglycerides, and LDL level (p<0.05) due to anthocyanin composition in the purple sweet potato yogurt. Previous studies reported the effect of anthocyanin from fruits in lowering de novo fatty acids synthesis, which is suspected due to the activation of AMP-activated protein kinase (AMPK).18,19 The activation of AMPK caused the inactivation of malonyl CoA decarboxylase (MCD), which led to the decreased of malonyl CoA level. Malonyl CoA itself was essential in de novo synthesize of fatty acids. Thus, de novo fatty acid synthesis in the liver was reduced due to the low level of malonyl CoA.20 Therefore, the activation of AMPK led to the inhibition of fatty acid synthesis as well as the oxidation of fatty acid. Thus, it was suggested that the anthocyanin from purple sweet potato could reduce de novo synthesize of fatty acid.
This study revealed that high fat diet group had significantly lower total lactic acid bacteria colony compared to other experimental groups. This finding was consistent with previous study by Turnbaugh and his colleagues, in which they also found lowering amount of 50% of Firmicutes microorganism such as Lactobacillus in obesity related diet study.19 Another study also reported that high fat diet intake decreased the composition of Bacteroidetes colony compared to low fat diet group.20
This study also found the decreasing of lactic acid bacteria colony in the mice intestine of the group which consumed higher dosage of PSPY. The possible reason for this finding was because in higher dose group, the bacteria created engagement with villi of small intestine where it propagated further until it exceeded its stationer phase. In this condition, the growth rate of the bacteria would be equal with its death rate, which means the colony would enter the death phase. Thus, the result of lower bacteria colony in higher dose group occur.21
Consumption of fermented milk would alter the gut microflora. The consumption of probiotic fermented milk and yogurt could increase colony amount of Bifidobacterium in adult human.22 Colony number of lactic acid bacteria was significantly high in the group that consume PSPY with dosage 0.3 mL/20 mg of mice’s body weight compared to other groups. This discovery showed that purple sweet potato could promote proliferation of lactic acid bacteria in high fat diet group study.
Probiotic which entered digestive system would be exposed to different type of environment including the acidity level of the environment. Therefore, only bacteria which could survived the harsh condition could reach and colonized in the intestine and colon.23 The isolation of microflora from human intestine had been done numerously and the most common type of bacteria identified was lactic acid bacteria. It is hypothesized that lactic acid bacteria had the tendency to be able to reach small intestine after underwent harsh environment condition changes in gastrointestinal track.24 Lactic acid bacteria harvested from mice small intestine was identified to be classified as bacillus gram positive bacteria with negative result on catalase test. These results was corresponded with the characteristic of lactic acid bacteria isolated from milk product.25 This study also demonstrated that effect of PSPY product in inhibiting weight gain, and decreasing visceral fat and liver weight in high fat diet mice model.
Conclusion
The best dosage of purple sweet potato yogurt to improve health condition of high fat diet mice model was 0.3 mL/20 gram of mice weight. The group treated with this dosage showed favorable outcome in term of total colony number of lactic acid bacteria, improvement of lipid profiles including total cholesterol, triglycerides and LDL level, with better visceral fat and liver weight. However, the product did not reveal anti-obesity effect due to insignificant body weight changed of the experimental model. Further study is needed to explore more detail on its molecular mechanism and also the toxicity dosage of the purple sweet potato yogurt product.
Acknowledgement
The authors thank to Neni Anggraeni and Irfan Anis Ahmad for technical assistance in Animal Laboratory of Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran. We are grateful to Widad Aghnia Shalannandia for her assistance, and also all technician in Advanced Biomedical Laboratory, Division of Microbiology and Parasitology Laboratory, Faculty of Medicine, Universitas Padjadjaran for all of the support during this experimental study.
Conflict of Interest
No conflict of interest has been declared by the authors.
Funding Source
The funding of this experimental study was supported by Ministry of Research and Technology/National Research and Innovation Agency; grant number: 3854/UN6.C/LT/2019
References
- Huriyati E, Luglio HF, Ratrikaningtyas PD, Tsani AFA, Sadewa AH, Juffrie M. Dyslipidemia, insulin resistance and dietary fat intake in obese and normal weight adolescents: The role of uncoupling protein 2 -866G/A gene polymorphism. Int J Mol Epidemiol Genet. 2016;7(1):67–73.
- Kang YJ, Wang HW, Cheon SY, Lee HJ, Hwang KM, Yoon HS. Associations of Obesity and Dyslipidemia with Intake of Sodium, Fat, and Sugar among Koreans: a Qualitative Systematic Review. Clin Nutr Res. 2016;5(4):290.
CrossRef - Anderson M, Aron D, Badell ML. Greenspan’s Basic & Clinical Endocrinology. 9th ed. Gardner DG, Shoback D, editors. McGraw Hill Medical Companies; 2011. 845–847 p.
- Li J, Wang J, Jia H, Cai X, Zhong H, Feng Q, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32(8):834–41.
CrossRef - Neish AS. Microbes in Gastrointestinal Health and Disease. Gastroenterology [Internet]. 2009;136(1):65–80. Available from: http://dx.doi.org/10.1053/j.gastro.2008.10.080
CrossRef - Baroutkoub A, Mehdi RZ, Beglarian R, Hassan J, Zahra S, Mohammad MS, et al. Effects of probiotic yoghurt consumption on the serum cholesterol levels in hypercholestromic cases in shiraz, southern iran. Sci Res Essays. 2010;5(16):2206–9.
- Tanaka M, Ishiguro K, Oki T, Okuno S. Functional components in sweetpotato and their genetic improvement. Breed Sci. 2017;67(1):52–61.
CrossRef - Cuevas E, Silke M, Peter H. Anthocyanins in Purple Sweet Potato ( Ipomoea batatas L .) Varieties. Fruit, Veg Cereal Sci Biotechnol. 2011;0–5.
- Kano M, Takayanagi T, Harada K, Makino K, Ishikawa F. Antioxidative activity of anthocyanins from purple sweet potato, Ipomoea batatas cultivar Ayamurasaki. Biosci Biotechnol Biochem. 2005;69(5):979–88.
CrossRef - Choi WJ, Dong HJ, Jeong HU, JUng, HH, Kim Y, Kim TH. Antiobesity effects of Lactobacillus plantarum LMT1-48 accompanied by inhibition of Enterobacter cloacae in the intestine of Diet-Induced Obese Mice. J Med Food. 2019;22(6):560-566
CrossRef - Fazilah NF, Ariff AB, Khayat ME, Rios-Solis L, Halim M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemical on the formulation of functional yogurt. Journal of Functional Foods. 2018;48:387-399.
CrossRef - Lauterio TJ, Bond JP, Ulman EA. Development and Characterization of a Purified Diet to Identify Obesity-Susceptible and Resistant Rat Populations. The Journal of Nutrition. 1994;124(11):2172–2178.
CrossRef - Ismail YS, Yulvizar C, Mazhitov, B. Characterization of lactic acid bacteria from local cow’s milk kefir. IOP Conf. Ser: Earth Environ. Sci. 2018;130:012019
CrossRef - Williamson C. Functional foods: what are the benefits? Br J Community Nurs. 2009;14(6):230–6.
CrossRef - Yang Z wei, Tang C e., Zhang J liang, Zhou Q, Zhang Z cheng. Stability and antioxidant activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) subjected to simulated in vitro gastrointestinal digestion. Int J Food Sci Technol. 2019;54(8):2604–14.
CrossRef - Chen X, Singh M, Bhargava K, Ramanathan R. Yogurt Fortification with Chickpea (Cicer arietinum) Flour: Physicochemical and Sensory Effects. JAOCS, J Am Oil Chem Soc. 2018;95(8):1041–8.
CrossRef - Islam M, Mazed M, Rahman S, Akter M. Study of Flavoured Dahi Incorporated with Banana Extract. J Environ Sci Nat Resour. 2015;6(1):153–8.
CrossRef - Wicaksono L, Yunianta, Widyaningsih T. Anthocyanin Extraction from Purple Sweet Potato Cultivar Antin-3 (Ipomoea batatas L.) using maceration, microwave assisted extraction, ultrasonic assisted extraction, and Their Application as Anti-Hyperglycemic Agents in Alloxan-Induced Wistar Rats. Int J PharmTech Res. 2016;9(3):181–92.
CrossRef - Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31.
- Zhang M, Yang XJ. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J Gastroenterol. 2016;22(40):8905–9.
CrossRef - Bertranda RL. Lag phase is a dynamic, organized, adaptive, and evolvable period that prepares bacteria for cell division. J Bacteriol. 2019;201(7):1–21.
CrossRef - Redondo-Useros N, Gheorghe A, Díaz-Prieto LE, Villavisencio B, Marcos A, Nova E. Associations of probiotic fermented milk (PFM) and yogurt consumption with bifidobacterium and lactobacillus components of the gut microbiota in healthy adults. Nutrients. 2019;11(3).
CrossRef - Derrien M, van Hylckama Vlieg JET. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol [Internet]. 2015;23(6):354–66. Available from: http://dx.doi.org/10.1016/j.tim.2015.03.002
CrossRef - Kok CR, Hutkins R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr Rev. 2018;76:4–15.
CrossRef - Setyawardani T, Rahayu WP, Maheswari R, Palupi N. Identification and Characterization of Probiotic Lactic Acid Bacteria Isolated from Indigenous Goat Milk Identification and Characterization of Probiotic Lactic Acid Bacteria Isolated from Indigenous Goat Milk. Anim Prod. 2011;13(1):57–63.







