Jyotirmayee Bahinipati1* and Rg Asutosh Mohapatra2
1Department of Biochemistry, Kalinga Institute of Medical Sciences, Bhubaneswar,751024, Odisha.
2Department of Orthopedics, SCB MCH, Cuttack,751024, Odisha.
Corresponding Author E-mail : jyotirmayee.bahinipati@kims.ac.in
DOI : https://dx.doi.org/10.13005/bpj/1894
Abstract
Low back pain is the leading cause of disability, interferes with the quality of life and affects the work performance. In most of them precise anatomic cause cannot be localized. LBP has been associated with lower levels of vitamin D. Magnesium is a N- methyl-D – Aspartate receptor antagonist, and hence has a role in management of chronic pain. Magnesium and vitamin D act in a coordinated manner. Its therefore essential to maintain adequate amount of magnesium for optimal benefits of vitamin D. Hence, this study is an attempt to measure serum vitamin D and magnesium and find their correlation in chronic LBP. Total 192 chronic LBP patients were recruited for the study. They were further divided into three groups according to their vitamin D levels ( ³ 30 ng/ml = Normal, 21-29 ng/ml=Insufficient, <20 ng/ml=Deficient). BMI, Serum vitamin D, magnesium, calcium, phosphorus was measured. Intensity of pain was measured by Visual analog scale. Correlation was found between serum vitamin D and serum magnesium in chronic LBP patients. Mean age in the study group was 46.4±9.43 years with female predominance (61.22%). BMI increases with decrease in serum vitamin D (p<0.001). 52.04% of chronic LBP were vitamin D deficient. Serum Magnesium in three groups were (1.90±0.11 mg/dl ,1.83±0.02 mg/dl ,1.76±0.34 mg/dl) respectively. The pain intensity as measured by VAS score was significantly higher with decrease in vitamin D levels. There was statistically significant relationship between serum vitamin D and serum magnesium in LBP. Hence, chronic LBP patients have significant decrease in vitamin D and serum magnesium levels. As there is significant correlation between vitamin D and serum magnesium in LBP, magnesium can play an important supplemental role in management of chronic LBP with vitamin D deficiency. This finding can be further confirmed by large sample prospective studies.
Keywords
BMI; Chronic LBP; Magnesium; Vitamin D, VAS
Download this article as:| Copy the following to cite this article: Bahinipati J, Mohapatra RG. A. Serum Magnesium and Vitamin D in Patients Presenting to the Orthopedics Out-Patient Department with Chronic Low Back Pain. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Bahinipati J, Mohapatra RG. A. Serum Magnesium and Vitamin D in Patients Presenting to the Orthopedics Out-Patient Department with Chronic Low Back Pain. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/39SQbwt |
Introduction
Low back pain(LBP) has now become major cause of disability in both developed and developing countries affecting the general well-being and performance at the work place imposing a high economic burden on individuals, families, communities and government. [1]. LBP is experienced in 60-80% of adults at some point in their lifetime [2]. The estimated worldwide lifetime prevalence of LBP varies from 54% to 84% [3]. The occurrence of LBP in India is alarming with nearing to 60% of people in India suffering from LBP at some time in their life span [2]. 2010 global burden of disease study estimated LBP among top 10 diseases that account for highest number of DALY worldwide [4].
LBP is generally not a disease but constellation of symptoms with an obscure cause of onset and difficulty in diagnosis. LBP restricts the mobility, interferes with normal functioning and results in lifelong pain and permanent disability. Less than 15% of patients with LBP have a specific cause behind the pain [5]. Numerous interventional studies have been done to investigate the cause behind non- specific LBP [6,7]. In addition to it, various conservative pharmacological interventions have also been tried [8]. At present LBP is mainly treated with analgesics. As the world population ages, the disease burden will also substantially increase.
Supplementation of vitamin D in LBP has gained interest in recent scenario because of its anti-inflammatory and neuromodulatory properties. Evidences have suggested that intracellular vitamin D receptors are present in skeletal muscle tissue mediating vitamin D response. Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyper innervation. There is rise in inflammatory markers in vitamin D deficient individuals and vitamin D is linked with reducing inflammation and LBP [9].
Adequate balance of magnesium and vitamin D is essential for maintaining the physiologic functions. Vitamin D and magnesium interact in a coordinated manner. Magnesium is needed for enzymatic activity of 25 hydroxylase and 1 ahydroxylase to form active form of vitamin D, in turn vitamin D helps in intestinal magnesium absorption [10]. The magnesium content of our diet is slowly declining because of food processing and soil conditions. Even use of fertilizers, pesticides change the soil quality, and in turn are reducing the magnesium levels [11]. Serum magnesium deficiency results in decrease in active form of vitamin D. Studies have shown the hypovitaminosis D associated risk of mortality could be modified by consumption of magnesium [11]. A positive association has been found between dietary magnesium intake and BMD (Bone Mineral Density) [12].
Current evidences have supported the role of magnesium in blocking central sensitization through its effect on N-methyl D Aspartate (NMDA) receptors. It blocks the neuronal influx of calcium through these receptors and prevents potentiation of pain signaling [13].
Many studies have suggested the role of vitamin D supplementation in low back pain and link has been found between serum vitamin D and magnesium [9,11]. Hence along with vitamin D there may be supplemental role of magnesium in LBP. So, this study is an attempt to find out the association between serum vitamin D and magnesium in individuals presenting to the outpatient department with the chief complaint of LBP.
Materials and Methods
This is a cross- sectional study conducted for a period of 1 year (August 2018-August 2019) in the Department of Biochemistry in collaboration with the Department of Orthopedics of Kalinga Institute of Medical Sciences.
Inclusion Criteria
Patients with age group 25-65 years presenting to the Orthopedics Outpatient Department with back pain were included for the study purpose.
Exclusion Criteria
Individuals with hepatic disorders, renal disorders, alcoholics, pregnant women, recent history of surgery or hospitalization and those taking drugs affecting the bone metabolism were excluded from the study. Patients with clinico-radiological evidences of rheumatoid arthritis, fractures, tuberculosis of spine, prolapse intervertebral disc, lordo-scoliosis were also excluded from the study.
Consent and Ethical Consideration
Informed consent was taken from all the study participants after proper explanation of the study objectives. Institutional ethical clearance was obtained before the commencement of the study.
Data Collection and Investigations
Total 196 patients presenting with chronic back pain (duration of pain >12 weeks) were recruited for the study purpose. Weight and height of the study participants were recorded and BMI (Body Mass Index) was calculated. 5 ml of venous blood sample was collected to estimate serum calcium, phosphorus, magnesium and vitamin D. Serum calcium was done by O- Cresolpthelein method and serum phosphorus was done by ammonium phosphomolybdate method in Cobas 400 plus autoanalyzer by Roche. Serum Magnesium was done in Cobas Integra (Roche) by Chlorophosphonazo III methodology. Vitamin D was analyzed by electrochemiluminiscence method in Cobas e411 (Roche). X ray of dorsolumbosacral spine in both AP (Anterior – Posterior) and lateral view were taken. MRI of Lumbosacral spine with screening of whole spine was done in selected cases to establish diagnosis and exclude any pathology.
According to the levels of vitamin D the patients were sub-grouped into three categories (Normal (Vitamin D = >30ng/ml), Insufficient (Vitamin D=21-29ng/ml) and Deficient (Vitamin D= < 20ng/ml). Intensity of pain was evaluated as nominal data which was determined by a 10cm visual analog scale (VAS). Patient with no pain was as “0” and where the pain is most severe was shown as “10”.
Statistical Analysis
All the statistical analyses were done by STATA software version 12. Descriptive statistics were presented as mean, standard deviation, percentage. To compare frequencies chi square test was used. Normality was tested by Kolmogorov Smirnov test. Association between vitamin D and magnesium was done by Pearson’s correlation coefficient. Statistical significance was accepted at p<0.05.
Results
Table 1 shows: Mean Age in the study group was 46.4±9.43 yrs. There were 38.77% males and 61.22% females in the study group. BMI in the study group was higher than normal with significant difference of BMI among the three groups. Serum calcium and phosphorus were significantly decreased. There was decrease in serum magnesium in all the groups with significant decrease in serum vitamin D. Mean vitamin D in normal group was 39±6.92ng/ml, Insufficient 23.31±3.23ng/ml and in deficient group was 9.63±6.21ng/ml. Pain scoring was significantly increased in deficient group.
Table 1: Demographic and biochemical parameters in the study group
| Parameters | Normal
(n=35) |
Insufficient
(n=59) |
Deficient
(n=102) |
p value | |
| Age(years) | 45.23±2.34 | 45.23± 6.66 | 46.67±2.34 | NS | |
| Gender | Male | 12 | 22 | 42 | NS |
| Female | 23 | 37 | 60 | ||
| BMI (Kg/m2) | 26.04±1.81 | 27.12±2.01 | 29.34±2.32 | <0.05 | |
| Calcium (mg/dl) | 9.12±2.12 | 8.75±2.00 | 7.89±2.31 | <0.001 | |
| Phosphorus(mg/dl) | 3.71±0.17 | 3.10±0.12 | 2.1±0.72 | <0.05 | |
| Magnesium(mg/dl) | 1.90±0.11 | 1.83±0.02 | 1.76±0.34 | <0.05 | |
| Vitamin D | 39±6.72 | 23.21±3.23 | 9.63±6.21 | <0.001 | |
| VAS Score | 4.34±0.51 | 5.12±1.11 | 7.88±1.25 | <0.001 | |
Table 2 shows that maximum participants in the study group complaining of low back pain (52.04%) were vitamin D deficient.
Table 2: Vitamin D deficiency in the study population
| Vitamin D | % Distribution |
| Normal (n=35) | 17.85 |
| Insufficient (n=59) | 30.11 |
| Deficient (n=102) | 52.04 |
Figure 1 shows that there is statistically significant correlation between vitamin D and serum magnesium levels in participants presenting with low back pain.Table 3 shows that there is significant correlation of vitamin D and Magnesium with VAS scores.
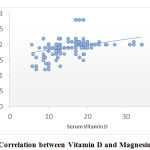 |
Figure 1: Correlation between Vitamin D and Magnesium in LBP |
Table 3: Correlation of Vitamin D and serum magnesium with VAS scores.
| VAS score | ||
| Parameters | r value | p value |
| Vitamin D | -0.43 | <0.001 |
| Mg | -0.61 | <0.001 |
Discussion
Pain localized below the costal margin and above the inferior gluteal folds is defined as the low back pain. With more than 12 weeks of persistent pain is referred to as the chronic LBP [14].
The mean age in our study participants was 46.4±9.43 yrs. In the study done by Ghai B et al, they found that the mean age group of 43.8 years [15]. Similarly, Lodh et al in their study found that 42% of their participants were of age group within 31-50 years with mean age of 46.19±15.69 years [9]. Hence, patients presenting to the OPD with the complain of Chronic low back pain without any precise anatomical cause are maximally presenting at middle ages. Our study suggested female predominance with (61.5%). Calik Y et al in their study also found, out of 145 patients 71% were females and 29% were males [16]. Lodh et al also found female gender predominance [9]. Hence, females mostly in their peri-menapausal age group predominantly present with chronic low back pain. Poomalar et al in their study found that 80% of females presenting with LBP were of peri-menapausal age group [17]. Biological response to pregnancy and childbearing, physical stress of child rearing and abdominal weight gain may be attributing to the cause behind increase prevalence of LBP among this group [18]. Besides, estrogen deficiency during this period may be leading to collagen wasting.
BMI in our study participants increased with decrease in vitamin D levels. Kumaratna M et al found in their study that higher BMI levels were associated with lower vitamin D levels [19]. In our study, vitamin D deficiency had higher BMI and VAS scores which was statistically significant. Su Ca et al and Choudhary D et al found elevated BMI is strongly associated with increase prevalence of vitamin D deficiency [20,21]. Obesity is recognized to be associated with vitamin D deficiency as adipose tissue sequesters 25(OH) Vitamin D, resulting in inverse relationship between vitamin D and BMI. Serum calcium and phosphorus decreased significantly in our participants with decrease in vitamin D. Lodh et al in their study found 6% of patients had calcium less than 8.5 mg/dl with a mean of 8.39±0.91mg/dl [9]. Calcium even showed a positive correlation with vitamin D levels. Similarly, serum phosphorus was also within lower range in low back pain patients (3.56±1.20 mg/dl).
Vitamin D deficiency in our study was seen in 52.04% and insufficiency in 30.11 %. Calik Y et al had vitamin D deficiency in 22.8% and insufficiency in 42.8% [16]. R Kain H et al found vitamin D deficiency was significantly more common in patients suffering from chronic low back pain compared to the controls (79% vs 61.4%, p< 0.05) [22]. Panwar et al also found that severe forms of vitamin D deficiency may be causally associated with chronic and subacute low back pain [23]. Meta-analysis by Babita G et al showed a strong association between LBP and low vitamin D levels [24].
Regeneration of nerve cells may be affected due to inadequate vitamin D levels. Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyper-innervation. Inadequate vitamin D induced inflammation may exacerbate pain, impairs healing and causes further structural damage and increase in pain intensity. Neuromuscular dysfunction can cause the pain to become chronic [9].
Serum magnesium in our study, significantly decreased with decrease in vitamin D levels. There was a significant positive correlation between serum magnesium and vitamin D levels. Kelishadi R et al found significant relationship between serum magnesium and vitamin D by linear regression analysis in a large nationally- representative sample [25]. Uwitonze AM et al in their survey explored that magnesium intake alone or in combination with vitamin D may improve the vitamin D status [10]. Similarly, Vojtkova et al found type I diabetic children with deficient vitamin D have lower serum magnesium in their blood [26].
Magnesium and vitamin D interact in a co-ordinated manner. 1,25(OH)2 D stimulates intestinal magnesium absorption. Magnesium acts as a cofactor for vitamin D binding protein and help in activation of vitamin D as 25 hydroxylation and renal 1a hydroxylation are magnesium dependent processes [10].
Lower serum magnesium in our study was significantly associated with intensity of chronic LBP. Similar findings were also observed by Yousef et al [27]. They investigated the use of iv magnesium followed by oral magnesium and compared with placebo in chronic LBP over 6 months and found that the group receiving magnesium treatment had significantly improved pain scores. Kirkland AE et al also found the role of serum magnesium in pain relief. Pain relieving effects of magnesium is dependent upon the blockade of NMDA receptors in the spinal cord. Hence, magnesium is thought to produce anti-nociceptive and analgesic effects in patients with chronic pain [13].
Hence, supplementation of serum magnesium along with vitamin D may alleviate the levels of vitamin D and may improve pain in chronic LBP patients with vitamin D deficiency.
This study was a cross- sectional study and done in a smaller sample so, further prospective studies in a larger number of patients should be done to further prove the role of magnesium supplementation in chronic LBP.
Acknowledgements
We are immensely grateful to all the participants who gave their consent for the study purpose. We thank the technicians of Biochemistry section of Central laboratory for their assistance.
Conflict of Interest
The authors declare that they do not have any conflict of interest.
Funding Source
There are no funding sources.
References
- Stewart Williams J, Ng N, Peltzer K, Yawson A, Biritwum R, Maximova T, et al.Risk Factors and Disability Associated with Low Back Pain in Older Adults in Low- and Middle-Income Countries. Results from the WHO Study on Global AGEing and Adult Health (SAGE). PLoS ONE.2015; 10(6): e0127880.
CrossRef - Ganesan S, Acharya A S, Chauhan R, Acharya S. Prevalence and Risk Factors for Low Back Pain in 1,355 Young Adults: A Cross-Sectional Study.Asian spine journal.2017; 11(4): 610–617.
CrossRef - Freburger JK, Holmes G M, Agans RP, Jackman A M, Darter JD, Wallace AS et al. The rising prevalence of chronic low back pain.Archives of internal medicine.2009; 169(3), 251–258.
CrossRef - Hurwitz HL, Randhawa K, Yu H, Cote P, Haldeman S. The Global Spine Care Initiative: a summary of the global burden of low back and neck pain studies. European spine Journal. 2018.https://doi.org/10.1007/s00586-017-5432-9.
CrossRef - Pereira M G, Roios E, Pereira M. Functional disability in patients with low back pain: the mediator role of suffering and beliefs about pain control in patients receiving physical and chiropractic treatment.Brazilian journal of physical therapy.2017; 21(6): 465–472.
CrossRef - Maher C, Underwood M, Buchbinder R. Non-specific low back pain. The Lancet.2017;389(10070):736-747.
CrossRef - Stubbs B, Koyanagi A, Thompson T, Veronese N, Carvalho AF, Solomi M. The epidemiology of back pain and its relationship with depression, psychosis, anxiety, sleep disturbances and stress sensitivity: Data from 43 low and middle-income countries.General Hospital Psychiatry.2016;43:63-70.
CrossRef - Kuijpers T, Middelkoop MV, Rubinstein SM, Osteo R, Verhagen A, Koes BW. A systematic review on the effectiveness of pharmacological interventions for chronic non-specific low back pain. European spine journal.2011;20(1):40-50.
CrossRef - Lodh M, Goswami B, Mahajan RD, Sen D, Jajodia N, Roy A. Assessment of vitamin D status in patients of chronic low back pain of unknown etiology. Indian Journal of clinical Biochemistry. 2015;30(2):174-179.
CrossRef - Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. The journal of American Osteopathic Association. 2018;118(3):181-189.
CrossRef - Marles RJ. Mineral nutrient composition of vegetables, fruits and grains. The context of reports of apparent historical declines. Journal of food composition and analysis. 2017; 56:93-103.
CrossRef - Marj M, Saneei P, Esmaillzadeh A. Dietary magnesium intake, bone mineral density and risk of fracture: a systematic review and meta-analysis. Osteoporosis International. 2016;27(4):1389-1399.
CrossRef - Kirkland AE, Sarlo GL, Holton KF. Role of magnesium in neurological disorders. Nutients.2018;10(6):730.
CrossRef - Vrbanic TS. Low back pain-from definition to diagnosis. Reumatizam.2011;58(2):105-107.
- Ghai B, Bansal D, Kapil G, Kanukula R, Lavudiya S, Sachdeva N. High prevalence of hypovitaminosis D in Indian chronic low back patients. Pain Physician 2015; 18: E853-62
CrossRef - Calık Y, Aygun U. Evaluation of vitamin D levels in patients with chronic low back-leg pain.Acta Orthopaedica et Traumatologica Turcica.2017; 51(3), 243–247.
CrossRef - Poomalar GK, Bupathy A. The quality of life during and after menopause among rural women.Journal of Clinical Diagnosis and Research. 2013; 7: 135–139.
- Wang YX, Wang JQ, Kaplar Z. Increased low back pain prevalence in females than in males after menopause age: evidences based on synthetic literature review.Quantitative imaging in medicine and surgery,6(2), 199–206.
CrossRef - Kumaratne M, Early G,Cisneros J. Vitamin D Deficiency and Association with Body Mass Index and Lipid Levels in Hispanic American Adolescents.Global pediatric health.2017. 4, 2333794X17744141.
CrossRef - Su CA, Kusin DJ, Li SQ, Ahn Um, Ahm Nu. The association between Body mass index and prevalence severity and frequency of low back pain: date from osteoarthritis Initiative. Spine.2018;43(12):848-852.
CrossRef - Chowdhury D, Sarkar S, Rashid MH, Rahaman A, Sarkar SK, Roy R. Influence of Body mass index on low back pain. Mymensingh Medicine Journal. 2014;23(1):125-129.
CrossRef - Rkain H, Bouaddi I, Ibrahim A, Lakhdar T, Abouqal R, Allali F et al. Relationship between vitamin D deficiency and chronic low back pain in post-menapausal women. Current Rheumatology Review. 2013;9(1):63-67.
CrossRef - Panwar, A., Valupadas, C., Veeramalla, M. Prevalence of vitamin D deficiency in chronic and subacute low back pain patients in India: a triple-arm controlled study Clinical Rheumatology. 2018; 37(5): 1367-1374.
CrossRef - Babita G, Vatte R, Boya CS, Bansal D. High Prevalence of Hypovitaminosis D in Patients with Low Back Pain: Evidence from Meta-Analysis. Pain Physician.2018;21(4): E389-399.
CrossRef - Kelishadi R, Ataei E, Ardalan G, Nazemian M, Tajadini M, Heshmat R,et al . Relationship of Serum Magnesium and Vitamin D Levels in a Nationally-Representative Sample of Iranian Adolescents: The CASPIAN-III Study.International journal of preventive medicine. 2014; 5(1): 99–103.
- Vojtkova J, Ciljakova M, Vojarova L, Janíková K, Michnová Z, Sagiová V. Hypovitaminosis D in children with type 1 diabetes mellitus and its influence on biochemical and densitometric parameters.Acta Medica. 2012; 55:18–22
CrossRef - Yousef A, Al-deeb A. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia. 2013; 68: 260–266.
CrossRef







