Meenakshi Bhardwaj, Rutuja Patil, Sugumar Mani, Malarvizhi R and A Hannah Rachel Vasanthi*
Natural Products Research Laboratory, Department of Biotechnology, School of Life Sciences, Pondicherry University, Puducherry, India 605014
Corresponding Author E-mail :hrvasanthi@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1893
Abstract
Systemic manifestations of infection due to bacteria or its toxins cause sepsis. Sepsis ultimately leads to multiple organ dysfunction syndrome and finally ends up in death and there are many experimental models to induce sepsis and study its prognosis. The present study aims to develop and standardize the effective concentration of Lipopolysaccharides (LPS) for induction of sepsis in male and female Sprague Dawley (SD) rat model as part of the refinement of animal protocols reducing pain and reduce stress in experimental rodents. Different concentrations of LPS (5, 10, 15 mg/ml) were administered to male and female rats (i.p.). Physical parameters like body temperature, heart rate and blood pressure along with hematological parameters (WBC, neutrophil, lymphocyte, platelet and hematocrit), oxidative stress markers (SOD, GSH and LPO), inflammatory markers (IL-2, TNF-α, NF-κB) and histopathology analysis were performed to confirm the induction of sepsis and its severity. Blood parameters like WBC, lymphocyte, platelet and hematocrit count significantly reduced whereas neutrophil count significantly increased in male SD rats in 10 and 15mg induction groups after 8h. LPS significantly increased generation of ROS, IL-2, TNF-α and NF-κB expression in the heart tissue at a dose of 10 and 15 mg in male SD rats than the female rats. Significant homogenous expression of blood, biochemical and molecular markers confirmed that 10 mg of LPS is sufficient to induce sepsis in experimental animals thereby reducing the manifestations and stress due to sepsis. However male rats are more suitable as compared to female rats, which might be resistant to such infectious toxins.
Keywords
Inflammation; Interleukin; LPS; Sepsis; TLR4
Download this article as:| Copy the following to cite this article: Bhardwaj. M, Patil. Rutuja, Mani S, Malarvizhi R, Vasanthi A H. R. Refinement of LPS induced Sepsis in SD Rats to Mimic Human Sepsis. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Bhardwaj. M, Patil. Rutuja, Mani S, Malarvizhi R, Vasanthi A H. R. Refinement of LPS induced Sepsis in SD Rats to Mimic Human Sepsis. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/3b2ZLgL |
Introduction
Sepsis is a life-threatening imbalance of physiological and biochemical outcomes caused by a dysregulated response by the host organ or body towards an infection. It is one of the major health concern in developed as well as in developing countries and is found to be accounting for more than $ 20 billion (5.2%) of the total cost of hospitals in the United States (1, 2). Globally, 20 to 30 million patients are estimated to be afflicted every year. In the developing world, sepsis accounts for 60-80% of lost lives in childhood, with more than 6 million neonates and children affected by sepsis annually (3). Although, the true incidences of sepsis are unknown, it is estimated to be one of the leading causes of mortality worldwide (4, 5).
Septic shock, a severe form of host systemic inflammatory reaction to infections, can be caused by bacterial, fungal, viral or parasite infections (6). The most profound evidence of sepsis are observed to be caused by bacteria like Staphylococcus aureus, Streptococcus pyogens, Escherichia coli, Klebshiella spp. and Pseudomonas aeruginosa (7). Various live bacteria and toxemia models which include injection of Lipopolysaccharides (LPS), peptidoglycan, lipoteichoic acid, CpG DNA, zymosan, and synthetic lipopeptides are typically used for induction of sepsis (8). Among these models, LPS causes a severe systemic inflammatory response in the absence of an ongoing infection by the initiation of an inflammatory cascade involving pro and anti-inflammatory mediators, finally leading to sepsis in vitro and in-vivo (9). The endotoxins also mediate cardiovascular changes that mimic sepsis in both experimental animals and human volunteers and are widely accepted. Further, LPS produces significant myocardial dysfunction and cardiovascular collapse. LPS stimulation leads to release of proinflammatory cytokine mediators like TNF-α and IL-6 which initiate a cascade of events leading to sepsis.
Past studies proved that difference in strain of LPS produces variable responses (10) in addition to it, different doses of LPS produce different immune responses (11). As part of the 3Rs principles in animal experimentation, the refinement of study protocols that means reducing the animal suffering as well as reducing experimental variability and enhancing translativity is imperative. This study focuses on the effective doses of LPS for the induction of sepsis in experimental male and female SD rats, thereby minimize the suffering of animals from septic shock. Animal sex and dose standardization study will be helpful in creating a standard model for studying sepsis in-vivo and translating the pathophysiology of sepsis in human beings.
Materials and Methods
Chemicals and Reagents
An Analytical grade EDTA di-potassium salt dehydrate, 2-propanol, chloroform and ethanol were purchased from Merck Lifesciences pvt. Ltd. Mumbai, India, Other reagents like tri-sodium citrate dihydrate, sodium chloride and sucrose were of analytical grade and obtained from Himedia Laboratories, Mumbai, India. LPS from Escherichia coli serotype O127:B8 was purchased from Sigma Aldrich and stored at -20˚C prior to use. Cholesterol and methionine were purchased from SRL SISCO laboratory, Mumbai. Choline chloride and Trizol reagent were purchased from Sigma. High capacity cDNA reverse transcription kit was purchased from Applied Bio systems, CA, USA and Fast start essential DNA green master mix was purchased from Roche Diagnostics GmbH, Mannheim Germany.
Experimental Animals
5-6 week-old male and female Sprague Dawley (SD) rats weighing 110-130 g were procured from Biogen Laboratory for the study. Animals were kept in a limited access rodent facility (CAHF, Pondicherry University) with controlled environmental conditions at a temperature of 20±2˚C, a relative humidity of 30-70% and with 12h light/12h dark cycle. The animals were acclimatized to laboratory conditions for 7 days prior to the initiation of the experiment. Approval from the Institutional Animal Ethics Committee (Approval No PU/CAHF/18th IAEC/2017/05) for the Purpose of CPCSEA was obtained prior to the experiments.
Experimental Designing and Grouping
After the acclimatization to the housing conditions, the animals were randomized into four groups with 12 animals each (n=12, 6 male and 6 female). Phase I study was executed to identify the dose of LPS necessary to induce sepsis in both male and female SD rats and thereby curtail under stress and develop refinement in the protocol.
Phase I
Group 1: HFD control rats administrated with 0.9% saline i.p.
Group 2: HFD + LPS 5 mg/kg b.w./i.p.
Group 3: HFD + LPS 10 mg/kg b.w./i.p.
Group 4: HFD + LPS 15 mg/kg b.w. i.p.
Phase II: Subsequently, in order to identify whether a normal diet or High-fat diet (HFD) has any influence on the induction of sepsis, the most appropriate dose of LPS with specific sex of animal appropriate for sepsis induction based on the results of phase I study was done.
(n=6, Male)
Group 1: Normal Diet rats administrated with 0.9% saline i.p.
Group 2: Normal Diet + LPS 10 mg/kg b.w./i.p.
Group 3: HFD control rats administrated with 0.9% saline i.p.
Group 4: HFD + LPS 10 mg/kg b.w./i.p.
Induction of Sepsis, Blood and Organ Collection
After 8 weeks of HFD, LPS (5, 10 and 15 mg/kg b.w. respectively i.p.) was injected to rats and blood was collected after the 8th h of LPS induction. Blood collection was carried out by retro-orbital venous plexus using heparin-coated glass capillary tubes into 2mL sterile eppendorf tubes. 0.5 ml of blood samples were transferred into anti-coagulant vials (11% tri-sodium citrate). Animals were observed and monitored till 48 h and then sacrificed and tissues were collected for further analysis.
Effect of LPS on Body Temperature and Blood Pressure
Rats were trained for 3 days prior to experimentation. Animals were placed in a restrainer connected to the non-invasive BP apparatus (IITC Life Science). Rectal temperature, arterial blood pressure and heart rate were recorded after 8h of LPS induction.
Hematological Analysis
In order to identify the extent and severity of sepsis induced in the animals, the hematological parameters were checked after both 4th and 8th h of LPS induction. For hematological analysis, blood samples were preserved with 10% sodium citrate to prevent blood coagulation and were analyzed within 1h of blood collection. The hematological parameters such as white blood cell (Total WBC, neutrophil, lymphocyte, monocyte, eosinophil and basophil), hemoglobin (HGB) concentration, hematocrit (HCT) and platelet (PCT) count were performed using a blood cell counter (BC-5000 Vet, Mindray Company, and China).
Assessment of Antioxidant Capacity in Tissues
The enzymatic endogenous antioxidants like Superoxide Dismutase (SOD); non-enzymatic endogenous antioxidants like reduced glutathione (GSH) and oxidative stress markers such as thiobarbituric acid reactive substances (TBARS) were measured in the heart tissue.
Assay of SOD
Superoxide dismutase was assayed by taking 50 µL of 10% tissue homogenate followed by the addition of 0.3 mL of sodium pyrophosphate buffer, 0.025 mL of PMS and 0.075 mL of NBT (Kakkar, Das et al. 1984). The reaction was started by addition of 0.075 mL of NADH. After incubation at 30 °C for 90 seconds, the reaction was stopped by the addition of 0.25 mL glacial acetic acid. Then the reaction mixture was stirred vigorously and shaken with 2.0 mL of n-butanol. The mixture was allowed to stand for 10 minutes followed by centrifugation. The color intensity of the chromogen was read at 560 nm. Results are expressed as Unit/ min/mg protein.
Assay of GSH
Glutathione content was estimated in 0.25 mL of 10 % tissue homogenate by the addition of equal volumes of ice-cold 5 % TCA and allowed for centrifugation at 4000 rpm for 10 minutes. Thereafter 0.25 mL of phosphate buffer and 0.5 mL of DTNB was added to 1 mL of supernatant and mixed well. The absorbance was read at 412 nmmusing a spectrophotometer. The results were calculated by interpolation with the pure glutathione and expressed as µmoles of GSH present /mg tissue (Ellman 1959).
Assay of TBARS
The method was initiated by heating the tissue homogenate with 0.8 mL saline, 0.5 mL of butylated hydroxytoluene and 3.5 mL thiobarbituric acid reagent for 90 seconds in a boiling water bath (Ohkawa, Ohishi et al. 1979). After cooling, the solution was centrifuged at 2,000 rpm for 10 minutes to remove the precipitate. The absorbance of the supernatant was determined at 532 nm using a spectrophotometer against a blank that contained all the reagents except the tissue homogenate. The results were calculated by interpolation with the standard malondialdehyde and the levels of TBARS are expressed as µmoles/mg tissue.
Total RNA extraction and Gene Expression Analysis
Total RNA was extracted from heart tissue samples using Trizol reagent (Sigma, USA) and reversely transcribed into cDNA using the super-Transcript kit, according to the manufacturer’s protocol (Genecopoeia, USA). Quantification of isolated RNA was carried out using a nano-drop (Thermoscientific) and cDNA synthesis was done using a commercial kit (high cDNA, Applied Biosystem). The concentration of RNA was adjusted to contain 100ng/µL uniformly in all the samples and cDNA was synthesized as per the manufacture’s instructions by employing random primers, reverse transcriptase and dNTPs suspended in the buffer. Quantitative real-time PCR (q-PCR) was performed on Roche Light Cycler 96 sequence detection system (Roche Pleasanton USA). The change in fluorescence of SYBR green dye in every cycle was monitored, and threshold cycle (ct) above background for each reaction was calculated. Expression levels of target genes were normalized to the reference gene β-actin. The sequence of the primers as follows- IL2 (F: 5’ATG ATG CTT TGA CAG ATG3’, R: 5’AGC GTG TGT TGG ATT TGA3’), TNF-α (F: 5’ACG CTCTTC TGT CTA CTG3’, R: 5’GGA TGA ACA CGC CAG TCG3’) and NF-κB (F: 5’CAC CAA AGA CCC ACC TCA3’, R: 5’GGA CCG CAT TCA AGT CAT3’) β- Actin (F: 5’ATG GAG AAG ATT TGG CAC C 3’, R: 5’ GGT CAT CTT TTC ACG GTT G 3’)
Histopathology
Sepsis generally leads to infection of the myocytes, therefore the heart tissues from each group were examined for histopathological changes. Tissues were fixed in 10% formalin solution and fixed overnight in cassettes. The paraffin-embedded tissues were sectioned on a microtome to a thickness of 5-7 mm, stained with hematoxylin and eosin (H&E), and observed under a light microscope for histo-architectural variations and documented by taking photomicrographs.
Statistical Analysis
The results of all data were statistically analyzed by the one-way ANOVA followed by Tukey’s multiple comparison test respectively. They were evaluated by Graph pad prism software (version 5.0) and the results were expressed as Mean ± SD, n=6 and a value of (p ≤ 0.05), (p ≤ 0.01) and (p ≤ 0.001) were accepted as significant comparing HFD and LPS induction groups.
Results
Effect of LPS on Body Temperature, Heart Rate and BP
Body temperature increased in 10 mg and 15 mg LPS induction groups compared to normal control in male and female rats after 8h of LPS induction. Similarly, heart rate significantly increased after 8h of LPS induction in male 10 (p < 0.01) and 15 mg groups (p< 0.001) and female rats in15mg LPS induction group. Further, a significant reduction in systolic and diastolic pressure was observed in male rats in 10 and 15mg LPS groups (Figure 1).
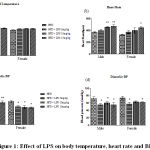 |
Figure 1: Effect of LPS on body temperature, heart rate and BP |
Effect of LPS on Hematological Parameters
In the present study, after LPS injection significant decrease in white blood cells count in 10 mg (p < 0.05) and 15 mg (p < 0.01) groups after 8th hour was observed in both male and female rats compared to HFD control (Table 1). Likewise, lymphocyte count significantly decreased in 5 mg, 10 mg and 15 mg (p < 0.001) groups after 8th h of LPS induction in both male and female rats compared to HFD control (Table 1). A significant increase in the neutrophil count was also observed in 10 mg (p < 0.001) and 15 mg (p < 0.001) groups after 8th h of LPS induction in male and female rats. A significant decrease was observed in platelet and HCT count in 5 mg (p<0.01 and p < 0.001) , 10 mg (p < 0.05 and p < 0.001) and 15 mg (p < 0.001) groups after 8th h of LPS induction in male SD rats compared to control group (Table 1).
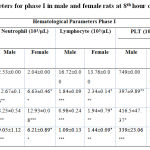 |
Table 1: Hematological parameters for phase I in male and female rats at 8th hour of LPS induction |
All the values were expressed in mean ± SEM, n = 6/group. Statistical analysis was performed by one-way ANOVA followed by Tukey’s multiple comparison test. HFD + 5mg, HFD +10 mg and HFD +15 mg were compared to HFD. The p values *, ** and *** indicates <0.05, 0.01 and 0.001 were considered significant.Subsequently, based on phase I results, 10mg of LPS was used to induce sepsis in only male rats. Accordingly, in phase II study, after LPS injection in normal diet (ND) and HFD rats, a significant decrease in white blood cells count (p < 0.05 and 0.01) after 4th and 8th hour were observed in the study rats compared to Normal diet and HFD control group (Table 2). However, a significant increase in the neutrophil count was observed in LPS induced (p < 0.001) groups after the 4th and 8th hour of LPS induction in male rats. In this experiment, lymphocyte count, platelet count and HCT were significantly decreased in 10 mg groups after 4th and 8th hour of LPS induction in male rats when compared to the control groups (Table 2). It is noteworthy to identify that HFD did not exhibit any difference compared to the ND fed animals.
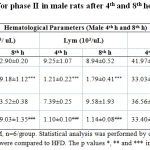 |
Table 2: Hematological parameters for phase II in male rats after 4th and 8th hour of LPS induction |
Effect of LPS on Oxidative Stress Markers
Oxidative damage caused during the infection was assessed by checking levels of oxidative stress markers in the heart tissue. Both an enzymatic antioxidant SOD (p <0.01) and non-enzymatic antioxidant GSH (p < 0.001) levels were significantly reduced in 10 mg LPS group rats compared to rats of the control group. Obviously, LPO levels were significantly increased in 10mg LPS group (p < 0.05) in SD rats compared to the controls confirming that sepsis leads to oxidative stress and vice versa (Figure 2).
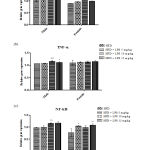 |
Figure 2: Relative gene expression in heart tissue in LPS induced sepsis |
Gene Expression Studies
Cytokines are effective regulators of the immune response to infection and play an important role in regulating inflammation. In the present study, expression of pro-inflammatory cytokines that regulates early responses in inflammation linked sepsis, like IL-2 and TNF-α and pro-inflammatory marker NFκB were studied. The results revealed, increased expression of IL-2 in 10mg and 15mg (p < 0.05) groups in male rats and 10mg (p < 0.05) group in female rats after LPS induction as compared to the control group (Figure 3 a). Moreover, an increase in the expression of TNF-α was noted in 10mg (p < 0.001) and 15mg (p < 0.05) groups when compared to the control group in male SD rats (Figure 3 b). Figure 3 c represents the increased expression of NF-κB in 10mg and 15mg (p < 0.01) groups in male rats and 10mg (p < 0.01) groups in female rats after LPS induction as compared to control group confirming the development of sepsis and sepsis-related complications. It is clearly noted that the male rats respond positively compared to the female rats in inducing infection as females may be resistant to infectious toxins.
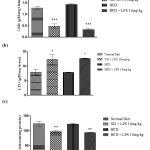 |
Figure 3: Oxidative stress markers in heart tissue in LPS induced sepsis |
Histopathological Changes in Heart Tissue
In order to identify the extent of damage caused in the heart tissue, Histopathological micrographs were assessed. Cardiac muscle fibers (myocardium), endocardium, myocardial blood vessels appeared normal in the control group. However, LPS induced (10 and 15 mg/kg) groups showed widened myocardial fiber and shrunken cells in heart tissue when compared to the uninduced control group confirming LPS induced inflammation of the cardiac cells (Figure 4).
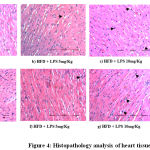 |
Figure 4: Histopathology analysis of heart tissue |
Discussion
Endotoxemia induced sepsis models are widely used to mimic and study sepsis in rodents and usually alters at least two of the following parameters – body temperature, heart rate, respiratory rate and white blood cell count (12). During sepsis, the innate immune system releases multiple inflammatory cytokines such as TNFα, IL-1β and IL-2 which results in a severe and persistent inflammatory response leading to cell and tissue damage. Cardiovascular dysfunction is one of the most common outcomes in sepsis. Sepsis-related myocardial dysfunction is noted in 20%–65% population (13). Alterations in blood flow are frequent in animal models of sepsis which reduces tissue oxygenation leading to hypoxia and oxidative damage, mainly in the heart tissue (14). Therefore, parameters like blood pressure and heart rate along with the level of oxidative stress and inflammatory markers in heart tissue were assessed to confirm the extent of sepsis. A significant increase in heart rate and decrease in BP was observed in 10 and 15mg LPS groups in male rats compared to HFD control. Previous studies show a significant increase in atrium volume during sepsis, leading to increased heart rate and reduced BP during sepsis and our results coincide with earlier studies (15, 16).
LPS is known to alter clinical and hematological variables (17). In our present study, total WBC, lymphocyte, platelet, hemoglobin and hematocrit count were significantly affected by LPS injection. A significant decrease in total WBC count was observed in 10 and 15mg LPS groups in male as well as female rats. This reduction in the total WBC count might be caused due to excessive NETosis during sepsis. This is in accordance with the previous study that indicated reduced WBC count in sepsis (18). Moreover, an increase in the circulatory neutrophil count is considered as a primary response during an inflammatory reaction in the early phase of sepsis (19). Neutrophil count was found to be significantly increased in 5,10 and 15mg LPS groups in male and female rats compared to the control, coinciding with the earlier reports (20). The overproduction of neutrophil might be caused by the activation of TLR4 cascade by LPS. Lymphocyte dysfunction is another predicted outcome during sepsis (21). A significant reduction in lymphocyte count was observed in 5, 10, 15 mg LPS groups compared to the control group in both male and female rats. This reduction in circulating lymphocyte might be because of apoptosis during septic shock (22). These results confirm that lymphocyte dysregulation plays a significant role in sepsis. Likewise, a reduced number of platelets, hemoglobin and hematocrit in the blood (thrombocytopenia) is one of the common outcomes of sepsis. In the present study, platelet, hemoglobin and Hematocrit count significantly reduced in 10 and 15mg LPS groups compared to the control group. Previous reports suggest that a decrease in platelet count is directly proportional to the increase in the severity of sepsis (23). LPS is found to be involved in hemolysis, which might be responsible for the decrease in hematocrit (HCT) during sepsis (24). Herein, decreased platelet, hemoglobin and HCT count confirm that RBC volume reduces during LPS induced sepsis. Although, significant results were observed in male and female rats, changes were more prominent in male rats compared to female rats. This might be because of female hormones, which are considered to increase the activity of humoral immune responses and thus protecting against infectious diseases and sepsis.
LPS causes oxidative stress-induced tissue and organ damage. Oxidative stress markers like LPO, SOD and GSH were assessed in the present study. In the phase II study, a significant increase in MDA levels (LPO) was observed in 10 mg LPS groups in male SD rats compared to the control groups indicating LPS induced oxidative damage (25). Moreover, enzymatic antioxidant SOD levels and non-enzymatic antioxidant GSH were significantly reduced in 10 mg LPS groups compared to the control groups in male rats. The reduced GSH level might be because of LPS induced cellular damage/apoptosis in the cells (26). GSH in heart showed a significant positive correlation with WBC and lymphocyte count after 4th (0.98 and 0.95 respectively) and 8th h (0.97 and 1.0) in male rats. SOD in the heart also showed a significant positive correlation with WBC and lymphocyte count in blood after 4th (0.99 and 0.99) and 8th h (0.98 and 0.99) of LPS induction respectively in male rats. It is interesting to note that GSH and SOD showed a strong negative correlation with the neutrophil count in the 4th (-0.95 and -0.97) and 8th h (-0.97 and -0.99) in male rats. These results are in line with previous literature, wherein the correlation of oxidative stress markers and hematological parameters in human subjects suggests that neutrophils may secrete trigger and increase oxidative stress (27). LPO showed a strong negative correlation with WBC and lymphocyte count after 4th (-0.99 and -0.98) and 8th h (-0.99 and -1.00) of LPS induction in male rats. However, a strong positive correlation was observed between LPO and neutrophil count after 4th (0.98) and 8th h (0.99) of LPS induction. These results corroborate with previous literature suggesting that LPS leads to oxidative stress as evidenced by the manifestation of lipid peroxidase products in organs and cause systemic inflammation (28).
An unbalanced pro and anti-inflammatory response leading to persistent inflammation play important roles in the acute and/or chronic phases of sepsis. Hence, in the present study, the expression of genes involved in the inflammatory cascade were studied using RT-PCR in heart tissue. LPS triggers an inflammatory response by binding and activating receptors like TLR4, further causing the release of cytokines and inflammatory markers (29). Similarly, the present study reveals a significant increase in the levels of inflammatory markers IL-2, TNF α and NFκB in LPS induced groups (10 mg and 15 mg) compared to the control group confirming the activation of inflammatory cascade during sepsis via TLR4 pathway. Data also suggests that the expression of inflammatory markers was better in male rats compared to female rats. Histopathological data are visual manifestations of the LPS effect on the physiologic functioning of the entire system (30). In 10 and 15 mg LPS groups, the heart tissues revealed widened cardiac fibers and swelling of myocardial cells, which supports the hematological, biochemical and molecular findings, confirming inflammation in the tissue. These changes in cell structure might be due to the formation of highly reactive radicals because of oxidative threat induced by LPS (31).
Conclusion
Phase I concludes that LPS 10 and 15 mg/kg produced moderate and severe sepsis. Therefore, 10mg LPS in experimental rats would be appropriate to study sepsis as it minimizes pain and suffering in animals as a higher dose of LPS may cause septic shock and even mortality in rats. Moreover, male animals were found to be more prone to infection compared to female rats as evidenced by oxidative stress and inflammatory marker expression. Hormones like estrogen might be responsible for this slight resistance to infection in female rats. Phase II study concludes that ND with 10 mg LPS is sufficient to induce an acute phase of sepsis and this study also establishes a relationship between hematological indices and oxidative stress markers. The present study, therefore, refines the existing sepsis model with additional information on sex as well as the influence of diet and the dose of LPS in the induction of sepsis.
Acknowledgment
The authors wish to thank the infrastructure facilities developed by DST- FIST and UGC-SAP grants in the Department of Biotechnology, Pondicherry University for executing the present study and the UGC fellowship from Pondicherry University to the first author and Dr. P. Shonima, Veterinarian, for helping in animal experimentation to execute the present work.
Conflict of Interest
The authors declare no conflict of interest.
Funding Source
This work was not funded by any funding agency.
References
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama. 2016;315(8):801-10.
- Torio CM, Andrews RM. National inpatient hospital costs: the most expensive conditions by payer, 2011: statistical brief# 160. 2006.
- Reinhart K, Daniels R, Machado FR. The burden of sepsis: a call to action in support of World Sepsis Day 2013. Revista Brasileira de terapia intensiva. 2013;25(1):3-5.
- Vincent J-L, Marshall JC, Ñamendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. The lancet Respiratory medicine. 2014;2(5):380-6.
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. American journal of respiratory and critical care medicine. 2016;193(3):259-72.
- Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. Bmj. 2016;353:i1585.
- Ramachandran G, Tulapurkar ME, Harris KM, Arad G, Shirvan A, Shemesh R, et al. A peptide antagonist of CD28 signaling attenuates toxic shock and necrotizing soft-tissue infection induced by Streptococcus pyogenes. The Journal of infectious diseases. 2013;207(12):1869-77.
- Lilley E, Armstrong R, Clark N, Gray P, Hawkins P, Mason K, et al. Refinement of animal models of sepsis and septic shock. Shock. 2015;43(4):304-16.
- Brandenburg K, Heinbockel L, Correa W, Lohner K. Peptides with dual mode of action: Killing bacteria and preventing endotoxin-induced sepsis. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2016;1858(5):971-9.
- Dogan MD, Ataoglu H, Akarsu ES. Effects of different serotypes of Escherichia coli lipopolysaccharides on body temperature in rats. Life sciences. 2000;67(19):2319-29.
- Burkovskiy I, Zhou J, Lehmann C. Use of Escherichia coli toxins in sepsis models. Advances in Bioscience and Biotechnology. 2013;4(03):424.
- Robertson CM, Coopersmith CM. The systemic inflammatory response syndrome. Microbes and Infection. 2006;8(5):1382-9.
- Vallabhajosyula S, Pruthi S, Shah S, Wiley B, Mankad S, Jentzer J. Basic and advanced echocardiographic evaluation of myocardial dysfunction in sepsis and septic shock. Anaesthesia and intensive care. 2018;46(1):13-24.
- De Backer D, Creteur J, Preiser J-C, Dubois M-J, Vincent J-L. Microvascular blood flow is altered in patients with sepsis. American journal of respiratory and critical care medicine. 2002;166(1):98-104.
- Hunter J, Doddi M. Sepsis and the heart. British journal of anaesthesia. 2009;104(1):3-11.
- Seemann S, Zohles F, Lupp A. Comprehensive comparison of three different animal models for systemic inflammation. Journal of biomedical science. 2017;24(1):60.
- Fairchild KD, Saucerman JJ, Raynor LL, Sivak JA, Xiao Y, Lake DE, et al. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2009;297(4):R1019-R27.
- Aminzadeh Z, Parsa E. Relationship between age and peripheral white blood cell count in patients with sepsis. International journal of preventive medicine. 2011;2(4):238.
- Brown K, Brain S, Pearson J, Edgeworth J, Lewis S, Treacher D. Neutrophils in development of multiple organ failure in sepsis. The Lancet. 2006;368(9530):157-69.
- Alves-Filho JC, Spiller F, Cunha FQ. Neutrophil paralysis in sepsis. Shock. 2010;34(7):15-21.
- de Pablo R, Monserrat J, Prieto A, Alvarez-Mon M. Role of circulating lymphocytes in patients with sepsis. BioMed research international. 2014;2014.
- Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18(6):487-94.
- Guclu E, Durmaz Y, Karabay O. Effect of severe sepsis on platelet count and their indices. African Health Sciences. 2013;13(2):333-8.
- Bateman R, Sharpe M, Singer M, Ellis C. The effect of sepsis on the erythrocyte. International journal of molecular sciences. 2017;18(9):1932.
- Şener G, Arbak S, Kurtaran P, Gedik N, Yeğen BÇ. Estrogen protects the liver and intestines against sepsis-induced injury in rats. Journal of Surgical Research. 2005;128(1):70-8.
- Yamada H, Arai T, Endo N, Yamashita K, Fukuda K, Sasada M, et al. LPS-induced ROS generation and changes in glutathione level and their relation to the maturation of human monocyte-derived dendritic cells. Life sciences. 2006;78(9):926-33.
- Asmah R, Yeboah G, Asare-Anane H, Antwi-Baffour S, Archampong T, Brown C, et al. Relationship between oxidative stress and haematological indices in patients with diabetes in the Ghanaian population. Clinical diabetes and endocrinology. 2015;1(1):7.
- SAMSURIA IK. Molecular basic of active smoking causes oxidative stress (LPO) and Inflammation (Netrophil). 2015.
- Liu SF, Malik AB. NF-κB activation as a pathological mechanism of septic shock and inflammation. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2006;290(4):L622-L45.
- Shi D-W, Zhang J, Jiang H-N, Tong C-Y, Gu G-R, Ji Y, et al. LPS pretreatment ameliorates multiple organ injuries and improves survival in a murine model of polymicrobial sepsis. Inflammation Research. 2011;60(9):841-9.
- Zhao L, Chen Y-H, Wang H, Ji Y-L, Ning H, Wang S-F, et al. Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. Toxicological sciences. 2008;103(1):149-57.
Abbreviations
BP: Blood pressure; GSH: Reduced glutathione; HCT: Hematocrit; HFD: High fat Diet; IL-2: interleukin 2; LPS: Lipopolysaccharide; NF-κB: nuclear factor kappa B; ND: Normal Diet; PCT: Platelet; q-PCR: Quantitative real-time PCR; SOD: Superoxide Dismutase; TNF-α: Tumor necrosis factor α; TLR4: Toll-like receptor; TBARS: Thiobarbituric acid Reactive Substances; WBC: White Blood Cell








