NA Siddiqi1*, Azeem Shalaby2, Mohammad Al Kindi2 and Fatima Al Ghafri2
1Department of Anatomy and Neurobiology, College of Medicine and Health Sciences, national University of Science and Technology, Sohar, Oman,
2Department of Pathology, Electron Microscope Laboratory, Sultan Qaboos University, Oman
Corresponding Author E-mail :najam@omc.edu.om
DOI : https://dx.doi.org/10.13005/bpj/1882
Abstract
This study was designed to measure the adverse effects of electromagnetic waves on the hepatocytes in vivo. For this purpose, a developing chick embryo model was selected. 40 fertilized chick eggs were used. An specific egg incubator was used for egg development. In the exposed group, 20 fertilized eggs were exposed to mobile phone electromagnetic waves by placing a mobile phone inside the incubator in silent mode, while in the control, the battery was removed. Mobile phone received calls from outside for 50 minutes in 24 hours. Electron microscopy of liver was conducted. Control group revealed normal hepatocytes with big central nucleus, well-developed cristae in normal looking mitochondria, rounded nucleus, scattered ribosomes, few glycogen vacuoles and rough endoplasmic reticulum. Normal looking sinusoids lined by simple squamous epithelium and few Kupffer cells. Sinusoid were containing many nucleated RBC. On tenth day of development, exposed group revealed marked proliferation of mitochondria. At day 15, electron-dense mitochondria which were either swollen or dumbbell shaped, degenerated invisible cristae were apparent. Marked infiltration of fatty vacuoles and myelin-like figures in the cytoplasm, and derangement of classic hepatic lobule and sinusoids was observed. We conclude that electromagnetic waves affected the proliferation of hepatocytes in the chick embryo.
Keywords
Electron microscopy; electromagnetic waves; Electron microscopic
Download this article as:| Copy the following to cite this article: Siddiqi N. A, Shalaby A, Al Kindi M , Al Ghafri F. Mobile Phone Electromagnetic Fields Affected the Hepatocytes in the White Leghorn Chicken Embryo: an Ultrastructural Study. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Siddiqi N. A, Shalaby A, Al Kindi M , Al Ghafri F. Mobile Phone Electromagnetic Fields Affected the Hepatocytes in the White Leghorn Chicken Embryo: an Ultrastructural Study. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2TSSI4j |
Introduction
Smart phones have added a new dimension into our lives. We are more preoccupied with our mobiles than talking to persons seated next to us. Parents give mobiles to their two-year olds to play with instead of toys. Before traveling we make sure that the mobile charger is not left behind. Kids and teenagers spend more time playing with mobiles than playing outside. Do we know that we are risking our health spending much time with smart phones? Smart phones use electromagnetic waves which are now believed to be harmful to living cells. WiFi is connected through electromagnetic waves while in use during calling and downloading data from the internet 1, 2. Newly born may be affected more due to the presence of high number of embryonic stem cells 3, 4,5,6,7. Now a days a newly born is exposed to these waves soon after birth and will continue until he dies. Prenatal and postnatal cell phone exposure leads to behavioral problems in children9.
Hypothesis of this research conducted at National University of Science and Technology, College of Medicine, is that the smart phone electromagnetic waves affect normal structure and function of liver cells in chick embryo model.
Objectives
To study the ultra-structure of the liver of the developing chick embryo in normal developing chick and compare it to liver exposed to mobile phone electromagnetic waves.
Material and Methods
Animal Experiment
Sohar poultry provided zero-day fertilized chicken eggs of breed ‘Cobb’ (Gallus gallus domesticus) for this experimental study. A strict pre-fixed inclusion and exclusion criteria was applied to these fertilized eggs.. The chick embryo model was previously extensively used as an animal model to access the effects of electromagnetic waves 10-19. Egg incubator Model EH-35, Sino-PFE Company, China, was made available for this experiment (Fig. 1a). All the eggs were placed in such a way that the farthest egg was within a radius of 16cm from the mobile phone placed inside the incubator. The temperature was set at 37 degrees, humidity at 50-60% and automatic egg rotation at ten rotations per day. 20 eggs were placed at one time in the egg holders. The experiment was performed twice and the specimens were sent for histological and electron microscopy preparations. It was partially blinded. The two groups will be randomly allotted a number of 20 eggs; control group and the experimental group. One incubator was used carrying 20 eggs at a time; one wave consisted of an exposed group experiment and one wave of control group. The eggs were exposed electromagnetic waves of the mobile phone during egg development while eggs were not exposed to waves in the control group. The mobile company and mobile phone set used for this study was using 1800 MHz frequency, power of 0.47 W/kg body and SAR 1.10 w/KG (head). Electromagnetic exposure and strength was confirmed by using a TriField Meter, model 100XE (Fig. 1b).
Experimental Group
20 fertilized eggs were exposed to electromagnetic field inside the incubator when the mobile was rung from outside. To prevent noise interference, the mobile was kept in silent mode and vibration disabled. All the eggs were within one wavelength (approximately 16.5 cm) of the mobile phone electromagnetic waves 14. A schedule was made with calling times and duration. 10 calls were made each 24 hours; one-call duration was 5 minutes. Nights were calling free. 50 minutes of exposure were given s in 24 hours starting from day 1; total exposure time was 500 minutes at day 10 and 750 minutes at day 15. 10 eggs each were scarified at day 10 and 15. After making a small hole in the shell, it was carefully cut by scissors and the embryos were carefully dissected from the membranes after cutting the umbilical cord. Survivability was noticed by observing the beating heart and movements of limbs (Fig. 1c). After exposure the abdominal cavity the liver was removed and fixed in 10% glutaraldehyde solution for EM preparation.
 |
Figure 1: a) Egg incubator b) Mobile phone in calling mode and the Trifield meter c) Developing chick embryo showing blood vessels and the membranes |
Control Group
Same experiment was repeated with 20 eggs but the mobile phone was not placed inside the incubator. The eggs were sacrificed at days 10 and 15, liver specimens were removed, placed in 10% glutaraldehyde solution for electron microscopic preparation.
Results
There was no mortality observed in all the groups at day 10 and 15.
Control Group
Electron microscopic findings both at day 10 and 15, revealed rows of hepatocytes forming hepatic lobules with lying in between the rows lined by simple squamous epithelium. (Fig.2). Hepatocytes well formed with a rounded nucleus in the center showing well developed chromatin. Nuclear double layered membrane with pores was clearly seen. Mitochondria with well-arranged cristae were also observed, surrounded by rough endoplasmic reticulum and free ribosomes. There were few glycogen vacuoles can been seen in the cytoplasm. Sinusoids can be seen in between the hepatocytes lined by simple squamous epithelium and Kupffer cells. Sinusoids were filled with RBCs having an oval nucleus. Canaliculi can also be seen in between the hepatocytes (Fig.3)
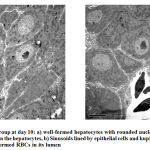 |
Figure 2: Control group at day 10 |
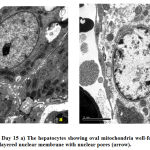 |
Figure 3: Control Day 15 a) The hepatocytes showing oval mitochondria well-formed rounded nucleus, b) double layered nuclear membrane with nuclear pores (arrow). |
Exposed Group
Hepatocytes revealed marked increased in number of mitochondria at day 10, some of them were swollen and surrounded by rough endoplasmic reticulum (Fig.4a). At day 15, mitochondria became electron-dense, some were rounded while others were elongated and dumbbell shaped. Few mitochondria and cristae showed degeneration. A prominent layer of rough endoplasmic layer around the mitochondria could also be seen. A rounded central nucleus could be seen clearly. The double layered nuclear membrane was blurred and pores were not clearly seen (Fig.5b). Few myelin-like figures were observed in the cytoplasm at day 15 (Fig.5b). Lipid filled vacuoles increased in number from day 10 to day 15 and sinuses were dilated (Fig.5a).
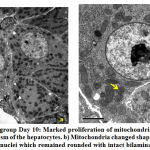 |
Figure 4: a) Exposed group Day 10 |
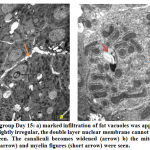 |
Figure 5: Exposed group Day 15 |
Discussions
Many studies used developing chick embryo to observe the effects of exposure of electromagnetic waves. Most of the studies studied gross morphological changes or histological effects on different tissues, however there are few ultrastructural studies. This is another study looking at the changes occurring inside the hepatocytes when exposed to radio waves. Liver is one of the organs which are affected very early in response to any external or internal stress. The radio waves are a source of external stress to the living cells. We have chosen day 10 and 15 day old chick embryo because most of the scientist has chosen these two days so it will be easy to compare with our study.
First observation was an increase number of mitochondria in the exposed group at both, day 10 and day 15. Oxidative stress induced by H2O2 in human lung is known to increase number of mitochondria and mtDNA 15. Electromagnetic wave exposure after 10 days affected the mitochondria which became swollen. Mitochondrial membrane permeability is dependent on interaction between ca+2 and ROS system, and an increased sympathetic activity is considered a primary cause of electromagnetic wave-induced calcium influx into the mitochondria 16. Mitochondria swelling is caused by free O+2 produced by ROS inside the mitochondria to invade the thiol protein which produces transition pores in the mitochondrial membrane to open and cause increase permeability16. Attia et al. also reported mitochondrial in the study done on rat hepatocytes after electromagnetic exposure17. Voyedovodskata reported that paramagnetic particles are naturally located in the mitochondria of liver cells which make the hepatocytes sensitive to magnetic field exposure and leading to modification of haemostasis by altering mitochondrial respiration in the hepatocytes18. Moreover, Gorczynska and Wegrzynowicz reported that mitochondria are the most sensitive organale to stress-generating factors and observed mitochondrial swelling in such conditions 19.
Most of the mitochondria became elongated; dumbbell shaped, and showed the process of degeneration at day 15 in the exposed group. Rough endoplasmic reticulum and free ribosomes were seen surrounding the mitochondria. Mitochondria are known for free radicals production in human sperm after electromagnetic wave exposure 20. Oxidative DNA damage may be due to electron leakage from the mitochondrial electron transport chain.
Lipid Droplets
Second Significant Observation was increase number of lipid filled vacuoles in the cytoplasm of the hepatocytes in the exposed groups. Disturbances in lipid inclusions and fat metabolisms were reported to increase the cytoplasmic vacuolation. In any pathological conditions, blockage of gluconeogenesis due to free radicals disturbs lipids and protein metabolisms and cause cell damage 16. Rough endoplasmic reticulum surrounding the mitochondria may be damaged and fragmented and contributing in fat droplets accumulation.
Hepatocytes of the developing chick embryos revealed fatty changes after electromagnetic waves exposure which is due to oxidative stress when exposed to electromagnetic waves 21, 22,23 . The damage is dose dependent 14. Similar results were reported by Lahijani et al 16. Similar results were also reported by other authors in rats and rabbits 17, 24. Radiation exposure may affect the breakdown of fat in the liver, similar to pregnancy, alcoholism, and malnutrition and poisoning. Fatty change is the initiation of damage to the hepatocytes, which is shown by an increase in the number of vacuoles filled with triglyceride fat. This is a sign of abnormal metabolism which may be due to an increase in Oxygen radicles species (ORS) production by the hepatocytes 21, 22, 23. Increase proliferation of mitochondria in hepatocytes and marked derangement of its internal structure in cardiac muscles is a sign of oxidative stress which was observed by other authors 15, 20, 25. Many authors have reported different effects of electromagnetic waves on the chick embryo which increases mortality of the developing chick embryo and resulted in malformations 10-14, 26-30.
Nuclei
Control group revealed rounded nuclei at both day 10 and 15. On the other hand, in the exposed group, it was circular at day 10, but at day 15 the shaped became oval or irregular but no change was observed in chromatin. Double layered nuclear membrane and pores was clearing seen control groups, however, it has become blurred and the pores were not clearly seen in the exposed groups. Electromagnetic exposure leads to a damage of pores in the nuclear membrane, or it becomes irregular which may result in release of nuclear material into the cytosol 16.
Different theories believe that mobile phone radiation produces reactive oxygen species (ROS) and DNA damage which was observed in human sperm. It also affects genes, cell membrane function and signal transduction 31, 32, 33. Rao et al recently provided new evidence that radio waves affect the plasma membrane 34. Radio waves also induce oxidative stress through NADH oxidase enzyme stimulation, which may be a major cause of various cellular adverse effects, observed in in vitro studies 35-42. As a consequence of increased levels of free radicals, various cellular and physiological processes can be damaged including gene expression, release of calcium from intracellular storage sites, cell growth, and apoptosis. Many scientist reported radio wave effects on genes resulting in signal transduction which resulted in alterations in membrane structure and function, and changes in metabolism associated with free-radical production 40-41, 42.
Conclusion
Electromagnetic wave exposure has induced many changes to the hepatocytes of chick embryo, especially mitochondria and nucleus, and increased fat deposition in the cytosol suggesting fatty change in the liver. Hence, it is scientifically documented that these electromagnetic waves are causing damage to living cells. Further studies should be carried out to fully understand the mechanism of this fatty change in hepatocytes mitochondrial damage when exposed to RFW.
References
- Samkange-Zeeb F, Blettner M, Emerging aspects of mobile phone use. Emerg Health Threats J, 2: 2-8 (2009).
CrossRef - Blake LB, Lai H, Biological effects from exposure to electromagnetic radiation emitted by cell tower base stations and other antenna arrays. Environ Rev; 18:369-397 (2010).
CrossRef - Leitgeb N, Mobile phones: are children at higher risk? Wien Med Wochenschr. 158 (1-2):36-41 (2008).
CrossRef - Kheifets L, Repacholi M, Saunders R, Van deventer E. The sensitivity of children to electromagnetic fields. Pediatrics; 116:303-13 (2005)
CrossRef - Maisch D, Children and mobile phones….Is there a health risks? Journal of Australian College of Nutritional and Environmental Medicine 22:3-8 (2003).
- Najam Siddiqi,Thomas Heming, Effects of mobile phone radiofrequency waves (RFW) on embryonic stem cells. The FASEB Journal 27:753.1 (2013).
CrossRef - Schüz J, Mobile phone use and exposures in children. Bioelectromagnetics Suppl 7:S45-50 (2005).
CrossRef - Abramson MJ, Benke GP, Dimitriadis C et al. Mobile telephone use in associated with changes in cognitive function in young adolescents. Bioelectromagnetics 30:678-86 (2009).
CrossRef - Divan HA, Kheifets L, Obel C, Olsen J, Prenatal and postnatal exposure to cell phone use and behavioral problems in children. Epidemiology. 19 (4):523-9 (2008).
CrossRef - Ingole I., Ghosh SK, Exposure to radio frequency radiation emitted by cell phone and mortality in chick embryo (Gallus domesticus). Biomedical Research 17:205-210 (2006).
CrossRef - Juutilainen J, Harri M, Saali K et al. Effects of 100 Hz magnetic fields with various waveforms on the development of chick embryo. Radiat Environ Biophys, 25:65-74 (1986).
- Ubeda A, leal J, trillo MA et al. Pulse shape of magnetic fields influences chick embryogenesis. J Anat, 137:513-536 (1983).
- Najam Siddiqi, Muthusami John C, Mark Norrish, Thomas Heming, Development of chick embryo exposed to a low dose of electromagnetic waves. J Ayub Med Coll; 28:224-228 (2016).
- Zareen N, Khan MY, Minhas LA, Dose related shifts in the developmental progress of chick embryo exposed to mobile phone induced electromagnetic fields. J Ayub Med Coll; 21(1):130–4 (2009).
- Hsin-Chen LEE, Pen-Hui YIN, Ching-You LU, Chin-Wen CHI and Yau-Huei WEI, Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J 348; 425-432 (2000).
CrossRef - Lahijani MS, Tehrani DM, Sabouri E, Histopathology and ultrastructural studies on the effects of electromagnetic fields on the liver of preincubated white Leghorn chicken embryo. Electromagnetic Biology and Medicine 28:391-413 (2009).
CrossRef - Attia AA, Yehia MA, Histological, ultrastructural and immunohistochemical studies of the low frequency electromagnetic field effect on thymus, spleen and liver of albino swiss mice. Pak J Bio Sci , 5(9):931-937 (2002).
CrossRef - Voyedovodskaya nv, Burbayev DS, Blyumenfeld LA. Detection of new paramagnegnetic centers in native mitochondria and animal tissues by the EPR. Biofizika, 26: 1-5, (1981).
- Gorczynska E and Wegrzynowicz R, Structural and functional changes in organelles of liver cells in rats exposed to magnetic fields, Env res, 55:188-198, (1991).
CrossRef - Geoffry N.De Iuliisw, Rhiannon J. Newey, Bruce V. King, R. John Aitken. Mobile phone radiation induces reaction oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE; 4: 1-9, (e6446) (2009).
CrossRef - Angulo P. Nonalcoholic fatty liver disease. N Engl J Med, 346:1221-1231 (2002).
CrossRef - Reddy JK. lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Amer J Physiol Gastrointest Liver Pgysiol, 290:852-858 (2006).
CrossRef - Ibrahim M, El-Ashry, Ali E. The influence of 50 Hz magnetic field on liver function. Rom J Biophys. 18:113-122
- Tarantino P, Lanubile R, lacalandra G, Dini L. Post-continuous whole body exposure of rabbits to 650 MHz electromagnetic fields: Effects on liver, spleen and brain. Biophysik, 44:51-59 (2005).
CrossRef - Ahmed M. Mohamadin, Ahmed A. Elberry, Amr D. Mariee, Gehan M. Morsy, Fahad A. Al-Abbasi, Lycopene attenuates oxidative stress and heart lysosomal damage in isoproterenol induced cardiotoxicity in rats: A biochemical study, Pathophysiology19: 121 (2012).
CrossRef - Ubeda A, Leal J, Trillo MA, Jimenez MA, Delgado JM, Pulse shape of magnetic fields influences chick embryogenesis. J Anat; 137: 513-536 (1983).
- Ubeda A, Trillo MA, Chacon L, et al. Leal J, Chick embryo development can be irreversibly altered by early exposure to weak extremely-low-frequency magnetic fields. Bioelectromagnetics; 15:385-398 (1994).
CrossRef - Youbicier-Simo BJ, Lebecq JC, Giaimis J, et al. Mortality of chicken embryos continuously exposed under GSM cell phone and validation of the effectiveness of a protective device. International conference on cell tower siting, Salzburg, Austria 233-235 (2000).
- Al-Qudsi F, Azzouz S, Effects of electromagnetic radiation on chick embryo development. Life Science Journal 9: 983-991 (2012).
- Farrell JM, Litovitz TL, Penafiel M, Montrose CJ, Doinov P, Barber M, Brown KM, Litovitz TA, The effect of pulsed and sinusoidal magnetic fields on the morphology of developing chick embryos. Bioelectromagnetics 18:431-438 (1997).
CrossRef - Aitken RJ, Bennetts LE, Sawyer D, Wiklendt AM, King BV, Impact of radio frequency electromagnetic radiation on DNA integrity in the male germ line. Int J Androl, 28: 171–179 (2005).
CrossRef - Shangcheng Xu, Zhou Zhou, Lei Zhang, Zhengping Yu, et al., Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons, Brain Research, 1311: 189-196 (2010).
CrossRef - Spadaro JA, Bergstrom WH, In vivo and in vitro effects of a pulsed electromagnetic field on net calcium flux in rat calvarial bone. Calcif Tissue Int 70: 496–502 (2002).
CrossRef - Rao VS, Titushkin IA, Moros EG, et. al. Non-thermal effects of radiofrequency-field exposure on calcium dynamics in stem cell-derived neuronal cells: elucidation of calcium pathways. Radiat Res, 169(3): 319-329 (2008).
CrossRef - Suleyman Dasdag, Mehmet Zulkuf Akdag, The link between radiofrequencies emitted from wireless technologies and oxidative stress, Journal of Chemical Neuroanatomy, 75: 85 (2016).
CrossRef - Megha K, Deshmukh PS, Banerjee BD, Tripathi AK, Abegaonkar MP, Low intensity microwave radiation induced oxidative stress, inflammatory response and DNA damage in rat brain, Neurotoxicology, 51: 158-65 (2015).
CrossRef - Mailankot M, Kunnath AP, Jayalekshmi H, Koduru B, Valsalan R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics (Sao Paulo). 64(6):561-5 (2009).
CrossRef - Merhan Mamdouh Ragy, Effect of exposure and withdrawal of 900-MHz-electromagnetic waves on brain, kidney and liver oxidative stress and some biochemical parameters in male rats, Electromagnetic Biology and Medicine, 34:279-284 (2015).
CrossRef - Verschaeve L, Heikkinen P, Verheyen G, Van Gorp U, et al. Investigation of co-genotoxic effects of radiofrequency electromagnetic fields in vivo. Radiat Res, 165: 598–607 (2006).
CrossRef - Donato A, Ceci P, Cannavo A. Tomei F, Naro F. Low power microwave interaction with phospholipase C and D signal transduction pathways in myogenic cells. Cell Biol Int; 28: 683–688 (2004).
CrossRef - Lantow M, Schuderer J, Hartwig C, Simkó M, Free radical release and HSP70 expression in two human immune-relevant cell lines after exposure to 1800 MHz radiofrequency radiation. Radiat Res, 165: 88–94 (2006).
CrossRef - Geoffry N De luliis, Rhiannon J Newey, Bruce V King, R John Aitken, Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in Vitro. Plos one 4(7), e6446 (2009).
CrossRef







