Manuscript accepted on :17-Jan-2020
Published online on: 10-02-2020
Plagiarism Check: Yes
Reviewed by: Waiel Al-Kahiry
Second Review by: Dr.Chaitany Patel
Final Approval by: Dr. H Fai Poon
Nafea Sami Enad Al-Esawi1, Abul Rahman Mohammed Geeran2, Muhammad Hammad Jasim Alajeely3 and Arkan Obaid Jasim Al-Isawi4
1Head of Pathology Department, College of Medicine, University of Anbar, Ramadi, Iraq
2Anatomy Department, College of Medicine, University of Anbar, Ramadi, Iraq
3Department of biochemistry , College of medicine , University of Anbar , Ramadi, Iraq
4Pathology Department, College of Medicine, University of Anbar, Ramadi, Iraq
Corresponding Author E-mail : drnsami@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1912
Abstract
Retraction of: Expression of CYT4Z1 in Breast Carcinoma; Correlating with Clinicopathological Parameters, 10.13005/bpj/1912 by Nafea Sami Enad Al-Esawi, Abul Rahman Mohammed Geeran, Muhammad Hammad Jasim Alajeely and Arkan Obaid Jasim Al-Isawi as the title have some medico-legal obstacles and serious mistakes in the statistical analysis.
Download this article as:| Copy the following to cite this article: Al-Esawi N. S. E, Geeran A. R. M, Alajeely M. H. J, Al-Isawi A. O. J. Expression of CYT4Z1 in Breast Carcinoma; Correlating with Clinicopathological Parameters. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Al-Esawi N. S. E, Geeran A. R. M, Alajeely M. H. J, Al-Isawi A. O. J. Expression of CYT4Z1 in Breast Carcinoma; Correlating with Clinicopathological Parameters. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2H6pUya |
Indrodution
Breast carcinoma is the most worrisome cancer , the commonly diagnosed and leading cause of cancer death in women worldwide with great heterogeneity in clinical behavior and it is related to 14% of tumor mortality [1,2]..
In Iraq ,according to the data from the Cancer Registry of Iraq 2016 , breast carcinoma is the most common malignancy affecting Iraqi women representing 19.5 % of all registered tumors with a trend for increase in incidence rate and propensity to affect younger generation , tendency to be diagnosed at advanced stages and likelihood prevalence of aggressive tumor behavioral forms and cancer mortality [3,4,5,6,7].
In Arab countries including Iraq ,it is the first malignancy among cancers diagnosed in women , present in earlier stages and in more advanced stages ,but studies in western Iraq are limited [ 8 ] P450 cytochrome (CYPs) are superfamily of membrane-bound enzymes which are found in high level in liver where it plays important roles in endogenous compounds biosynthesis and metabolism in addition to oxidative metabolism and detoxification of various exogenous compounds . In this aspect it can bioactivate endogenous substrate to active metabolites, so it can facilitate cancer development by activation of compounds consumed in food , converting procarcinogens to carcinogens thus it has various potentially significant role in tumor biology , thus it has various potentially vital role in tumor biology , progression and prognostic significance [9,10]. Recently , a variety of CYPs have been found to expressed in increasing number of cancer tissue and normal tissues [11].
CYP4Z1 is a fatty acid hydroxylase that is involved in steroid metabolism and it is a type of CYP4 which is the least well characterized and specifically expressed in mammary tissue and hypothesized role in breast cancer through formation of the signaling molecule 20-Hydroxyeicostatetraenoic acid (20-HETE) so it could promote or suppress tumor that is sensitive to estrogen hormones level through its influence on hormonal control .CYP4Z1 have genetic variability with polymorphism that influence on this process . [12,13,14] .
An expanded understanding cancer biology has led to support the possible influence of CYP4Z1 which contributes to malignancy in many organs and potentiate the violent biological behavior and poor clinical prognosis . [15,16,17 ] .
Originally ,CYP4Z1 was identified in mammary tissue and up regulated in breast carcinoma .It is the least well described type of the CYP4 enzymes , so the local unique expression of CYP4Z1 appears to be very significant because it provides the basis for the development of new novel diagnostic and therapeutic strategies since expression of CYPs in cancers may be involved in activation and /or inactivation of anticancer drugs in addition to its possible related prognostic influence. [18]. In addition the stable CYP4Z1 overexpression in breast cancer cells has been stated to stimulate angiogenesis and growth of tumor in mice with attendant increase in cellular 20-HETE [19].
Aim of Study
The study aims to evaluate the immunohistochemical CYP4Z1 expression in breast carcinoma and correlate its score with other conventional clinicopathological parameters .
Patients and Methods
This is a descriptive study carried out on archived tissue specimens which were surgically obtained from 128 patients ( 120 of invasive ductal carcinoma and 8 patient of benign tumor ) during 3 years work in Anbar province , west of Iraq , examined with respect to immunohistochemistry CYP4Z1 expression .
All personal data from samples were gained from the regional oncological centers . The study was properly approved by the ethics committee , College of medicine ,University of Anbar . Paraffin embedded blocks of breast carcinoma for patient underwent modified radical mastectomy with axillary clearance were processed for CYP4Z1 assessment .
For 128 tissue specimens , we had access to two slices from different areas of the same tumor . These were compared to assess homogeneity regarding the expression pattern. Regarding Immunohistochemical analysis the examined breast carcinoma specimens were derived from formalin-fixed , paraffin-embedded tumors in 4-µm thick sections .
One 4-µm section from each paraffin block was stained with a routine hematoxylin and eosin to verify the presence of carcinoma and fixation adequacy which was sufficient for IHC analysis and the best preserved and stained areas of the sections were assessed for carcinoma grade according to modified Bloom-Richardson grading system .Then assigning IHC score was performed on the invasive carcinoma component only.
All slices were evaluated without clinical outcome knowledge . For each staining run , positive control slides were prepared from breast carcinoma known to be positive for the patient studied .The results of semi quantitative CYP4Z1 staining scores were recorded according to the system established by Allred et al [20].The criteria of positive reaction for CYP4Z1 were assessed by scoring the proportion and intensity in 100 malignant cells performed at X 40 objective in 25 malignant fields on carcinoma.
The scoring for proportion of membrane staining pattern of malignant cells and the intensity of staining were taken in account and the staining being graded on the basis of a four point scoring systems which are ranged from 0-3 . The intensity of CYP4Z1 staining was defined by a visual scale from 0 to 3 ( grade 0=no staining , grade I=weak , grade 2 = moderate , grade 3=intense staining ).
IHC analysis of CYP4Z1 expression was performed using the avidin-biotin-peroxidae complex technique (Vectastain Rabbit IgG abc-KIT ,Vector Labs ,Burlingame ,California ,USA) and the CYP4Z1 polyclonal antibody. The tumor slides were de-paraffinized in xylene and rehydrated in ethanol and thereafter incubated for 5 minutes in a 3% hydrogen peroxide to inhibit the endogenous peroxidase activity .To avoid non-specific background staining , the sliders were blocked with 1.5% goat serum for 20 minutes in citrate buffer ( 10 mM citrate buffer ,PH 6.O), followed by incubation with CYP4Z1 antibody , at 4°C overnight .
The antibody was used at 1:1000 dilution .Then the sample was rinsed and incubated with biotinylated secondary antibodies and afterward rinsed and incubated with avidin-biotin-peroxidase complex (Vector laboratories Ltd ,pet ,Peterborough UK) for 30 minutes at room temperature . Visualization of immunostaining was achieved by immersion of slides in 0.05% 3,3-Diaminobenzidine tetrahydrochloride chromogene (DAB ) , subsequently counterstaining with hematoxylin .
Evaluation of immunohistochemistry Scoring :Each time a set of tumor samples was stained , references slices were involved as well as one negative slice incubated with pre-immune serum . The whole tumor slide was scored . The scoring was based on the highest intensity found in the tumor that covered at least 5% of the tumor area . Two independent investigators (N.S and A.O), blinded to clinical data , scored the specimens . Scoring divergences were resolved by consent after re-examination .
For CYT4Z1-expression , cells were considered positive if they demonstrated clear membranous and/or cytoplasmic immunolabeling .The scoring result were calculated as opposed to strength of staining in an individual cells . The scoring results of each immunlabeling of specific cells types were presented as non(0) ,weak(1) , moderate(2) and intense (3) .
The sections were ‘weak’ CYP4Z1 score when less than 33% of cells had expression . A score of ‘moderate’ was used to cells which had expression on 33% to 66% of the section while the score ‘high’ denoted sections had expression on more than 67% of the cells .Scores 0 and 1 were grouped together as negative-weak expression while scores 2 and 3 were grouped as medium – high expression .
The resulting slides were viewed and analyzed by using a Leica BMRM microscope ( Leica DMRB , Wetzler , Germany ) with images digitally captured and processed using a Leica MPS52 Camera (Q imaging , Germany ) and Ac Quis imaging capture system ( synoptics , Cambridge , UK ) respectively . Statistical analysis of data was carried out using the available statistical package of SPSS-25 (Statistical Packages for Social Sciences- version 25). Data were presented in simple measures of frequency & percentage.
The significance of difference of different percentages (qualitative data) were tested using Pearson Chi-square test ((2-test) with application of Yate’s correction or Fisher Exact test whenever applicable. Statistical significance was considered whenever the P value was equal or less than 0.05 .
Results
All the demographic and pathological data for study cases are shown in Table -1 and Table-2.
CYP4Z1 Expression
The staining was predominately localized to cell membrane/ cytoplasm without any significant nuclear staining and the staining was uniform across all sections with varying degree of staining intensity ( Figure .1).
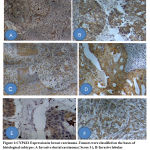 |
Figure 1: CYP4Z1 Expression in breast carcinoma . |
The results showed frank difference in CYP4Z1 expression in malignant samples in contrast to benign samples , where 85 cases (70.8 %) of malignant samples showed a moderate-intense expression which is considered as high score overexpression , 35 (29.2%) of malignant samples showed no or weak CYP4Z1 expression , while no benign case showed moderate-intense expression . The infiltrative ductal carcinoma showed moderate-strong expression in 69 cases (80.2% ) out of 86 studied samples in contrast to other subtype of mammary carcinoma which were more lower than in other carcinoma subtypes with statistic significant difference ( P =000.1) . In our study, patients were classified into two main age groups ,the first which were younger than 50 years old were 69/128 (53.9%) ,27 (39.1%) of them were with weak or negative score and 42(60.9%) were with high score of CYP4Z1 expression .The other 59 (46%) of studied cases were older than 50 years ,16 (27%) of them were with weak or negative expression and 43 ( 72.9% ) were with high CYP4Z1 expression .
Among 43 negative cases , 27 cases ( 62.8%) were younger than 50 years old and the 16 cases (37.2%) were older than 50 years . Among 85 positive cases , 42 cases (49.4%) were younger than 50 year old and 43 cases ( 50.6%) were older than 50 years old with no statistic significant difference ( P=0.152). Family history were documented in 34 cases of those with breast carcinoma ( 28.3%) with strong CYP4Z1 expression seen in 30 cases of them (88.2%) with significant statistic difference ( P=0.008) . In our study the mean patients was age 47.5 years , 53.9% of them were younger than 50 year , 60% of them show CYP4Z1 overexpression .The peak age frequency of 50-59 year was reported in our study which included 28.1% of breast carcinoma patients , 69.4% show moderate-intense expression . The mean age for breast carcinoma patients with CYP4Z1 overexpression was 49 years ,54.5% of them show moderate-intense expression .and 78% of those over 60 year old and we find a statistic significant association between the age and the tumor expression of CYP4Z1 which reflects gradual increase of CYP4Z1 expression of with age ( P=0.038). (Table-1).
In our study , the most common histological subtypes were invasive ductal carcinoma -not otherwise specified ( 67.2%),followed by intraductal carcinoma (9.4%) , invasive lobular carcinoma (6.3%) and mucinous carcinoma (4.7%) .(Table-1) . The CYP4Z1 overexpression was high in carcinoma of grade II ( 83%) , lower in grade I ( 63.6%) and intermediate in those of grade III cases with no statistic significant difference (P=0.100) .(Table-1) Regarding TNM stage of breast carcinoma, the tumor size ranged from 0.9 cm to 6.3 cm with mean size of 2.8 cm and CYP4Z1 expression show no statistic significant difference regarding TNM stage and lymph nodes involvements ( P=0.708 ) in spite of expression was more frequently seen in T3(80%) . The majority of carcinoma cases seen in T2 stage 63( 52.5%) with no statistic clinical significance between tumor size and CYT4Z1 were expression (P =0.702).
Among patients with tumor size more than 5cm (T3), 80% had overexpression of CYP4Z1 , compared to 73% for those of T2 and 61% for those tumors smaller than 2 cm (T1) , i. e . CYP4Z1 expression increases intensity gradually with tumor size with no statistic significant difference (P=0.702). Lymph node status was evaluated for patients with mastectomy and axillary clearance ,85 cases( 70.8%) were with no lymph node involvements , only 35(29.2%) have lymph nodes metastasis , 80% of them (28/35) cases show moderate-intense CYP4Z1 overexpression in contrast to 67.1% ( 57/85 ) of studied samples showing no lymph nodes involvements with no statistical significance difference ( P =0.156) .
Table 1: Correlation of clinicopathological parameters and CYP4Z1 expression
| Z1 Cytochrome4z1 | P value | ||||
| Total | 0—1 | 2—3 | |||
| No (%) | No (%) | No (%) | |||
| Type | Malignant | 120 (93.7) | 35 (29.2) | 85 (70.8) | 0.0001* |
| Benign | 8 (6.3) | 8 (100) | – | ||
| Histopatholgoy | Invasive ductal CA | 86 (67.2) | 17 (19.8) | 69 (80.2) | 0.0001* |
| Intra ductal CA | 12 (9.4) | 4 (33.3) | 8 (66.7) | ||
| Invasive lobular CA | 8 (6.3) | 6 (75.0) | 2 (25.0) | ||
| Invasive papillaryCA | 2 (1.6) | 1 (50.0) | 1 (50.0) | ||
| Mucinous adeno CA | 6 (4.7) | 3 (50.0) | 3 (50.0) | ||
| Lobular CA insitu | 2 (1.6) | 1 (50.0) | 1 (50.0) | ||
| Metaplastic CA | 1 (0.8) | 1 (100) | – | ||
| Medullary CA | 1 (0.8) | 1 (100) | – | ||
| Paget’s disease | 1 (0.8) | – | 1 (100) | ||
| Undifferentiated CA | 1 (0.8) | 1 (100) | – | ||
| IDC *others; | Yes | 99 (82.5) | 21 (21.2) | 78 (78.8) | 0.0001* |
| No | 21 (17.5) | 14 (66.7) | 7 (33.3) | ||
| Age (years) | <30y | 6 (4.7) | 5 (83.3) | 1 (16.7) | 0.038* |
| 30—39 | 30 (23.4) | 7 (23.3) | 23 (76.7) | ||
| 40—49 | 33 (25.8) | 15 (45.5) | 18 (54.5) | ||
| 50—59 | 36 (28.1) | 11 (30.6) | 25 (69.4) | ||
| 60—69 | 14 (10.9) | 3 (21.4) | 11 (78.6) | ||
| =>70y | 9 (7.0) | 2 (22.2) | 7 (77.8) | ||
| Mean±SD | 48.3±13.0 | 46.3±13.0 | 49.3±12.9 | ||
| Age (years) | <50y | 69 (53.9) | 27 (39.1) | 42 (60.9) | 0.152 |
| =>50y | 59 (46.1) | 16 (27.1) | 43 (72.9) | ||
| Grade | [ I ] | 11 (9.2) | 4 (36.4) | 7 (63.6) | |
| [ II ] | 47 (39.2) | 8 (17.0) | 39 (83.0) | ||
| [ III ] | 54 (45.0) | 19 (35.2) | 35 (64.8) | ||
| Not | 8 (6.7) | 4 (50.0) | 4 (50.0) | 0.100
|
|
| Family history | Yes | 34 (28.3) | 4 (11.8) | 30 (88.2) | 0.008* |
| No | 86 (71.7) | 31 (36.0) | 55 (64.0) | ||
| *Significant difference between proportions using Pearson Chi-square test at 0.05level. | |||||
Table 2: Correlation of cancer stage and CYP4Z1 expression
| Cytochrome 4Z1 | P value | ||||
| Total | 0—1 | 2—3 | |||
| No (%) | No (%) | No (%) | |||
| TNM Staging | T1N0M0 | 16 (13.3) | 7 (43.8) | 9 (56.3) | 0.708 |
| T1N1M0 | 2 (1.7) | – | 2 (100) | ||
| T2N0M0 | 44 (36.7) | 13 (29.5) | 31 (70.5) | ||
| T2N1M0 | 16 (13.3) | 4 (25.0) | 12 (75.0) | ||
| T2N2M0 | 2 (1.7) | – | 2 (100) | ||
| T2N3M0 | 1 (0.8) | – | 1 (100) | ||
| T3N0M0 | 7 (5.8) | 2 (28.6) | 5 (71.4) | ||
| T3N1M0 | 8 (6.7) | 1 (12.5) | 7 (87.5) | ||
| T4N0M0 | 5 (4.2) | 1 (20.0) | 4 (80.0) | ||
| T4N1M0 | 2 (1.7) | – | 2 (100) | ||
| T4N2M0 | 1 (0.8) | 1 (100) | – | ||
| TisN0M0 | 13 (10.8) | 5 (38.5) | 8 (61.5) | ||
| TisN1M0 | 3 (2.5) | 1 (33.3) | 2 (66.7) | ||
| Tumor size | T1 | 18 (15.0) | 7 (38.9) | 11 (61.1) | 0.702 |
| T2 | 63 (52.5) | 17 (27.0) | 46 (73.0) | ||
| T3 | 15 (12.5) | 3 (20.0) | 12 (80.0) | ||
| T4 | 8 (6.7) | 2 (25.0) | 6 (75.0) | ||
| Tis | 16 (13.3) | 6 (37.5) | 10 (62.5) | ||
| LN involvement | Yes | 35 (29.2) | 7 (20.0) | 28 (80.0) | 0.156 |
| No | 85 (70.8) | 28 (32.9) | 57 (67.1) | ||
| LN involvement | LN0 | 85 (70.8) | 28 (32.9) | 57 (67.1) | 0.356 |
| LN1 | 31 (25.8) | 6 (19.4) | 25 (80.6) | ||
| LN2 | 4 (3.3) | 1 (25.0) | 3 (75.0) | ||
| *Significant difference between proportions using Pearson Chi-square test at 0.05 level. | |||||
Discussion
Breast carcinoma is a disease with a great heterogeneity in its clinical behavior and its prognosis is related to a large variety of clinical and pathological factors . Despite the growing evidence for the contribution of CYP4Z1 to tumor malignancy ,there were limited studies looking for CYP4Z1 expression in breast carcinoma and no study looking for CYP4Z1 in metastatic breast carcinoma. (18).
While the CYP4Z1 substrate is unknown, CYP4Z1 mRNA was detected in normal mammary gland tissue and breast carcinoma tissue , whereas only minimal expression was found in all other tissues [9]. In the present study , CYP4Z1 overexpression was seen in 70.8% of Iraqi female patients’ breast carcinoma with more predominant in T3(80%) . This reflects the correlation between CYP4Z1 expression and development of tumor in contrast to benign tumors that show no CYP41 expression. This CYP4Z1 overexpression is associated with high stage and it correlates with poor prognosis .
These results came in accordance with preceding study concerned with CYP4Z1 expression in breast carcinoma (20).This overexpression is verified by other studies which show that CYP4Z1 overexpression is concomitant with increased production of 20-hydroxyeicosatetraenoic acid (20-HETE) in breast carcinoma , and it has been assumed that CYP4Z1 metabolizes arachidonic acid to 20-HETE resulting in enhanced growth and spread of breast cancer cells [15,16].
The current study which includes 120 patients with breast carcinoma of various types and stages proved that CYP4Z1 is expressed to various extent in breast carcinoma and that 70.8% exhibit an overexpression and this expression was heterogeneous in the tumor as there were areas with stronger staining and other areas with weaker staining within the same tumor slice .
IHC scoring is a semi quantitative method , which represents a weakness of our study and we tried to standardize the analysis by grading the slide using the most strongly stained area ,exceeding 5% of the tumor area on each slide . Numerous evidence indicates the importance of CYP4Z1 expression in breast carcinoma where it is increasingly recognized as a potential marker or a target for advance of new tumor therapy . [17] .In the present study , we present convincing evidence of IHC CYP4Z1 expression in breast carcinoma and we demonstrate generally absent or weak expression in benign breast lesions .
As the sample size of our study was to small , strong statistically significant correlation between CYP4Z1 expression and some of clinicopathologic features were not possible .However , some clear significant statistic correlation , particularly with prognostic factors of carcinoma (Age , family history and carcinoma subtypes ) is documented and this reflects a difference in correlation of CYPp450 in certain families and role of family history in women who have a first degree relative with breast carcinoma which show a statistically significant difference with CYP4Z1 expression that supports the previous data that verify the polymorphism of CYP4Z1 in populations that reflected in susceptibility of them for various types of malignancies and so our result data suggest that CYP4Z1 may be associated with the breast cancer health disparity among the societies.
The result confirms the suggested progressive effect of CYP4Z1 with age specially in invasive ductal carcinoma subtype of carcinoma and all the metastatic carcinoma expression CYP4Z1 which had unfavorable disease prognosis markers so that further sample size may be better delineate the relationships . The statistic significant association between the age and the tumor expression of CYP4Z1 which reflects an expression of CYP4Z1 increases gradually with age and plays an essential role in promoting carcinogenesis , angiogenesis and tumor aggressiveness.
Although the accurate role of CYP4Z1 in tumor progression is unclear , these data indicate that CYP4Z1 has been involved in the pathogenesis of tumor progression. The low number of analyzed lymph nodes that were identified by axillary dissection may cause underestimation of the number of stage III tumors . Many clinical studies have established that alteration in CYP4Z1 expression predicts poor prognosis for breast cancer and it is associated with features of tumor aggressiveness and also a predictive marker of responsiveness to selected forms of therapy [ 17] .
Additionally, it was described that expression of CYP4Z1 gene is up regulated by activated glucocorticoid and progesterone receptors [21]. In our study , all benign breast lesion demonstrated negative or weak CYP4Z1 expression and this may be attributed to low protein expression of CYP4Z1 , protein conformational changes ,or protein degradation and these results agree with those reported in previous studies [20] .
Our study showed a propensity of CYP4Z1 overexpression to be more associated with larger tumor size (Table-2).Although this difference was not statistically significant but the portion of tumors larger than 5cm have higher rate of CYP4Z1 overexpression than those smaller than 5cm in size ( 80% in T3 versus 61% in T1) .This result may reflect CYP4Z1 role in enhancing progressive proliferation of malignant cells that result in increase in tumor mass size ,which is in concordance with data reported in other literature that show significant increase of tumor weight and microvessel density with CYP4Z1 overexpression by 2.6-fold and 1.9-fold in human tumor xenograft models, respectively [17]. The highest CYP4Z1 expression was seen in invasive ductal carcinoma, NOS (80%), which is known to have an aggressive clinical course , very often resulting in early recurrence and death .This difference is significant statistically . 66.7%% of intraductal carcinoma show overexpression of CYP4Z1 , while 25% of those of infiltrative lobular carcinoma .This lower CYP4Z1 expression may reflect the lower aggressiveness of infiltrative lobular carcinoma in contrast to invasive ductal carcinoma .
This supports the view that CYP4Z1 overexpression is correlated with tumor aggressiveness , tumor progression and statistic significance that seen in other literatures [15] in spite of some limitations that should be considered when interpreting our results which are limited by relatively small number of the non ductal subtype of mammary carcinoma .
Our study has correlating CYP4Z1 overexpression with grade (83% in grade II ,63% in grade I and 64% in grade III ) with no statistically significant difference in contrast breast carcinoma with no grade classification ( mucinous carcinoma , metaplastic carcinoma , medullary carcinoma ) that has lower CYP4Z1 expression in 50% . This verifies the correlation between the tumor aggressiveness and CYP4Z1 expression .This is in concordance with a previous study that showed CYP4Z1 overexpression which was specifically associated with increasing tumor grade of breast cancer as well as inferior patient outcome in mammary and ovarian cancer [2,6,20) .
The prognostic importance of CYP4Z1 has also been investigated in the context of patients with or without lymph nodes involvements ,where overexpression was seen in 67% for those with no lymph nodes involvements(N0) and more with N1(80%) and N2( 75%). Although there is no significant statistic difference ,it still reflects the correlation of higher expression of CYP4Z1 with higher tumor stages including lymph nodes involvement and so aggressiveness of tumor .
Conclusion
This study has defined the expression profile of CYP4Z1 in mammary cancer and it may offer the potential application as biomarkers to distinguish between benign and malignant breast disease growths and be a prognostic biomarker for malignant progression in these tumors . The underlying mechanism at the cellular level remains unclear , but it seems to play an important role in controlling the functions of other molecules enhancing tumor development and metastasis ,Therefore , the impact of CYP4Z1 expression on other molecules functions is interesting to be investigated.
Funding
The authors received no financial support for the research , authorship , and/or publication of the article
References
- Jemal, A., Bray, F., Center, M.M., Ferlay, J., Ward, E. and Forman, D .Global Cancer Statistics. CA: A Cancer Journal for Clinicians. 2011; 61: 69-90.
- Lauby-Secretan, B. , Scoccianti, C. , Loomis, D., Benbrahim-Tallaa, L., Bouvard, V., Bianchini, F.et al. Breast-Cancer Screening—Viewpoint of the IARC Working Group. NELM 2015; 372(24): 2353-8 .
- Najjar, H. and Easson, A .Age at Diagnosis of Breast Cancer in Arab Nations. International Journal of Surgery.2010; (8) : 448-452.
- Republic of Iraqi .Ministry of Health and Environment . Iraqi cancer board .Annual Report :Iraqi cancer registry 2016 .Available at :http://moh.gov.iq/upload/upfile/ar/882.pdf .
- Majid, R.A., Mohammed, H.A., Hassan, H.A., Abdulmahdi, W.A., Rashid, R.W. and Hughson, M.D. A Population-Based Study of Kurdish Breast Cancer in Northern Iraq: Hormone Receptor and HER2 Status. A Comparison with ARABIC Women and United States SEER Data. BMC Women’s Health.2012; 12. 16.
- Tiwari, V., Pande, S.C. , Verma, K. and Goel, S. Is Multiparity a Protective Mechanism in Iraqi Females with Breast Cancer? Need for Detailed Analysis. Clinical Cancer Investigation Journal.2015;4: 292-293.
- Al Alwan, N . Iraqi Initiative of a Regional Comparative Breast Cancer Research Project in the Middle East. Journal of Cancer Biology & Research .2014 ;2: 1016
- Assi, H.A., Khoury, K.E., Dbouk, H.,Khalil, L.E., Mouhieddine, T.H. and El Saghir, N.S. Epidemiology and Prognosis of Breast Cancer in Young Women. Journal of Thoracic Disease . 2013;5 :S2–S8.
- Downie D, Mc Fadyen MC, Rooney PH, Cruickshank ME, Parkin DE, Miller ID et al. Profiling cytochrome P450 expression in ovarian cancer: identification of prognostic markers. Clinical Cancer Research . 2005; 11: 7369–7375 .
- Murray GI, Patimalla S, Stewart KN, Miller ID, Heys SD. 2010 Profiling the expression of cytochrome P450 in breast cancer. Histopathology. 2010; 57:202–211.
- Hannemann F, Bichet A, Ewen KM, Bernhardt R. Cytochrome P450 systems-biological variations of electron transport chains. Biochim Biophys Acta .2007; 1770: 330–344.
- Rieger MA, Ebner R, Bell DR, Kiessling A, Rohayem J, Schmitz M et al. Identification of a novel mammary-restricted cytochrome P450, CYP4Z1, with overexpression in breast carcinoma. Cancer Research 2004; 64: 2357–2364.
- Zöllner A, Dragan CA, Pistorius D, Muller R, Bode HB, Peters FT et al. Human CYP4Z1 catalyzes the in-chain hydroxylation of lauric acid and myristic acid. Biological Chemistry 2009; 390: 313–317
- YangX, Hutter M ,Goh WWB , Bureik M . Curr Pharm Des. CYP4Z1-A human cytochrome P450 enzyme that might hold the key to curing breast cancer . Current Pharmaceutical Design, 2017 ; 23(14): 2060-2064.
- Yu W, Chai H, Li Y, Zhao H, Xie X, Zheng H, et al . Increased expression of CYP4Z1 promotes tumor angiogenesis and growth in human breast cancer. Toxicol Appl Pharmacol . 2012; 264(1):73–83.
- Zheng, L., Li, X., Gu, Y., Lv, X. & Xi, T. 2015 The 3′UTR of the pseudogene CYP4Z2P promotes tumor angiogenesis in breast cancer by acting as a ceRNA for CYP4Z1. Breast Cancer Research and Treatment .2015;150: 105–118 .
- Yu W, Chai H, Li Y, Zhao H, Xie X, Zheng H, et al . Increased expression of CYP4Z1 promotes tumor angiogenesis and growth in human breast cancer . Toxicology and Applied Pharmacology. 2012 Oct 1; 264(1): 73–83.
- Rieger MA, Ebner R, Bell DR, Kiessling A, Rohayem J, Schmitz M, et al. Identification of a novel mammary-restricted cytochrome P450, CYP4Z1, with overexpression in breast carcinoma. Cancer Research .2004; 64:2357–2364.
- Allred DC, Harvey JM, Berardo M , Clark GM : Prognostic and predictive factors in breast cancer by immunhistochemical analysis .Modern Patholology .1998 ; 11:155-68 .
- Murray GI , Patimalla S, Stewart KN, Miller ID, Heys SD . Profiling the expression of cytochrome P450 in breast cancer. . Histopathology. 2010 ;57(2):202-11.
- Li Y, Steppi A, Zhou Y, Mao F, Miller PC , He MM, et al . Tumoral expression of drug and xenobiotic metabolizing enzymes in breast cancer patients of different ethnicities with implications to personalized medicine . Sci Rep . 2017 ;7(1) :4747 .
Abbreviation
CYP4Z1 : Cytochrome 4 Z 1.
20-HETE : 20-hydroxyeicosatetraenoic acid .
SPSS-25 : Statistical Packages for Social Sciences- version 25.
CYPp450 : Cytochrome P450







