Ezz El Din Mostafa Abd El Wahed Shalaby1*, Iman F. Gaballah1 and Asmaa Anwar Kadry 2
1Forensic medicine and clinical toxicology department, faculty of medicine- Cairo university, Egypt
2National Egyptian center of environmental and toxicological Research (NECTR) , faculty of medicine Cairo university, Egypt.
Corresponding author E-mail: aakfamily2020@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1903
Abstract
Phthalates are widely used in softening plastics especially it is cheap. But due to its harmful effect on health in many countries non phthalate plasticizers they are used instead in many products. As Phthalates rapidly metabolized and excreted in urine and feces.in the form of Monoesters (mono butyl phthalate) which can be measured in urine and breast milk using High Performance Liquid Chromatography (HPLC), So aim of our study to detect and counting of Mono-n-Butyl phthalate (MnBp) in the breast milk and urine of 20, randomly selected, lactating females house wife’s with mean age of mothers from 17-39 years from Helwan Primary Health Center – Cairo – Egypt after their consent. . with detailed history regarding Sample analysis was conducted in The Micro Analytical Center – Faculty of Science – Cairo University using HPLC machine . As high non-occupational human exposures due to wide uses of phthalates is reported, so in our study we assess phthalate level in breast milk and urine in correlation with uses of personal care products(Cosmetics),Vinyl use in walls and in home flooring , drinking and eating in plastics containers and in relation to smoking. Our study shows a high statistically significant relation between phthalate level in mothers' urine and in breast milk with PVC or Vinyl use in walls and in home flooring, while its level in mothers' urine was highly significant in cases of drinking in plastic bottles and in eating in plastic containers. shows a high statistically significant relation between phthalate level in mothers' urine and in breast milk with PVC or Vinyl use in walls and in home flooring, while its level in mothers' urine was highly significant in cases of drinking in plastic bottles and in eating in plastic containers.
Keywords
Mono-N- Butyl Phthalate; HPLC; Breast Milk; Urine
Download this article as:| Copy the following to cite this article: Shalaby E. E. D. M. A. E. W, Gaballah I. F, Kadry A. A, Evaluation of Phthalate in Breast Milk and Urine of Lactating Women in Egypt. Biomed Pharmacol J 2019; 13(1). |
| Copy the following to cite this URL: Shalaby E. E. D. M. A. E. W, Gaballah I. F, Kadry A. A, Evaluation of Phthalate in Breast Milk and Urine of Lactating Women in Egypt. Biomed Pharmacol J 2019; 13(1). Available from: https://bit.ly/2QS5iPp |
Introduction
Phthalates,1 or phthalate esters are added to plastics making them more flexible, durable, transparent and long lived; therefore they are used in softening polyvinyl chloride (PVC). Due to low cost, di(2-ethylhexyl) phthalate (DEHP) was the main plasticizer and benzyl butyl phthalate (BBP) is used in the manufacture of flooring material. In the US, Canada and European Union, they are replaced by many products due to health concerns where non phthalate plasticizers are used instead 2
They are also found in modern electronics, catheters, enteric coatings of pharmaceutical pills, blood transfusion devices as well as nutritional supplements and personal-care products (perfumes, eye shadows, moisturizers, nail polish, liquid soap, hair spray).3 This leads to high non-occupational human exposure (ingestion, dermal contact and,4 parenteral exposure).5
They are rapidly metabolized and excreted in urine and feces.4 Monoesters (mono butyl phthalate) are excreted unchanged in urine and feces or may undergo biotransformation to produce more water soluble glucuronide conjugates increasing urinary excretion.4,6 Monoesters can be measured in urine, breast milk, serum, saliva, seminal plasma and amniotic fluid using High Performance Liquid Chromatography (HPLC).7,8,9
During gestation, phthalates may cause male infertility where semen quality and volume are decreased and sperms show increased damaged DNA and decreased motility.10,11,12,13,14 Exposure may be also associated with diabetes,15,16 breast cancer, obesity, metabolic disorders, and immune function.17 It may also develop ADHD, autistic behaviors in children.18,19
So in our study, detection of Monoesters (mono butyl phthalate) by HPLC is very important to be detected in breast milk and urine of lactating women is important to avoid potential risk of phthalate toxicity to mother as well as her newborn infant.
Aim of the Study
Since phthalates are proven harmful to the health of human beings and newborn infants (especially neurodevelopment), so our study is concerned about assessing and detecting phthalates in milk of lactating women in different stages of lactation as milk is the main nutritional source for newborns and infants compared to urinary detection of exposure level of mothers.
Methodology
Mono-n-Butyl phthalate (MnBp), a metabolite of di-n-butyl phthalate was detected in the breast milk and urine of 20, randomly selected, lactating females house wife’s (to exclude occupational exposures) from Helwan Primary Health Center – Cairo – Egypt.
Written informed consents from all participant in the study with no disclosure for names or any data of patients as we use serial number in all our samples and the consent include acceptance for publication of the data as well as the approval of Human Research Ethical Committee were done. Sample analysis was conducted in The Micro Analytical Center – Faculty of Science – Cairo University.
History
Personal history à name, age, marital status, special habits of medical importance.
Medical history à hypertension, diabetes, kidney or liver disease, any neurological symptoms as well as history of blood transfusion or intravenous drug intake.
Exposure history à smoking (active or passive) and duration of exposure, cosmetic use, drinking in plastic bottles and eating in plastic containers.
Surgical history.
Questionnaire
Table 1: English translated questionnaire:
| Name: | |||
| Age: | |||
| Occupation: | |||
| Present history: | Complaint | ||
| System related to complaint | |||
| Past history | Similar problems: | ||
| previous illness (DM, HTN, bronchial asthma, others) | |||
| operations | |||
| blood transfusion | |||
| hospital admissions | |||
| Drug history | Medications or over the counter drugs à regular or irregular intake and duration of intake. | ||
| Illicit drug use. | |||
| Family history | Similar problems. | ||
| DM, HTN, bronchial asthma. | |||
| Social history | Daily activity. | ||
| Smoking, alcohol. | |||
| Family. | |||
| Potential Sources of Exposure to Phthalates | |||
| Occupational Exposure | |||
| PVC plastic factories | |||
| Perfume industry | |||
| Cosmetic industry | |||
| Dietary Exposure | |||
| Container type | Frequency/week | ||
| Consumer Products | |||
| Household cleaners 2hours/ week | |||
| Perfumes | |||
| Soaps , shampoos | |||
| Material Used in Building | |||
| Home built time period | |||
| 1969 or earlier | |||
| 1970-1979 | |||
| 1980-1989 | |||
| 1990-1999 | |||
| 2000-2010 | |||
| In last year | |||
| Flooring material | |||
| Poly vinyl flooring | |||
| Wood | |||
| Wall materials | |||
| Wall paper | |||
| Vinyl | |||
| Wood | |||
| Textile | |||
| Ventilation system | |||
| Natural | |||
| Mechanical exhaust | |||
| Mechanical exhaust and supply | |||
| Medical Exposure | |||
| Chronic need for blood transfusion | |||
| Chronic use of any IV preparations | |||
| Do you agree to share in research about phthalate exposure | NO | YES | |
Laboratory Investigations
Comparative assessment of MnBP in breast milk and urine obtained from 20 lactating females.
MnBp (>99.9%) à from FLUKA, Inc.
Methanol, acetonitrile, ethyl acetate and dichloromethane à from Carlo Erba group, Inc. All solvents are HPLC grade.
β-glucuronidase (Escherichia coli-K12) à from Roche Biomedical.
Water was purified using a direct-Q gradient 8 UV system (Millipore).
Sample collection
Urine Specimens
Urine specimens were labeled with the subject identification number.
Turbid samples or those containing blood were excluded.
They were then analyzed by HPLC in the laboratory of Faculty of Science – Cairo University.
Collected urine samples (pooled from individuals) were stored at -40 °C.
In this study, plastic equipment’s were not used to avoid contamination; all glass apparatuses were washed with chromic acid solution and rinsed with deionized water and methanol before drying.
Breast Milk Specimens
A single sample was collected from each lactating female.
To assess average exposure, samples consisted of many small aliquots collected over successive infant feeds up to a volume of 50ml.
Samples were collected and stored in 250-mL Pyrex glass bottles with Teflon-coated caps.
Oral and written instructions were given to mothers to feed their baby first before milk aliquot collection (hind milk).
This collection method was chosen to ensure that breastfeeding had been well established beforehand.
- Each sample was frozen consecutively in a household freezer at -20°C in a single glass bottle as additive aliquots and delivered frozen for analysis.
- Breast milk samples with a volume > 50 mL were included in the analyses. [20]
HPLC apparatus
A high-pressure isocratic system was used, consisting of a Dionex Ulti Mate 3000 UHPLC; RS pump, auto sampler, column compartment, and Diode Array detector (2012).
Column C8
Mobile phase à acetonitrile + 0.1% acetic acid = 100% UV/VIS detector
WL 254nm
Flow rate 1ml/minute
Injection volume 20 UL
 |
Figure 1: Pump |
 |
Figure 2: Auto Sampler |
 |
Figure 3: Column Compartment |
 |
Figure 4: Diode Array Detector |
Chromatographic column reversed phase 150 mm × 4.6 mm Hypersil BDS, C18 particle size 5U.
 |
Figure 5: BDS Hypersil Column |
Solid Phase Extraction
Hypersep glasses block 16 port vacuum manifolds and vacuum pump ROCKER 400 Thermo scientific.
SPE columns were purchased from THERMO SCIENTIFIC. HYPERSEP C8 500MG/3ML/50PKG.
 |
Figure 6: Vacuum Manifold and Vacuum Pump |
 |
Figure 7: Solid Phase Extraction Column |
 |
Figure 8: Balance |
 |
Figure 9: Milli-Q Water Purification System |
Dimension RxL Max analyzer (Siemens Healthcare GmbH-HenKestr. 127, 91052 Erlangen, Germany) by colorimetric techniques.
Stock Solution
Stock solution was prepared using 10mg analytical standard added to 10ml of acetonitril.
Each 1ml of the solution contains 1mg of mono butyl phthalate.
Sample Pretreatment (Solid Phase Extraction)
Urine samples were thawed and vortexed homogeneously.
Each 950-µL urine sample was transferred into a glass tube. Then, 5 µL of β-glucuronidase (200 U/mL) and 245 µL ammonium acetate buffer (1 M, pH 6.5) were added to the tube and vortexed in turn.
Samples were then incubated at 37 °C for 90 min. [21]
Solid Phase Extraction
Conditioning àwith 1mL methanol, 1mL acetonitrile, and 1mL phosphate buffer solution (pH 2.0) were added successively.
Loading à 1mL urine sample was diluted with 1mL phosphate buffer solution (pH 2.0) and added to the SPE column.
Wash à cartridges were then washed with 2mL formic acid solution (0.1M) and 1mL water. Cartridges were dried under negative pressure. The target analytes were?
Elution à sequentially with 1mL acetonitrile and 1mL ethyl acetate, the eluent was collected together, concentrated, and evaporated.
The dry residue was reconstituted with 200 µL of 1:9 (v/v) acetonitrile–water. [22]
Chromatographic conditions
Mobile Phase (0.1% acetic acid in acetonitrile).
To make 1L, 1.0 mL of acetic acid is added to 1000 mL HPLC grade acetonitrile.
This solution is stored at room temperature in an amber bottle.
Column temperature was set at 40 °C.
Sample injection volume was 20µL.
Flow rate was 0.3 mL/min.
UV 254.23
Method Validation
The analytical method was validated to demonstrate:
Linearity
LOD & LOQ
Accuracy
Precision
Statistical analysis
Recorded data was analyzed using the statistical package for social sciences, version 20.0 (SPSS Inc., Chicago, Illinois, USA).
Quantitative data was expressed as mean ± standard deviation (SD).
Qualitative data was expressed as frequency and percentage.
The following tests were done:
Independent-samples t-test of significance was used when comparing between two means.
Pearson’s correlation coefficient (r) test was used to assess the degree of association between two sets of variables
The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant as the following (probability: P-value):
– P ≤ 0.05 à significant.
– P ≤ 0.001 à highly significant.
– P > 0.05 à insignificant.
Table 2
| Total number (n) = 20 | |
| Age of mother (years) | 17-39 yrs. [27.80±5.70] |
| Age of infant (months) | 2-24 months [9.75±6.89] |
| PVC, Vinyl use in walls and in home flooring | |
| Yes | 4 (20.0%) |
| No | 16 (80.0%) |
| Active and passive smoking | |
| Yes | 15 (75.0%) |
| No | 5 (25.0%) |
| Cosmetic use | |
| Yes | 4 (20.0%) |
| No | 16 (80.0%) |
| Drinking in plastic bottles | |
| Yes | 14 (70.0%) |
| No | 6 (30.0%) |
| Eating in plastic containers | |
| Yes | 14 (70.0%) |
| No | 6 (30.0%) |
| Phthalate level in mothers’ urine (mg/L) | 0-24.22[12.52±6.65] |
| Phthalate level in breast milk (mg/L) | 0.02-8.80[2.51±2.92] |
Result
Distribution of all examined parameters of the study group.
As observed in table (2), the mean age of mothers (years) was 17-39 years [27.80±5.70], the mean age of infants in months was 2-24 months [9.75±6.89], number of those using PVC and/or Vinyl in home walls and flooring are 4 (20.0%), number of active and passive smokers was 15 (75.0%), those using cosmetics were 4 (20.0%), mothers drinking in plastic bottles were 14 (70.0%) while those eat in plastic containers were 14 (70.0%). Mean phthalate level in mothers’ urine was 0-24.22µg/l [12.52±6.65] and in breast milk 0.02-8.80µg/l [2.51±2.92].
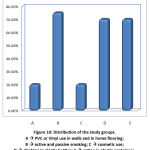 |
Figure 10: Distribution of the study groups. A à PVC or Vinyl use in walls and in home flooring; B à active and passive smoking; C à cosmetic use; D à drinking in plastic bottles; E à eating in plastic containers.Click here to view Figure |
Table 3: Correlation between phthalate level in mothers’ urine (µg/L) and in breast milk (µg/L) with all parameters, using Pearson Correlation Coefficient of the study group.
| Phthalate level in mother urine (µg/L) | Phthalate level in breast milk (µg/L) | ||
| Age of mother (years) | r | 0.381 | 0.518 |
| p-value | 0.098 | 0.019* | |
| Age of infant (months) | r | 0.334 | 0.244 |
| p-value | 0.150 | 0.299 | |
| Phthalate level in mother urine (µg/L) | r | — | 0.459 |
| p-value | — | 0.028* | |
r-Pearson Correlation Coefficient;
p-value>0.05 NS; *p-value <0.05 S; **p-value <0.001 HS
as seen in table (3), there was a positive correlation and a statistical significance between phthalate level in mothers’ urine in relation to their age and a significant correlation was detected between phthalate level in breast milk and in mothers’ urine (µg/L).
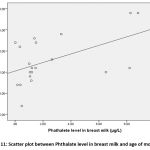 |
Figure 11: Scatter plot between Phthalate level in breast milk and age of mothers. |
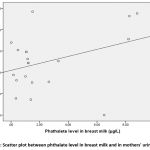 |
Figure 12: Scatter plot between phthalate level in breast milk and in mothers’ urine (µg/L). |
Table 4: Relation between phthalate level in mothers’ urine (mg/L) and in breast milk (mg/L) with PVC or Vinyl use in walls and in home flooring, active and passive smoking, cosmetic use, drinking in plastic bottles and eating in plastic containers of the study group.
| Parameters | Phthalate level in mother urine (µg/L) | Phthalate level in breast milk (µg/L) | |||
| Mean | ±SD | Mean | ±SD | ||
| PVC or Vinyl use in walls and in home flooring | Yes | 22.25 | 3.00 | 6.69 | 3.47 |
| No | 10.09 | 4.77 | 1.46 | 1.59 | |
| Independent Sample t-test | t-test | 23.089 | 21.225 | ||
| p-value | <0.001** | <0.001** | |||
| Active and Passive smoker | Yes | 13.25 | 6.70 | 2.58 | 3.11 |
| No | 10.35 | 6.72 | 2.29 | 2.53 | |
| Independent Sample t-test | t-test | 0.699 | 0.037 | ||
| p-value | 0.414 | 0.850 | |||
| Cosmetic use | Yes | 13.71 | 8.48 | 1.29 | 0.19 |
| No | 12.23 | 6.42 | 2.81 | 3.21 | |
| Independent Sample t-test | t-test | 0.152 | 0.868 | ||
| p-value | 0.702 | 0.364 | |||
| Drink in plastic bottles | Yes | 15.49 | 5.27 | 2.81 | 3.16 |
| No | 5.60 | 3.70 | 1.81 | 2.36 | |
| Independent Sample t-test | t-test | 17.213 | 0.474 | ||
| p-value | <0.001** | 0.500 | |||
| Eat in plastic containers | Yes | 15.49 | 5.27 | 2.81 | 3.16 |
| No | 5.60 | 3.70 | 1.81 | 2.36 | |
| Independent Sample t-test | 17.213 | 0.474 | |||
| <0.001** | 0.500 | ||||
p-value>0.05 NS; *p-value <0.05 S; **p-value <0.001 HS
table (4) shows a high statistically significant relation (p<0.001) between phthalate level in mothers’ urine and in breast milk with PVC or Vinyl use in walls and in home flooring, while its level in mothers’ urine was highly significant (p<0.05) in cases of drinking in plastic bottles and in eating in plastic containers.
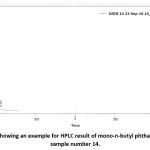 |
Figure 13: A curve showing an example for HPLC result of mono-n-butyl phthalate level in urine in sample number 14. |
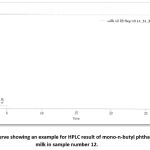 |
Figure 14: A curve showing an example for HPLC result of mono-n-butyl phthalate level in milk in sample number 12. |
Discussion
In this study, 20 lactating females were randomly selected from Helwan – Cairo – Egypt, with mean age of mother 17-39 years [27.80±5.70] and mean age of infants 2-24 months [9.75±6.89]. Mothers having PVC and/or Vinyl in walls and in home flooring were 4 (20.0%), active and passive smokers were 15 (75.0%), those using cosmetics were 4 (20.0%), mothers drinking in plastic bottles were 14 (70.0%) while those eating in plastic containers were 14 (70.0%).
Mean phthalate level in mothers urine was 0-24.22 µg/l [12.52±6.65] and in breast milk was 0.02-8.80 µg/l [2.51±2.92].
There was a positive correlation and a statistical significance between phthalate level in mothers’ urine in relation to their age and a significant correlation was detected between phthalate level in breast milk and in mothers’ urine (µg/L).
The current study shows a high statistically significant relation between phthalate level in mothers’ urine as compared to its level in breast milk using PVC or Vinyl in the walls and home flooring. This goes with Allan C. Just et al., (2015) which showed an increase in urinary metabolites of phthalate in Vinyl flooring in homes with an increased risk of bronchial asthma.24
Phthalate levels were highly significant in the urine of mothers drinking in plastic bottles and eating in plastic containers which was consistent with Dong Rui Hua et al., (2017) which showed that diet was a major exposure source for phthalates and that it is likely that plastic containers contributed to phthalate contamination of foods.25
On the other hand, results were not consistent with Rose O. Sulentic et al., (2018) who did not identify water consumption or consumer product use as major sources contributing to phthalate exposure.26
No significant correlation was observed between active and passive smoking and cosmetic use with mono-butyl-phthalate level in mothers’ urine and in breast milk samples; on the contrary, Lauren E. Parlett and colleagues (2013) showed that personal care products (PCPs) use was widespread in this group of recently pregnant females. Female’s use of PCPs, particularly perfumes and fragrance products, was positively associated with urinary concentration of multiple phthalate metabolites,27 that mostly explained that in our study random sample of lactating females where only 20% were using cosmetics like shampoos, perfumes in a sporadic manner in a rate of once per week or less.
Conclusion
In our study which was conducted on 20 lactating mothers in Egypt for assessment of mono-butyl-phthalate in breast milk and urine, phthalate level in mothers’ urine and in breast milk showed a highly significant increase with PVC or Vinyl use in walls and in home flooring, while the level in mothers’ urine was highly significant in mothers drinking in plastic bottles and eating in plastic containers. For further research for better health of lactating mothers and their fetuses, especially that phthalate is highly toxic material to both mother and fetus.
Acknowledgments
I want to thank The Micro Analytical Center – Faculty of Science – Cairo University for their support and providing HPLC work up.
References
- “ACC Addresses Phthalates Safety” on YouTube: video of Steve Risotto of the American Chemistry Council, uploaded by user American Chemistry on 23 October 2009, retrieved 23 December 2011.
- Phthalates Assessing and Managing Chemicals Under TSCA. www.epa.gov. 21 September 2015. Retrieved 7 April 2017.
- https://en.wikipedia.org/wiki/Phthalate
- Toxicological profile for di-n-butyl phthalate (DBP). Atlanta, GA: Agency for Toxic Substances and Disease Registry. (2001)
- Adibi JJ, Perera FP, Jedrychowski W, et al. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environ Health Perspect J,111:1719–22. (2003)
- Rudel R, Camann D, Spengler JD, et al., Household exposure to phthalates, pesticides, alkylphenols, pbdes, and other endocrine active compounds Toxicol Sci J;72:184. (2003)
- Koch HM, Bolt HM, Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol J; 78:123–30. (2004)
- Blount BC, Milgram KE, Silva MJ, et al. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem J;72:4127–34. (2000)OpenUrl
- Takatori S, Kitagawa Y, Kitagawa M, et al. Determination of di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate in human serum using liquid chromatography-tandem mass spectrometry. J Chromatogr B; 804:397–401. (2004)
- Alzaga R, Pena A, Bayona JM. Determination of phthalic monoesters in aqueous and urine samples by solid-phase micro extraction-diazomethane on- fiber derivatization-gas chromatography-mass spectrometry. Journal of Separation Science; 26:87–96. (2003)
- Silva MJ, Slakman AR, Reidy JA, et al. Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J Chromatogr B; 805:161–7. (2004)
- Salam, Maya. “Sperm Count in Western Men Has Dropped Over 50 Percent Since 1973, Paper Finds”. The New York Times. ISSN 0362-4331. (Retrieved 15 November 2018).
- Hauser, R; Calafat, AM. “Phthalates and human health”. Occupational and Environmental Medicine.62(11):806–18. (2005).
- Lyche, Jan L.; Gutleb, Arno C.; Bergman, Åke; Eriksen, Gunnar S.; Murk, Alber Tinka J.; Ropstad, Erik; Saunders, Margaret; Skaare, Janneche U.”Reproductive and Developmental Toxicity of Phthalates”. Journal of Toxicology and Environmental Health, Part B. 12 (4): 225–249. (2009).
- Jurewicz, Joanna; Hanke, Wojciech.”Exposure to phthalates: Reproductive outcome and children health. A review of epidemiological studies”. International Journal of Occupational Medicine and Environmental Health. 24 (2): 115–41. (2011).
- Giulivo, Monica; Lopez de Alda, Miren; Capri, Ettore; Barceló, Damià . “Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review”. Environmental Research. 151: 251–264. (2016).
- Bansal, Amita; Henao-Mejia, Jorge; Simmons, Rebecca A. “Immune System: An Emerging Player in Mediating Effects of Endocrine Disruptors on Metabolic Health”. Endocrinology. 159 (1): 32–45. (2018).
- Factor-Litvak, Pam; Insel, Beverly; Calafat, Antonia M.; Liu, Xinhua; Perera, Frederica; Rauh, Virginia A.; Whyatt, Robin M.; Carpenter, David O. “Persistent Associations between Maternal Prenatal Exposure to Phthalates on Child IQ at Age 7 Years”. PLoS ONE. 9 (12): e114003. (2014).
- Balalian, Arin A.; Whyatt, Robin M.; Liu, Xinhua; Insel, Beverly J.; Rauh, Virginia A.; Herbstman, Julie; Factor-Litvak, Pam. “Prenatal and childhood exposure to phthalates and motor skills at age 11 years”. Environmental Research. 171: 416–427. (2019).
- Katharina M. Main Gerda K. Mortensen Marko M. Kaleva Kirsten A., et al., Human Breast Milk Contamination with Phthalates and Alterations of Endogenous Reproductive Hormones in Infants Three Months of Age. Environ Health Prospect J. 114(2): 270–276. (2006).
- Benson, R., Phthalates. Toxicol. Pharmacol. 53(2), 90–101. (2009).
- Xiao, X., Mruk, D. D., Cheng, C. Y,”Intercellular adhesion molecules (ICAMs) and spermatogenesis”. Human Reproduction Update 19 (2): 167–86. (2013).
- Latini,G.,Monitoring phthalate exposure in humans. Clin Chim Acta. 361: 20-29. (2005).
- Allan C. Just, Rachel L. Miller, Matthew S. Perzanowski, et al., Vinyl flooring in the home is associated with children’s airborne butyl benzyl phthalate and urinary metabolite concentrations. J Expo Sci Environ Epidemiol.; 25(6): 574–579. (2015)
- Dong Rui Hua, Zhang Han, Zhang Mei Ru, et al., Association between Phthalate Exposure and the Use of Plastic Containers in Shanghai Adults. Biomed Environ Sci J; 30(10): 727-736. (2017)
- Rose O. Sulentic, Irina Dumitrascu, Nicole C. Deziel, et al., Phthalate Exposure from Drinking Water in Romanian Adolescents. Int J Environ Res Public Health.; 15(10): 2109. (2018)
- Lauren E. Parlett, Antonia M. Calafat, and Shanna H. Swan, Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol. 2013 Mar; 23(2): 197–206. (2013)








