Muhammad Yulis Hamidy1* , Huriatul Masdar2
, Huriatul Masdar2 and Darmawi2
and Darmawi2
1Department of Pharmacology, Faculty of Medicine, Universitas Riau, Pekanbaru, Indonesia
2Department of Histology, Faculty of Medicine, Universitas Riau, Pekanbaru, Indonesia
Corresponding Author E-mail :yulis.hamidy@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1902
Abstract
To evaluate mangrove (Rhizophora sp) fruit extract effect on foam cell formation at the initiation stage of atherosclerosis. The study was conducted on 18 rats divided into 3 groups (n=6). Group 1 was given a standard diet, Group 2 was given a high cholesterol diet, and Group 3 was given a high cholesterol diet and mangrove fruit extract 500 mg/kg. The mangrove fruit extract effect on foam cell formation was assessed by histopathologic examination using hematoxylin eosin staining. The results showed that the number of foam cells was increased significantly in high cholesterol diet-fed rats compared to standard diet-fed rats (21.17 vs. 11.75). Mangrove fruit extract reduced this number remarkably (21.17 vs. 12.67). In conclusion, mangrove fruit extract inhibits the formation of foam cells at the initiation stage of atherosclerosis.
Keywords
Atherosclerosis; Foam Cell; Mangrove Fruit Extract
Download this article as:| Copy the following to cite this article: Hamidy M. Y , Masdar H, Darmawi D. Effect of Mangrove (Rhizophora sp) Fruit Extract on Foam Cell Formation at the Initiation Stage of Atherosclerosis. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Hamidy M. Y , Masdar H, Darmawi D. Effect of Mangrove (Rhizophora sp) Fruit Extract on Foam Cell Formation at the Initiation Stage of Atherosclerosis. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2UkbzW5 |
Introduction
Atherosclerosis is a major cause of cardiovascular disease and is a significant contributor to death in the world, including in Indonesia [1]. Atherosclerosis is a vascular inflammatory disease characterized by lipid accumulation, cell death and fibrosis in the arteries [2]. Inflammation has an important role and is the main mechanism underlying atherosclerosis pathogenesis. The formation of free radicals that trigger inflammatory reactions in the vascular lining is the basis of the course of this disease. Exogenous antioxidants are useful for eliminating these free radicals so they can prevent further vascular damage [3].
The early stage of atherosclerosis begins with the entry of low-density lipoprotein (LDL) into the intima of artery. The LDL will undergo oxidation to oxidized LDL (ox-LDL) which will activate endothelial cells. Activated endothelial cells express leukocyte adhesion molecules such as Vascular Cell Adhesion Molecule 1 (VCAM-1) on the surface of the arteries. VCAM-1 then interacts with monocytes in the circulation. Monocytes that have been attached to vascular endothelial cells then migrate into the arterial intima layer mediated by Monocyte Chemoattractant Protein-1 (MCP-1)[4]. Monocytes will then differentiate into macrophages in response to Macrophage-Colony Stimulating Factor (M-CSF)[5,6]. These macrophages then devour excessive cholesterol via scavenger receptors which will lead to foam cell formation [4,5]. Formation of foam cells that occurs in the initial stages of atherogenesis is a major hallmark of atherosclerotic disease [6].
One of the antioxidants that can be developed is mangrove (Rhizophora sp), whose habitat is abundant in Riau Province, Indonesia, especially in coastal areas. Mangrove (Rhizophora sp) contains flavonoid, tannin, saponin, polyphenols, sterols and alkaloid found in both stems, leaves and fruit so that various studies have been carried out to determine the pharmacological effects of this natural product. Based on previous studies Rhizophora sp has been shown to have pharmacological effects as anti-inflammatory, anti-oxidant, and anti-hypercholesterolemia [7-10]. The existence of these effects provides an opportunity to be used as an anti-atherosclerosis [11].
The purpose of this study was to evaluate the effect of mangrove (Rhizophora sp) fruit extract on foam cell formation at the initiation stage of atherosclerosis.
Materials and Methods
Experimental Animals
Eighteen healthy male Wistar rats (purchased from School of Pharmacy, Universitas Riau, Pekanbaru, Indonesia), 150-250 g body weight, 10 weeks of age were placed in cages individually in a room with proper ventilation, room temperature between 20–26 ̊C, and allowed for food and water ad libitum. Lighting of the room was regulated light and dark alternately for 12 hours. Animals underwent an acclimatization period at least 7 days before use in our study. During the acclimatization period the rats got a normal diet. Rats were treated in accordance with the Helsinki convention. Ethical approval was obtained from the Ethical Review Board for Medicine & Health Research, Faculty of Medicine, Universitas Riau (No:099/UN.19.5.1.1.8/UEPKK/2019).
Experimental Design
Rats were randomly divided into 3 groups (n=6), Group 1 was given a standard diet. Group 2 was given a high cholesterol diet of vitamin D3 (cholecalciferol) 700.000 IU/kg (Sigma, St. Louis, MO, USA) on the first day followed by 5% goat fat, 2% cholesterol, 0.2% cholic acid (Sigma, St. Louis, MO, USA) and 92.8% standard diet for 3 days to induce atherosclerosis initiation stage [11,12]. Group 3 was given a high cholesterol diet and treated with mangrove fruit extract 500 mg/kg. Mangrove fruit extract was given orally once a day using a gastric tube.
Evaluation of Foam Cell Formation
At the end of the experiment, the rats were sacrificed with ether anesthesia. Subsequently, abdominal aortic tissues were rapidly excised. We fixed the aortic sample in 10% neutralized formaldehyde in 0.1 M phosphate buffer and embedded in paraffin. A hematoxylin and eosin (H&E) staining was performed to count the number of foam cells in the abdominal aortic in rats. Foam cells were calculated at 10 fields of view with ×400 magnification. Images were obtained using a light microscope equipped with a digital camera (Leica, Wetzlar, Germany) connected to a PC monitor.
Statistical Analysis
Data are presented as mean ± SEM.
Results
The H&E staining showed that in Group 1 receiving standard diet and Group 3 receiving a high cholesterol diet and treated with mangrove fruit extract demonstrated few foam cells (Figures 1A and 1C), while in Group 2 receiving a high cholesterol diet showed abundance of foam cells in the aortic lesion (Figure 1B). These results demonstrated that the administration of high cholesterol diet induced foam cell formation and mangrove fruit extract treatment inhibited the accumulation of these cells in aortic lesion.
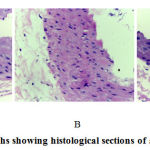 |
Figure 1: Photomicrographs showing histological sections of aorta abdominal of rats. |
(A) Group 1 was given a standard diet, (B) Group 2 was given a high cholesterol diet, (C) Group 3 was given a high cholesterol diet and treated with mangrove fruit extract 500 mg/kg, at magnification of ×400 stained with H&E. The red arrow indicated foam cell.
Figure 2 shows the number of foam cells among different experimental groups. High cholesterol diet-fed animals exhibit increased number of foam cells in the aortic lesions compared to control rats (21.17 + 2.21 vs. 11.75 + 3.33). Mangrove fruit extract treatment significantly reduced the accumulation of foam cells in the aortic lesions (21.17 + 2.21 vs. 12.67 + 4.62).
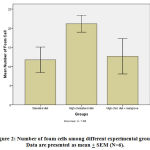 |
Figure 2: Number of foam cells among different experimental groups. |
Discussion
During the recent years, many researchers have paid attention to phytochemicals, which are regarded as important sources for drug development [13]. Mangroves are plants that live on the coastline and live in tropical and subtropical regions. This plant is known as halophyte which is very tolerant of high salt content and easily adapts to environments with low oxygenation and muddy. In Indonesia, approximately 47 species of mangrove plants were found, both of which belong to the family Rhizophoraceae, Sonneratiaceae, Avicenniaceae and Meliaceae [14]. The results of this study showed that mangrove fruit extract may reduce foam cell formation in the early stages of atherosclerosis.
The formation of foam cells involves various processes such as taking ox-LDL, cholesterol esterification and cholesterol efflux. Ox-LDL uptake by macrophages is carried out through phagocytosis and pinocytosis processes mediated by CD36 and SR-A. About 75-90% of ox-LDL uptake by macrophages was carried out through both receptors [15]. Manning-Tobin et al. reported the results of his study which showed that SR-A suppression can prevent the formation of atherosclerotic lesions [16], while research conducted by Xie et al. showed that down-regulation of CD36 can inhibit foam cell formation [17].
The process of cholesterol esterification involves Acyl coenzyme A: cholesterol acyltransferase-1 (ACAT1) and neutral cholesteryl ester hydrolase (nCEH) [18]. The level of cholesterol esterification determines the number of macrophages that will turn into foam cells [19]. Xu et al. showed ACAT1 inhibition can suppress foam cell formation and inhibit the development of atherosclerosis [20].
The process of cholesterol efflux involves the ATP-binding cassette (ABC) transporter A1 (ABCA1), ABCG1 and BI-receptor scavenger (SR-BI) [21,22]. Disruption in the process of cholesterol efflux results in cholesterol accumulation in macrophages [23], whereas ABCA1 stimulation can inhibit foam cell formation [24].
The balance between cholesterol uptake, cholesterol esterification and cholesterol efflux plays a role in preventing lipid accumulation in macrophages [25]. At the stage of atherosclerosis initiation there is an increase in CD36 and SR-A expression, an increase in cholesterol esterification due to an increase in ACAT1 levels or a decrease in nCEH levels and a decrease in cholesterol efflux due to a decrease in the expression of ABCA1, ABCG1 and SR-BI. This condition causes the accumulation of cholesterol esters in the macrophages which will result in the formation of foam cells [26]. The progression of atherosclerosis depends on the accumulation of monocytes on the walls of the arteries, the more monocytes/macrophages accumulate, the more foam cells are formed so that the development of atherosclerotic lesions will also accelerate [27].
The initiation stage of atherosclerosis is characterized by the formation of foam cells. Foam cells found at the initiation stage of atherosclerosis are derived from monocytes found in the circulation. Monocytes that have been attached to vascular endothelial cells then migrate into the intima layer of arteries [28]. The mechanism of mangrove fruit extract in inhibiting foam cell formation is thought to be through the inhibition of monocyte migration into the arterial wall.
Conclusion
In conclusion, mangrove fruit extract could inhibit the formation of foam cells at the initiation stage of atherosclerosis. Thus mangrove fruit extract is very potential to be developed as anti-atherosclerosis.
Acknowledgements
The authors gratefully acknowledge that the present research is supported by Research Development Centre University of Riau.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Funding Source
The present research is funded by University of Riau (Grant No. 899/UN.19.5.1.3/PT.01.03/2019).
References
- Libby P, Ridker P.M, Maseri A. Inflammation and atherosclerosis. Circulation, 2002; 105: 1135–1143.
- Kones R, 2011. Primary prevention of coronary heart disease: integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug design, development and therapy 5: 325-380
- Herrmann J, Lerman LO, Lerman A. On to the road to degradation: atherosclerosis and the proteasome. Cardiovasc Res. 2010. 85:291-302
- Libby P, Okamoto Y, Rocha V.Z, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circulation Journal, 2010; 74: 213-220.
- Li Y, Guo Y, Chen Y, Wang Y, You Y, Yang Q, et al. Establishment of an interleukin‑1β‑induced inflammation‑activated endothelial cell‑smooth muscle cell‑mononuclear cell co‑culture model and evaluation of the anti‑inflammatory effects of tanshinone IIa on atherosclerosis. Molecular Medicine Reports, 2015; 12: 1665-1676.
- Namiki M, Kawashima S, Yamashita T, Ozaki M, Hirase T, Ishida T, et al. Local overexpression of monocyte chemoattractant protein-1 at vessel wall induces infiltration of macrophages and formation of atherosclerotic lesion: Synergism with hypercholesterolemia. Arterioscler Thromb Vasc Biol, 2002; 22: 115-120.
- Das SK, Samantaray D, Patra JK, Samanta L, Thatoi H. Antidiabetic potential of mangrove plants: a review. Frontiers in Life Sci. 2016. 9(1):75-88.
- Amin AM, Zakaria, MB, Mahmood AA, Mohamad F, Maskam MF. Hypocholesterolemic effect of methanol extract from Avicennia alba leaves in atherosclerotic-induced new zealand white rabbit. Malays Appl Biol. 2011. 40(2):51-4.
- Simlai A, Rai A, Mishra S, Mukherjee K, Roy A. Antimicrobial and antioxidant activities in the bark extracts of Sonneratia coseolaris, a mangrove plant. EXCLI Journal. 2014. 13:997-1010.
- Krishnamoorthy M, Sasikumar JM, Shamna R, Pandiarajan C, Sofia P, Nagarajan B. Antioxidant activities of bark extract from mangroves, Bruguiera cylindrica (L.) Blume and Ceriops decandra Perr. Indian J Pharmacol. 2011;43(5):557-62.
- Hamidy M .Y, Oenzil F, Yanwirasti Y, Aldi Y. Effect of Andrographolide on Monocyte Chemoattractant Protein-1 Expression at the Initiation Stage of Atherosclerosis in Atherogenic Diet-Fed Rats. Biomed Pharmacol J 2019;12(3).
- et al. (2016). Changes in expression of proteasome in rats at different stages of atherosclerosis, Anat Cell Biol. 49:99-106.
- Mishra BB. et al. (2011). Natural products: an evolving role in future drug discovery, Eur J Med Chem. 2011;46:4769–807.
- Bakar A, Purnama P, Rahmayuni R. Pengelolaan hutan mangrove dan pemanfaatannya dalam meningkatkan ekonomi masyarakat pesisir pantai Provinsi Riau. Kutubkhanah. 2013. 16(2):94-103.
- Kruth HS. (2013). Fluid-phase pinocytosis of LDL by macrophages: a novel target to reduce macrophage cholesterol accumulation in atherosclerotic lesions, Curr Pharm Des. 19: 5865–72.
- Manning-Tobin JJ. et al. (2009). Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice, Arterioscler Thromb Vasc Biol. 29:19 –26.
- Xie C. et al. (2011). Phenolic acids are in vivo atheroprotective compounds appearing in the serum of rats after blueberry consumption, J Agric Food Chem. 59: 10381–7.
- Ghosh S. et al. (2010). Macrophage cholesteryl ester mobilization and atherosclerosis, Vascul Pharmacol. 52:1–10.
- Ghosh S. (2012). Early steps in reverse cholesterol transport: cholesteryl ester hydrolase and other hydrolases, Curr Opin Endocrinol Diabetes Obes. 19: 136–41.
- Xu G. et al. (2009). Preventive effects of Heregulin-b1 on macrophage foam cell formation and atherosclerosis, Circ Res. 105:500-10.
- Westerterp M. et al. (2014). ATP-binding cassette transporters, atherosclerosis, and inflammation, Circ Res. 114: 157–70.
- Cho W. et al. (2015). Corticotropin-releasing hormone (CRH) promotes macrophage foam cell formation via reduced expression of ATP binding cassette transporter-1 (ABCA1), PLoS One 10(6):e0130587.
- Wu M. et al. (2015). Polydatin inhibits formation of macrophage-derived foam cells, Evid Based Complement Alternat Med. 2015:729017.
- Oram JF. et al. (2006). ATP-binding cassette cholesterol transporters and cardiovascular disease, Circ Res. 99:1031–43.
- Cheng LC. et al. (2011). α-Lipoic acid ameliorates foam cell formation via liver X receptor α-dependent upregulation of ATP-binding cassette transporters A1 and G1, Free Radic Biol Med. 50:47–54.
- Yu XH. et al. (2013). Foam cells in atherosclerosis, Clinica Chimica Acta. 424:245–52.
- Libby P. et al. (2002). Inflammation and atherosclerosis, Circulation. 105:1135–43.
- Wang Q. et al. (2016). Iron together with lipid downregulates protein levels of ceruloplasmin in macrophages associated with rapid foam cell formation, Journal of Atherosclerosis Thrombosis. 23(10):1201–11.








