Department of Nursing Sciences, Faculty of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, KSA, Zip code 18616
Corresponding Author E mail: sara.saleh17@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1854
Abstract
Aim of the study; to determine the diabetic foot ulcer infection rate, bacterial etiology and antibiotic susceptibility. Research design: A cross sectional study design was utilized to fulfill the aim of this study. Setting: The study was conducted in out-patient diabetes clinic at Asyut University Hospital. Patients: A total of two hundred adult patients (both males and females) having type I or type II diabetes, with a foot ulcer. One tool was used for data collection of this study; structured diabetic patient interview questionnaire sheet with the following parts: Part I: Diabetic Patients demographic characteristics, Part II: Medical profile of the studied patients and Part III: Laboratory tests: Glycosylated Hemoglobin (Hg A1C) and Pus culture and sensitivity results. Results: The present study revealed that 53.5 % of the studied sample was female, 56.5 % were married, 45.5 % were in the age group between 50 to less than 65 years and 39.5 % were illiterate. 56 % of the sample was having type II diabetes, mean duration of diabetes in years was 13.14 ± 7.36, mean body mass index was 26.95 ± 6.75, regarding treatment regimen; 46.5 % were taking insulin, 53 % were in poor glycemic control, 28 % fair and 19 % were in good glycemic control. 51 % of the studied patients their duration of foot ulcer was less than a month, 27 % from 1 – 2 months, 69 % of the studied sample was having a superficial ulcer. 89 % was having a positive pus culture result out of which 23 % was related to pseudomonas aeruginosa, followed by Escherichia coli (20 %), Staphelococcus aureus (19 %) and the least common organism was Citrobacter isolates (2 %). 37 % of the causative organisms were sensitive to Piperacillin tazobactam, 22 % were sensitive to Gentamicin, 16 % to Vancomycin, 13 % to Azithromycin and 12 % were sensitive to Levofloxacin.
Keywords
Antibiotic Susceptibility; Bacterial Etiology; Diabetic Foot Ulcer; Prevalence
Download this article as:| Copy the following to cite this article: Mohsen S. A. Abd-El. Diabetic Foot Ulcer Infection Rate, Bacterial Etiology and Antibiotic Susceptibility: A Cross Sectional Study. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Mohsen S. A. Abd-El. Diabetic Foot Ulcer Infection Rate, Bacterial Etiology and Antibiotic Susceptibility: A Cross Sectional Study. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/38j4da8 |
Introduction
World Health Organization (WHO) defined Diabetes Mellitus (DM) as a metabolic disorder of multiple etiology characterized by chronic hyperglycemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action, or both (WHO,1999). It leads to long-term complications affecting almost every system in the body and often leading to blindness, heart and blood vessel disease, stroke, kidney failure, amputations, and nerve damage (Kahsu, et al., 2015).
Approximately one half of all patients with foot ulcers have peripheral arterial disease (PAD) which is considered an important predictor of outcome (Prompers et al., 2008). Patients in whom their foot ulcer progress to diabetic foot infections suffer from prolonged hospitalization, amputations of their foot which increases the rates of mortality (Nyamu et al., 2003).
Foot ulcers can lead to lifelong disability and substantially diminished quality of life, put restrictions on mobility, poor psychosocial adjustment, and lower self-perceptions of health than patients who do not have ulcers, moreover the survival rate of patients with diabetic foot ulcer was decreased compared to diabetic patients without foot ulcer (Spanos et al., 2017).
It is imperative that appropriate antibiotic therapy is instituted as the infection of foot ulcers is often due to more than one organism (Viswanathan et al., 2002). Morbidity and the finances involved will increase to a significant extent if the infection was caused by multidrug resistance organisms (Hartemann-Heurtier et al., 2004).
Aim of the Study
This study aimed to determine the diabetic foot ulcer infection rate, bacterial etiology and antibiotic susceptibility.
Significance of the Study
It is estimated that 415 million people are living with diabetes worldwide; this is estimated to be 1 in 11 of the world’s adult population. 46% of people with diabetes are undiagnosed. This number is expected to rise to 642 million people by 2040. Diabetic foot ulcers are a major complication of diabetes which can lead to amputation of the foot or limb. Treatment of this complication is a global major health care problem resulting in high care costs and mortality rate. Early recognition of infection is highly important to determine factors that predict the healing progress of DFU and the risk of amputation.
Materials and Method
Research Design
A cross sectional study design was utilized to fulfill the aim of this study.
Setting
The study was conducted in out-patient diabetes clinic at Asyut University Hospital.
Patients
A total of two hundred adult patients (both males and females) having type I or type II diabetes with foot ulcer, exclusion criteria; patients who had traumatic ulcers due to other than diabetes causes like motor car accidents, burn and any injury due to sharp materials, also patients on broad spectrum antibiotics were excluded from the study. Data were collected over a period of 8 months from October 2017 till May 2018. The sample size was determined based on the epi info program using 10 % acceptable error, and 95 % confidence coefficient.
Tool
One tool was used for data collection of this study; structured diabetic patient interview questionnaire sheet with the following parts
Diabetic Patients demographic characteristics
Age, gender, marital status, educational level, occupation, and area of residence.
Medical profile of the studied patients
Type of diabetes, duration of diabetes, body mass index, smoking status, treatment regimen, duration and depth of ulcer.
Laboratory tests: 1.Glycosylated Hemoglobin (Hg A1C)
To measure the control level of the blood glucose level.
Pus culture and sensitivity results
It included the laboratory results of the pus culture taken from the diabetic foot
ulcer causative agent and the antibiotic sensitivity.
Method
Permission for data collection was obtained from the responsible authorities after explaining the aim of the study.
Validation of the tool was assessed before starting data collection by a jury of 5 experts in medicine, research, and nursing fields for content validity to ascertain the appropriateness of items for measuring what they supposed to measure.
Tool reliability was calculated using Cronbach,s Alpha test which equal 0.96.
A pilot study was carried out on 10 % (20 diabetic patients) to ascertain the relevance, clarity, and applicability of the research tool, no modifications were needed.
Data were collected through an individual interview. An informed agreement was obtained from patients after explaining the purpose, and nature of the study to gain their cooperation.
The researcher met with each patient individually for filling the questionnaire, after this a sample of venous blood was taken for analysis of the Hg A1C and a wound swab was obtained from the floor of the ulcer. Direct microscopic examination and aerobic cultures were done by standard methods, the bacteriological spectrum and the sensitive antibiotics were noted for each patient.
Ethical Considerations
The purpose of the study was explained to each diabetic patient and an informed written consent to participate in the study was obtained. Confidentiality of the collected data and the right to withdraw at any time were ensured.
Statistical Analysis of the Data
Data were fed to the computer and analyzed using SPSS software package version 20. Qualitative data were described using number and percent. Quantitative data were described using minimum and maximum, mean and standard deviation.
Results and Discussion
The present study revealed that 53.5 % of the studied sample was female; this study result comes in accordance with Bagdady, 2014 who found that two thirds of her studied sample was females. 56.5 % were married, 45.5 % were in the age group between 50 to less than 65 years, This finding was in the same line with El-Nahas et al., 2008, Faris et al., 2012 and Hurley, et al 2013 who reported that, the peak incidence of foot ulcer was in age group from 50 ≥ 65 years. 39.5 % were illiterate, 51 % were not working and this comes in the same line with Hurley et al., 2013 who illustrated that the majority (48%) of their sample has primary education and the university graduates comprised only 6% of their subjects and were not working. 58 % of them were living in rural areas.
56 % of the sample was having type II diabetes, and this result is supported by Akhil et al., 2015 who reported that type II diabetes represented 82.6 % of their study sample, duration of diabetes in years ranged from 2 to 40 years with a mean and standard deviation of 13.14 ± 7.36 this result comes in disagreement with Akhil et al., 2015 who reported than the mean duration of diabetes in years was 18 years, body mass index ranged from 16.67 – 58.32 with a mean and standard deviation of 26.95 ± 6.75 this finding is comparable to the results by El-Nahas et al 2008, 52.5 % were smokers; this result is in line with Khalil et al., 2014 and Al Kafrawy et al., 2014 findings and regarding treatment regimen; 46.5 % were taking insulin, 40 % oral hypoglycemic agents and 13.5 % were using combined therapy this comes in line with the results of Nyamu et al., 2003 who reported that there was an observed high proportion of patients on insulin.
Regarding glycemic control; 53 % were in poor glycemic control, 28 % fair and 19 % were in good glycemic control, this result comes in accordance with the results by Kathirvel et al., 2018 who reported that 44.66 % of the sample were in poor glycemic control, 36 % fair and 19.33 % in good control.
51 % of the studied patients their duration of foot ulcer was less than a month, 27 % from 1 – 2 months, 20 % from 2 – 3 months and 2 % were more than 3 months, 69 % of the studied sample was having a superficial ulcer while 31 % were having a deep foot ulcer, these results agree with the results of Kathirvel et al., 2018 who reported that 68 % of the ulcers were <1 month, 22.7 % from 1-2 months, 6 % from 2-3 months and 3.3 % >3 months and the highest percentage in their sample (61.33 %) had a superficial ulcer.
Out of the 200 cases included in the present study 178 patients (89 %) was having a positive pus culture result while 11 % of the sample was having negative pus culture result, this study result is in the same line with the results of Vaddadhi et al., 2019 who reported that out of 100 samples processed 90(90%) were culture positive.
Out of the 178 (89 %) positive cases 23 % was related to pseudomonas aeruginosa, followed by Escherichia coli (20 %), Staphelococcus aureus (19 %), Klebseilla spp. (17 %), Enterococci (16 %), Proteus (3 %) and the least common organism was Citrobacter isolates (2 %). Out of the causative organisms 37 % were sensitive to Piperacillin tazobactam, 22 % were sensitive to Gentamicin, 16 % to Vancomycin, 13 % to Azithromycin and 12 % were sensitive to Levofloxacin. These study results comes in agreement with Vaddadhi et al., 2019 who documented that Pseudomonas aeruginosa 23(23%), was the most common isolate causing diabetic foot infections, followed by Escherichia coli and Out of 23(23%) Pseudomonas isolates, 22(95%) were sensitivity to Piperacillin tazobactam. 13(56%) were sensitive to Gentamicin and Only 2 (8 %) were least sensitive to Azithromycin.
Table 1: Patients’ distribution according to socio-demographic characteristics (n = 200):
| Patient characteristics | N. | % |
| Sex: | ||
| – Male | 93 | 46.5 |
| – Female | 107 | 53.5 |
| Marital status: | ||
| – Single | 17 | 8.5 |
| – Married | 113 | 56.5 |
| – Divorced | 25 | 12.5 |
| – Widowed | 45 | 22.5 |
| Age: | ||
| – 18 > 30 | 13 | 6.5 |
| – 30 > 40 | 26 | 13 |
| – 40 > 49 | 70 | 35.5 |
| – 50 > 65 | 91 | 45.5 |
| Educational level: | ||
| – Illiterate | 79 | 39.5 |
| – Reading and writing | 47 | 23.5 |
| – Preparatory school | 33 | 16.5 |
| – Secondary school | 25 | 12.5 |
| – University | 16 | 8 |
| Occupational status: | ||
| – Non-working | 102 | 51 |
| – Professional work | 80 | 40 |
| – Farmer | 18 | 9 |
| Residence: | ||
| – Rural | 116 | 58 |
| – Urban | 84 | 42 |
Table 2: Medical profile of the studied patients (n = 200).
| Patient’s related Medical Data | N. | % |
| Type of diabetes: | ||
| – Type 1 | 88 | 44 |
| – Type 2 | 112 | 56 |
| Duration of diabetes (years): | ||
| – Range | 2.0 – 40.0 | |
| – Mean ± SD. | 13.14 ± 7.36 | |
| Body mass index (BMI): | ||
| – Range | 16.67 – 58.32 | |
| – Mean ± SD. | 26.95 ± 6.75 | |
| Smoking status: | ||
| – Smokers | 105 | 52.5 |
| – Non-smokers | 95 | 47.5 |
| Treatment regimen: | ||
| – Insulin | 93 | 46.5 |
| – Oral hypoglycemic agent | 80 | 40 |
| – Combined | 27 | 13.5 |
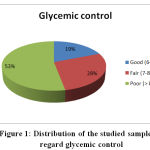 |
Figure 1: Distribution of the studied sample as regard glycemic control |
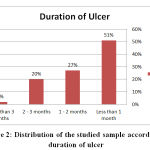 |
Figure 2: Distribution of the studied sample according to duration of ulcer |
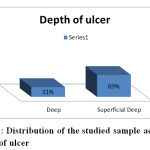 |
Figure 3: Distribution of the studied sample according to depth of ulcer |
Table 3: Distribution of the studied sample according to pus culture result
| Result of culture | N. | % |
| Positive | 178 | 89 |
| Negative | 22 | 11 |
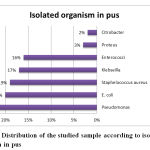 |
Figure 4: Distribution of the studied sample according to isolated organism in pus |
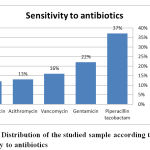 |
Figure 5: Distribution of the studied sample according to sensitivity to antibiotics |
Conclusion
The current study concluded that out of the 200 cases included, 178 patients (89 %) was having a positive pus culture result out of which 23 % was related to pseudomonas aeruginosa, followed by Escherichia coli (20 %), Staphelococcus aureus (19 %), Klebseilla spp. (17 %), Enterococci (16 %), Proteus (3 %) and the least common organism was Citrobacter isolates (2 %). 37 % of the causative organisms were sensitive to Piperacillin tazobactam, 22 % were sensitive to Gentamicin, 16 % to Vancomycin, 13 % to Azithromycin and 12 % were sensitive to Levofloxacin.
Acknowledgement
Thanks to Dr. Ghada Hassan who helped in data collection.
Conflict of Interest
No conflict of interest.
Funding Source
None
References:
- Akhil M, Mohammad R, Varshochi A, Pirzadeh T, Memar M, Bialvaei A, Sofla H, and Alizadeh N. Bacterial etiology and antibiotic susceptibility pattern of diabetic foot infections in Tabriz, Iran. GMS Hygiene and Infection Control, 2015; 10: 1-6.
- Al Kafrawy N, Mustafa E A, Dawood A, Ebaid O, and Zidane Study of risk factors of diabetic foot ulcers. M. M. J., 2014 27 (1): 28-34.
CrossRef - Bagdady E. Screening Techniques to Identify Occurrence of Diabetic Foot Ulceration among People at High Risk. Unpublished doctorate thesis. Faculty of Nursing, Port Said University, 2014.
CrossRef - El-Nahas M, Gawish H, Tarshoby M, and State M. The prevalence of risk factors for foot ulceration in Egyptian diabetic patients. Pract. Diab. Int., 2008; 25: 362–366.
CrossRef - Faris G, Ali H, Yousef S, Nidal A, and Kamel M. Prevalence of Diabetic Foot Ulcer and its Associated Risk Factors among Diabetic Patients in Jordan. J Med J 2012; 46 (2):118-125
- Hartemann-Heurtier A, Robert J, Jacqueminet S, Ha Van G, Golmard JL, and Jarlier V,. Diabetic foot ulcer and multidrug-resistant organisms: risk factors and impact. Diabetic Med. J. 2004; 21: 710 – 715.
CrossRef - Hurley L, Kelly L, Garrow A.P, Glynn L.G, Mcintosh C., Alvarez A, Avalos G, and Dinneen S. A prospective study of risk factors for foot ulceration. The West of Ireland Diabetes Foot Study. 2013;106(12):1103-10
CrossRef - Gebrekirstos K, Gebrekiros S and Fantahun A. Prevalence and Factors Associated With Diabetic Foot Ulcer among Adult Patients in Ayder Referral Hospital Diabetic Clinic Mekelle, North Ethiopia. J Diabetes Metab., 2015; 6 (8).
- Kathirvel M, Prabakaran V, Jayarajan J, Sivakumar A, and Govindan V. Risk factors for the diabetic foot infection with multidrug-resistant microorganisms in South India. Int Surg J., 2018; 5(2): 675 – 682.
CrossRef - Khalil S, Zaki A, Rehim A, Megallaa M, Gaber N, Gamal H, and Rohoma K. Prevalence of diabetic foot disorders and related risk factors among Egyptian subjects with diabetes. Prim Care Diabetes, 2015; 9(4):297-303.
CrossRef - Nyamu P, Otieno C, Amayo E, and McLigeyo S. Risk factors and prevalence of diabetic foot ulcers at Kenyatta National Hospital, Nairobi. East Afr. Med. J., 2003; 80(1): 36-43.
CrossRef - Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio d, Uccioli L, Urbancic V, Bakker K, Holstein P, Jirkovska A, Piaggesi A, Ragnarson-Tennvall G, Reike H, Spraul M, Van Acker K, Van Baal J, Van Merode F, Ferreira I, and Huijberts Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia., 2008; 51(5): 747–755.
CrossRef - Spanos K, Saleptsis V, Athanasoulas A, Karathanos C, Bargiota A, Chan P, Giannoukas AD. Factors Associated With Ulcer Healing and Quality of Life in Patients With Diabetic Foot Ulcer. Angiology. 2017; 68(3):242-50.
CrossRef - Vaddadhi S, Kumari R and Kumari L. Study of bacterial pathogens with their antibiogram causing diabetic foot ulcers. Int. J. Adv. Res. 2019; 7(4): 908-914
CrossRef - Viswanathan V, Jasmine JJ, Snehalatha C, and Ramachandran A. Prevalence of pathogens in diabetic foot infection in South India type 2 diabetic patients. J Ass Physic India. 2002; 50:1013-6.
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus. 1999. 2.pdf








