Inoyat Jumayev1* , Pulat Usmanov1, Shavkat Rustamov1 and Sherzod Zhurakulov2
, Pulat Usmanov1, Shavkat Rustamov1 and Sherzod Zhurakulov2
1Institute of Biophysics and Biochemistry, National University of Uzbekistan, Tashkent, Uzbekistan.
2Institute of Chemistry of Plant Substances, Uzbek Academy of Sciences, Tashkent, Uzbekistan.
Corresponding Author E-mail : inoyat8585@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/1892
Abstract
In this study, mechanisms of inotropic action of some isoquinoline alkaloids 1-(4-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (F-24), 1-(2-chloro-4,5-methylenedioxyphenyl)-2-hydroxyethyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (N-14) and 1-(2-сhloro-4,5-methylenedioxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (F-14) were studied. The F-24, F-14, and N-14 alkaloids have been shown to have a negative effect on papillary muscle contraction activity, IC50 value -16,8 µM, 14,03 µM and 12 µM. Са2+L-channel blocker - nifedipine, adenylate cyclase (AС) activator–forskolin, β-adrenoreceptor (β-AR) blocker – propranolol, protein kinase С (PKС) activator – phorbol 12-myristate 13-acetate and SR RyR2 activator caffeine were used. Inotropic effects of F-24, F-14 and N-14 isoquinoline alkaloids on cardiomyocytes were suggested, based on results obtained in experiments carried in cardiomyocytes β-AR → [cAMP] → PKA → [Са2+]in ↑ cascade, PKC, RyR2 and Na+/Ca2+ modulation.
Keywords
Inotropic Effect; Isoquinoline Alkaloids; Papillary Muscle
Download this article as:| Copy the following to cite this article: Jumayev I, Usmanov P, Rustamov S, Zhurakulov S. Comparative Inotropic Effects of the Some Isoquinoline Alkaloids. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Jumayev I, Usmanov P, Rustamov S, Zhurakulov S. Comparative Inotropic Effects of the Some Isoquinoline Alkaloids. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/2wiLvSi |
Introduction
In the world, there has been a trend towards the spread of cardiovascular diseases in recent years, which continues to be a leader in general disease and death structure1,2. Worldwide, cardiovascular diseases are a major problem in the medical, social and economic context, leading to disability and premature deaths3,4,5. According to the World Health Organization’s statistical data, about 17,300,000 people die of cardiovascular diseases each year and makes up ~31% of all deaths under general conditions6,1.
Cardiovascular disease is associated with dysfunctional changes in cardiomyocytes [Са2+]in homeostasis, and myocardial rhythmic activity is provided by the function of Са2+ transport system, located in the cardiomyocyte sarcolemma and sarcoplasmic reticulum (SR) membrane. Са2+ entrance to potassium-activating L-type Ca2+ -channel (Ca2+L-channel) in the sarcolemma during the formation of Na+ -channel activation and action potential (AP) in Cardiomyocyte activates CR Ca2+ -channel (RyR2; ryanodine receptor type 2). Ca2+ affinity (Ca2+ -spark) type cardiomyocytes cytosol increases the concentration of Ca2+ and ions Ca2+ in troponin-C in myocardium. Myocardial contraction is resulted by increases of Са2+ concentration in Са2+–oscillation (Са2+–spark) type cardiomyocytes cytosol and further formation with troponin-С in the myofilament. During the diastole, Сa2+ are normalized by Сa2+ -ATPase (SERCA2a, Sarco (endo) plasmic reticulum calcium-ATPase type 2) in the sarcoplasmic reticulum membrane and Na+/Ca2+ -exchanger type 1 function. These ion-transport systems are at the center of the normal physiological function of myocardium. Breakage in function of these systems in cardiomyocytes leads to disruption of [Сa2+]in dynamics and development of arrhythmia-type cardiopathology7,8,9. The effect of “target” on effective antiarrhythmic and cardiotropic drugs, currently used in clinical cardiology practice, is directed to the pharmacological correction of the function of these systems (Figure 1).
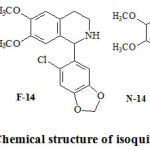 |
Figure 1: Chemical structure of isoquinoline alkaloids. |
The disorders in the RyR2 structure have been reported to cause cardiac dysfunction and arrhythmias10.
Specifically, increase in the spontaneous activation of RyR2, as well as increases of [Ca2+]in concentration induced by hyperphosphorylation of RyR2 through β–AR – PKA and the development of pathogenesis of post-depolarization-type arrhythmia were established11.
The RyR2 functional activity is regulated mainly by three mechanisms: [Ca2+]SR, [Са2+] in concentration changes, and by regulatory proteins and adaptive mechanisms regulated by RyR2 activation/inactivation12.
In β–AR– [cAMF] in-PCA reactions cascade, under the effect of cervical oscillation of RyR2-FKBP12.6, RyR2 activity increases; thereby the probability of formation of arrhythmia rises13.
Adenylate cyclase (AС) and protein kinase A (PCA) system are important in the functional activity of cardiomyocytes.
In the case of β–adrenoreceptor (B-AR) activation in cardiomyocytes sarcolemma, AС enzyme activation via guanine-dependent activating transmembrane Gs-protein (guanine nucleotide-binding proteins) and [сAMF] value increase. PCA is activated by cAMF, and functional protein molecules including PLB, troponin-I, Ca2+L-channel are phosphorylated14,15.
Regulating the functional activity of cardiomyocytes through the modulation of the above-mentioned mechanism is significant to establish mechanism of action of biologically active substances, creation of potential pharmaceutical preparations on their basis, and treatment/prevention of cardiopathology.
Among chemical compounds, containing heterocyclic structure, isoquinoline alkaloids are characterized by a wide range of physiological spectra. Isoquinoline alkaloids possess antiarrhythmic and cardiotropic effects on cardiovascular system, spasmolytic, pain-relieving and anti-inflammatory effects16,17. Spasmolytic drugs included in the list of isoquinoline alkaloids, such as Papaverine and No-Shpa, are widely used in medical practice. Besides, papaverine analogs – salsolin, salsolidin, guanethidine, have a strong hypotensive effect18.
The berberine (Berberine) isoquinoline alkaloid derived from Rhizoma Coptidis plant species has been reported to possess activity against pathogenic bacteria, ischemia, and thrombus formation, and hepatoprotective and antiarrhythmic effects19.
Aim of this study was to establish mechanism of action of 1-(4-dimethylaminophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (F-24), 1-(2-chloro-4,5-methylenedioxyphenyl)-2-hydroxyethyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (N-14) and 1-(2-сhloro-4,5-methylenedioxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (F-14) alkaloids on cardiomyocytes ion- transport systems, by registering papillary muscular contraction activity of rat heart, in vitro conditions.
Material and Methods
Solvents and Chemicals
All reagents, used in experiments, were of analytic–grade (NaCl, KCl, CaCl2, MgSO4, KH2PO4, glucose, NaHCO3). (±)-propranolol hydrochloride, nifedipine hydrochloride, forskolin, phorbol 12-myristate 13-acetate (PMA), caffeine were obtained from Sigma Chemical (St. Louis, Missouri, USA).
Isoquinoline alkaloids, the effects of which were studied, were synthesized by researchers group from the Institute for Plant Substances of Academy of Sciences of Uzbekistan.The isoquinoline alkaloids were synthesized on the basis of the Pictet -Spengler, and Bischler-Napieralski reactions. The chemical structures of the synthesized isoquinoline alkaloids were established using IR and NMR N1 spectroscopy.
Tissue Preparation and Measurement of Contractility
In the experiments, the standard mechanography was used to screen the inotropic effect of isoquinoline alkaloids.
The prepared papillary muscle was connected to a force transducer for signal recording. In experiments, the papillary muscle preparations were isolated from the right atrium of adult albino rats’ hearts. The papillary muscles were 0.4–1.3 mm in diameter and 2.5–3.8 mm in length. The papillary muscles samples were prepared according to Sonnenblick, and the muscle was placed in a special horizontal tissue chamber (Type 813; Hugo Sachs Elektronik, March-Hugstetten, Germany), designed for in vitro study in standard pharmacological experiments for measuring contraction force response of papillary muscle preparations. The top of the system was open and it is provided with the organ chamber, volume – 5 ml, the Thermo–circulator for flow heater physiological solution and the wire holder for the force transducer (Type F30/Model D-79232; Hugo Sachs Elektronik, March-Hugstetten, Germany), with a precision micrometer control. In the experiments, modified the physiological Krebs–Henseleit solution containing (in mM): 118 NaCl; 4.7 KCl; 2.5 CaCl2; 1.2 MgSO4; 1.1 KH2PO4; 5.5 glucose and 25 NaHCO3; pH 7.4 were used. This Krebs-Henseleit solution which was continuously bubbled with 95% O2 and 5% CO2 and kept at a temperature of +36±0.5 °C by means of water heating system controlled by temperature controller U8 (Bulgaria), and flowed in and out of the organ bath at a rate of 3-5 ml/min with the peristaltic pump LKB Bromma (Sweden).
The isometric force transducer F30 was connected to a transducer amplifier (Type TAM-A; Hugo Sachs Elektronik, Harvard Apparatus GmbH, Germany). The papillary muscle was lifted with electric impulses higher than a threshold (~20%), rectangular, electrical pulses of frequency 0.5 Hz; 5 ms and 5 V amplitude, delivered via a pair of platinum electrodes placed in the muscle-mounting organ chamber by using stimulator ESL-2 (Russia). Thus, wires of a pair of platinum electrodes were placed as parallel to the organ; the physiological solution of Krebs-Henseleit provided shortening the electrical contact distance between the electrodes and the preparation of the papillary muscles. After 60 min of incubation period, papillary muscles were stimulated by an initial electrical pulse of frequency 0.5 Hz, amplitude 5 V, and 5 msec pulses. The obtained signals were given from the transducer F30 to amplifier and sent to a computer by using a pen chart recorder (Type TZ 4620; Czech Republic) or a personal computer with analog-digital converter LabPro Logger Lite 1.2 software (Vernier Software & Technology, Beaverton, USA).
Data Analysis
Papillary muscle contractions were plotted as a percentage of the force before the drug application in each muscle. Data were analyzed by OriginPro 7.0 (MicroCal Software, Northampton, MA). Pooled data were given as means ±S.E.M. of observations (n). Concentration-response curves were fitted to the logistic equation: , where Emax – is the maximum effect, k – is a factor which represents the slope of the curve, and pD2 – is the drug concentration exhibiting 50% of the Emax expressed as negative log molar. Values are expressed as mean ±S.E.M. The values were considered as significantly different when p<0.05.
Results and Discussion
Inotropic Action of Isoquinoline Alkaloids
The alkaloids of F-24 (10-60 µM), F-14 (5 to 40 µM) and N-14 (5 to 25 µM) isoquinoline in the experiments showed a negative inotropic effect on the papillary muscle contraction. Pupillary muscle contraction force decreased up to 92.4±3.8%, 72.7±4.4% and 66.5±3.3% respectively. Semi-maximum concentrations of F-24, N-14 and F-14 alkaloids (IC50) were established to be 15.1 µM, 18.6 µM, and 23.9 µM, respectively (Figure 2).
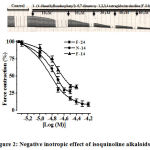 |
Figure 2: Negative inotropic effect of isoquinoline alkaloids. |
The ordinate axis shows the pulsating force of the papillary muscle, expressed as a percentage of the maximum value of 100%. The stimulation frequency is 0.5 Hz (t=+36±0.5ºC). Р<0.01 (n=3-4).
The Role of Ca2+L-Channels in the Inotropic Effect of Isoquinoline Alkaloids
Many negatively inotropic agents cause the Ca2+L-channel blocking in cardiomyocytes to reduce the concentration of [Ca2+]in the cytosol20. Therefore, in recent experiments, we have investigated negative inotropic effects of F-24, N-14, F-14 alkaloids in the current state of the Ca2+L-channel blocker nifedipine (IC50=0,01 µМ) in the incubation medium. The negative effect of isoquinoline alkaloids: F-24 (15.1 μM), N-14 (18.6 μM) and F-14 (23.9 μM) in nifedipine (0.01 μM) containing incubation medium compiled 44.7±6.3%, 39.7±4.1% and 33.7±3.3% respectively (Figure 3).
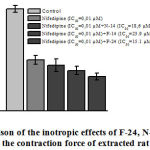 |
Figure 3: Comparison of the inotropic effects of F-24, N-14, F-14 . |
Stimulation: 0.5 Hz, 5 V, 5 msec, +36±0.5 °C, resting tension = 10 mN. P<0.05 indicates value compared to control.
The results show, that negative effects of F-24, N-14, F-14 alkaloids, in partial correlation, blockade Ca2+L-channel in cardiomyocytes
The Cascade of β–AR–AC Reactions in the Inotropic Action of Isoquinoline Alkaloids
Proteinasease A (PCA) in cardiomyocytes is regulated by phosphorylation of functional protein macromolecules, for example, by the phosphorylation of PCA, RyR2 is activated and the value of [Са2+]in cytosol increases21. Cardiomyocyte protein kinase A – linked molecule (AKAP, A-kinase-anchor protein) ensures that signal transduction via PCA is performed correctly and the presence of mACAP (muscle-specific AKAP) molecules in the RyR2 phosphorylation22. The Beta-adrenergic receptors (β-ARs) stimulation enhances contractility through protein kinase-A (PKA) substrate phosphorylation. This PKA signaling is conferred in part by PKA binding to A-kinase anchoring proteins (AKAPs). AKAPs coordinate multi-protein signaling networks that are targeted to specific intracellular locations, resulting in the localization of enzyme activity and transmitting intracellular actions of neurotransmitters and hormones to its target substrates. In particular, mAKAP (muscle-selective AKAP) has been shown to be present on the nuclear envelope of cardiomyocytes with various proteins including PKA-regulatory subunit (RIIα), phosphodiesterase-4D3, protein phosphatase-2A, and ryanodine receptor23.
In further experiments, on the basis of inotropic effects on the papillary muscular contraction in the rats, we studied the putative effects of isoquinoline alkaloids (F-24, N-14, and F-14) on β–АR–АC reactions cascade.
It has been revealed, that the positive inotropic effects of adenylate cyclase activator – forscolin (10 μM) decrease for 8.1±2.4%, 39.3±6.4% and 87.6±5.2% by F-24 (IC50=15.1 μM), F-14 (IC50=23.9 μM) and N-14 (IC50 = 18.6 μM) alkaloids, respectively (Figure 4).
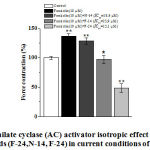 |
Figure 4: Adenilate cyclase (AC) activator isotropic effect of isoquinoline sequence |
The ordinate axis shows the pulsating force of the papillary muscle, expressed as a percentage of the maximum value of 100%. The stimulation frequency is 0.5 Hz (t=36±0.5ºC). * – p <0.05, ** – p <0.01 (n=4-5) for control.
The inotropic effect of isoquinoline alkaloids (N-14, F-14, and F-24) in the medium of β-AR blocker propranolol (10 μM) + AC activator forskolin (IC50 = 3.4 μM) on AT-activation in cardiomyocytes. It was registered that under the effects of forskolin (IС50=3.4 μM), in the medium propranolol (10 μM) incubation, the β-AR blocker, papillary muscle contraction activity significantly increased by 23.2±3.5%, compared to control. The alkaloids N-14 (IC50=18.6 µM), F-14 (IC50=23.9 µM) and F-24 (IC50= 15.1 µM) reduced propranolol (10 µM) + forskolin (IС50=3.4 μM) influence by 2.8±2.3%, 13.5±3.4% and 69.5±6.2%, respectively (Fig. 5).
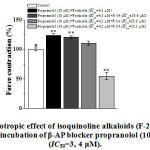 |
Figure 5: The inotropic effect of isoquinoline alkaloids (F-24, N-14, F-24) |
The ordinate axis shows the pulsating force of the papillary muscle, expressed as a percentage of the maximum value of 100%. The stimulation frequency is 0.5 Hz (t =36±0.5ºC). * –for control ** – р<0.01 (n=5-6).
In the case of incubation of propranolol (10 µM) + forskolin (IC50 = 3,4 µM), the significant changes were detected by N-14, F-14 alkaloids, and there was no change by the negative inotropic effect of F-24 alkaloid. The results show that the negative inotropic effect of N-14 and F-14 alkaloids on the inhibition of inactivity is directly related to the modulation of AC activity.
Further, in order to clarify the mechanism of negative inotropic action, inotropic effects of the studied alkaloids were investigated in the incubation condition of phorbol 12-myristate-13 acetate (FMA), PKC activator. In the experiments, the heartbeat of the FMA concentration (0,01-1 µM) had a negative effect on papillary muscle contraction and its IC50 value equaled 0,1 μM. The negative inotropic effects of F-24 (IC50), F-14 (IC50) and N-14 (IC50) in the FMA (EC50=0.1 µМ) incubation conditions compiled 30.7±6.4%, 21.4±3.5% and 14.6±4.2%, respectively (Fig. 6).
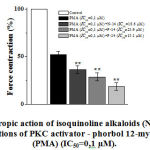 |
Figure 6: The inotropic action of isoquinoline alkaloids (N-14, F-14, F-24) |
The ordinate axis shows the pulsating force of the papillary muscle, expressed as a percentage of the maximum value of 100%. The stimulation frequency is 0.5 Hz (t=36±0.5ºC). *– for control ** – р<0,01 (n=4–5).
Based on these results, it is suggested that the effects of negligible inotropic effects of the studied isoquinoline alkaloids were partially related to the change of [Са2+]in concentration by modulating PKC activity.
Effects of Isoquinoline Alkaloids on RyR2 Activity
In the series of subsequent experiments, we have studied the effects of isoquinoline alkaloids (N-14, F-14) on possible RyR2 activity. Methods, including RyR2 activator – caffeine (20 mM) in case of non-stimulus incidence of single contraction, allows us to estimate the amount of [Са2+]SR24. Under these conditions, no post-rest potentiation is observed for about 30 seconds after 15 minutes, which is explained due to caffeine (20 mM) [Ca2+]SR excretion into cytosol completely [Bouchard, 1990]. In this case, caffeine (20 mM) causes a single contraction due to Ca2+ excretion from SR through RyR2 in non-stimulating conditions.
The concentration increase of [Ca2+]in formed by the action of caffeine (20 mM) is normalized by the Na+/Ca2+ exchange function25. In incubation condition when [Na+]out=0, Na+/Ca2+ exchanger exporting function is blocked, and subsequently [Ca2+]in ions concentration and amplitude force of contraction are maintained in a stable state under caffeine (20 μM) 26.
In our experiments when [Na+]out=0, caffeine (20 mM), without stimulus, was found to increase papillary muscle force contraction by 28±4.4%, compared to control. The amplitude force contraction of the papillary muscle, generated by caffeine (20 mM) in the presence of F-14 (40 μM) and N-14 (30 μM) isoquinoline alkaloids in the incubation environment, decreased for 18±3.7% and 35.6±4.1%, in comparison with the control group, respectively (Figure 7).
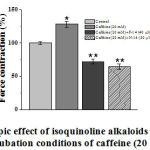 |
Figure 7. Inotropic effect of isoquinoline alkaloids (F-14 and N-14) |
The ordinate axis shows force contraction of the papillary muscle, expressed as a percentage of the maximum value of 100%. The stimulation frequency is 0.5 Hz (t=36±0.5ºC). * – for control ** – р<0.01 (n=3–5).
The results show, that the concentrations of Са2+ ions in SR under the influence of F-14 and N-14 isoquinoline alkaloids are negatively affected. The experiments also revealed, that in F-24 isoquinoline alkaloid (60 µM) incubation condition, single-contraction changes into tonic contraction and its amplitude remains constant by caffeine (20 mM) (Fig. 8).
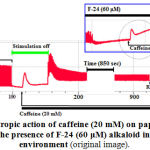 |
Figure 8: Inotropic action of caffeine (20 mM) on papillary |
The time caffeine (20 mM) added to the medium containing F-24 (60 µM) was indicated with a pointer. The frequency of Initial Stimulation is 1 Hz.
This can be explained with the increase of [Ca2+]in concentration through Na+/Ca2+ exchanger blockade together with the modulation of RyR2 under the influence of F-24, and constancy of [Ca2+]in concentration and the contraction force amplitude with caffeine (20 mM)27.
Conclusion
Thus, the negative inotropic effect of N-14, F-14 and F-24 isoquinoline alkaloids on the papillary muscle contraction were established to be in association with partial blockade of Ca2+L-channel in cardiomyocytes. It has been determined that negative inotropic effect of N-14, F-14 alkaloids occur depending on PKC modulation. The negative inotropic effect of F-24 isoquinoline alkaloid was suggested to arise from Na+/Ca2+ exchanger blockade together with RyR2 modulation.
Conflict of Interest
The authors have declared that no conflict of interest exists.
Acknowledgements
This work was supported by a grant FA-F6-T083 from the Coordinating Committee for Development of Science and Technology under the Cabinet of Ministers of the Republic of Uzbekistan.
References
- Heart disease and stroke statistics – 2017 update: A report from the American heart association. http://circ. ahajournals.org/content/135/10/e146 Date of the application: 15.08.2017.
- Nichols, N.Townsend, P.Scarborough, M.Rayner. Cardiovascular disease in Europe 2014: Epidemiological update. European Heart Journal. – 2014. – Special article. – P.2–10.
- R.Cowie, A.Mostead, D.A.Wood et al. The epidemiology of heart failure. Eur. Heart J. – 1997. – V.18. – P.208–225.
- J.V.McMurray, S.Stewart. Epidemiology, aetiology, and prognosis of heart failure. Heart. – 2000. – V.83. – P.596–602.
- F.Mendez, M.R.Cowie. The epidemiological features of heart failure in developing countries: A review of the literature. Int. J. Cardiol. – 2001. – V.80. – P.213–219.
- B. Efficacy of herbal remedies in the treatment of cardiovascular diseases in human and animals. Kocatepe Vet. J. – 2003. – V.61. – P.63–68.
- Marks. Ryanodine receptors in heart failure and sudden cardiac death. J. Mol. Cell. Cardiol. – 2001. – V.33. – P.615–624.
- D.Bers, S.Despa. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. Journal of Pharmacological Sciences. – 2006. – V.100. – P.315–322.
- Santonastasi M., Wehrens X.H. Ryanodine receptors as pharmacological targets for heart disease. Pharmacol. Sin. – 2007. – V. 28 (7). – P. 937–944.
- Lynda M. Blayneyand Anthony Lai. Ryanodine receptor-mediated arrhythmias and sudden cardiac death. Pharmacol Ther. 2009 Aug; 123(2): 151–177.
- Jian Shan,1Matthew J. Betzenhauser,1 Alexander Kushnir,1 Steven Reiken,1 Albano C. Meli,1 Anetta Wronska,1Miroslav Dura,1 Bi-Xing Chen,1 and Andrew R. Marks1,2. Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice.J Clin Invest. 2010 Dec 1; 120(12): 4375–4387.
- Zhang JZ1, Waddell HM1, Jones PP1. Regulation of RYR2 by sarcoplasmic reticulum Ca(2+).Clin Exp Pharmacol Physiol.2015 Jun;42(6):720-6.
- Isaac N. Pessah, Gennady Cherednichenko, and Pamela J. Lein. Minding the Calcium Store: Ryanodine Receptor Activation as a Convergent Mechanism of PCB Toxicity//Pharmacol Ther. 2010 Feb; 125(2): 260–285.
- Maria Magocsi,1E Sylvester Vizi,2 Zsolt Selmeczy,2 Anna Brózik,1 and Judith Szelenyi2. Multiple G-protein-coupling specificity of β-adrenoceptor in macrophages.Immunology. 2007 Dec; 122(4): 503–513.
- Viola Kooij,1Ger J. M. Stienen,2 and Jolanda van der Velden2. The role of protein kinase C-mediated phosphorylation of sarcomeric proteins in the heart-detrimental or beneficial? Biophys Rev. 2011 Sep; 3(3): 107.
- Israilov I.A. The results of the study of alkaloid plants. – Tashkent .: Fan, 1993. –P. 155
- Suau R. et al. Phytochemical variations within populations of Platycapnossaxicola Willk. Biochemical systematics and ecology. – 2004. – Т. 32. 6. – 565-572.
- Mashkovsky M.D. Medicines Tashkent: Publishing house of medical literature, 1998.-Т – P. 393-394.
- Fan-Cheng Meng,1Zheng-Feng Wu,1 Zhi-Qi Yin,2 Li-Gen Lin,1 Ruibing Wang,1 and Qing-Wen Zhang1. Coptidis rhizoma and its main bioactive components: recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin Med. 2018; 13: 13.
- Gidalevitz T1, Prahlad V, Morimoto RI. The stress of protein misfolding: from single cells to multicellular organisms. Cold Spring Harb Perspect Biol.2011 Jun 1;3(6).
- Alexander Kushnir*and Andrew R. Marks*. The Ryanodine Receptor in Cardiac Physiology and Disease. Adv Pharmacol. 2010; 59: 1–30.
- Rababa’h A1, Singh S2, Suryavanshi SV2, Altarabsheh SE3, Deo SV4, McConnell BK5. Compartmentalization role of A-kinase anchoring proteins (AKAPs) in mediating protein kinase A (PKA) signaling and cardiomyocyte hypertrophy. Int J Mol Sci. 2014 Dec 24;16(1):218-29.
- Michael S. Kapiloff, Nicole Jackson, Nathan Airhart. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. Journal of Cell Science 2001 114: 3167-3176
- Huihui Kong, Peter P. Jones, Andrea Koop, Lin Zhang, Henry J. Duff, and R. Wayne Chen*. Caffeine Induces Ca2+Release by Reducing The Threshold for Luminal Ca2+ Activation of the Ryanodine Receptor.Biochem J. 2008 Sep 15; 414(3): 441–452.
- Noriko Saegusa, Emma Moorhouse, Richard D. Vaughan-Jones, Kenneth W. Spitzer. Influence of pH on Ca2+ current and its control of electrical and Ca2+ signaling in ventricular myocytes. J. Gen. Physiol. Vol. 138 No. 5 537–559
- Bassani R.A., Bassani J.W.M., Bers D.M. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]in during caffeine contractures in rabbit cardiac myocytes. Journal of Physiology. – 1992. – V.453. – P.591–608.
- Inoyat Jumayev, Pulat Usmanov, Shakhnoz Kurbonova, Adilbay Еsimbetov, Shavkat Rustamov, Sherzod Jurakulov, Valentina Vinogradova. Characteristics of inotropic activity of some isoquinoline alkaloids. Universum – 2019. V 5(59). – P. 5–8.
Abbreviations Used in This Paper are as Follows
- AC = Adenylate cyclase;
- ATP = Adenosintriphosphat;
- Са2+L-channels = The L-type Ca2+–channels;
- [cAMP]in = The intracellular concentration of Cyclic Adenosine 3′,5’–monophosphate;
- [Са2+]in = The intracellular concentration of Ca2+ ions;
- + dF/dtmax = The maximum velocity of force development;
- –dF/dtmax = The maximum velocity of relaxation;
- EC50 = The values of concentration for 50% of the maximal effect;
- Gs = Guanine nucleotide-binding proteins;
- HSE = Hugo Sachs Elektronik;
- PKA = Protein kinase A;
- SR = Sarcoplasmic reticulum;
- RyRs = Ryanodine Receptors or Sarcoplasmic reticulum Ca2+–channels;
- CICR = “Ca2+ induced Ca2+ release”;
- SERCA = Са2+–ATPasa of sarcoplasmic reticulum;








