Praveen A N1 , Praveen Panchaksharimath1*
, Praveen Panchaksharimath1* and Karthik Nagaraj2
and Karthik Nagaraj2
1Department of Pharmacology, Bangalore Medical College and Research Institute (BMC and RI), Bengaluru, 560002, Karnataka, India
2Department of Neurology, Super speciality hospital (PMSSY) BMC and RI, Bengaluru 560002, Karnataka, India
Corresponding Author E-mail : praveengowdadr@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/1898
Abstract
Focal seizures account for 60% of all the epilepsies, 1/3rd of patients do not respond to monotherapy; making it necessary to try a combination of drugs for better seizure control. Levetiracetam has good safety profile, less drug interactions and no requirement of plasma monitoring making it a better adjunctive. To compare the efficacy and safety of levetiracetam as add-on to carbamazepine and phenytoin in treatment of focal seizures. Prospective, open label, comparative study conducted in the department of Neurology (PMSSY) from November 2016 to May 2018. 60 out-patients with focal seizures, already on phenytoin or Carbamazepine tablet were given Levetiracetam as add-on. Seizure count, adverse effects, and Quality of life (QOL) were monitored at 4th, 8th and 12th week. The mean age was 33.38 years and 63% were males. Levetiracetam as add-on had more than 50% reduction in seizure count from baseline in 90% and 100% of patients in Carbamazepine and phenytoin groups respectively. 66% in Carbamazepine group and 56% patients in Phenytoin group had no seizure episode during the course of treatment. Mild adverse effects like headache, giddiness, tiredness and slurring of speech were observed in both the groups. There was improvement in QOL in both the groups. However there were no significant differences in seizure count, QOL between groups across different time periods, when levetiracetam was used as add-on to both the drugs. Levetiracetam can be a better add-on to focal seizures patients who were not controlled with monotherapy.
Keywords
Carbamazepine; Focal Seizures; Levetiracetam; Phenytoin; Responder Rate
Download this article as:| Copy the following to cite this article: Praveen A. N, Panchaksharimath P, Nagaraj K. A Comparative Study to Evaluate the Efficacy and Safety of Levetiracetam as an Add-on to Carbamazepine and Phenytoin in Focal Seizures at a Tertiary Care Hospital. Biomed Pharmacol J 2020;13(1). |
| Copy the following to cite this URL: Praveen A. N, Panchaksharimath P, Nagaraj K. A Comparative Study to Evaluate the Efficacy and Safety of Levetiracetam as an Add-on to Carbamazepine and Phenytoin in Focal Seizures at a Tertiary Care Hospital. Biomed Pharmacol J 2020;13(1). Available from: https://bit.ly/3dxriJ8 |
Introduction
Focal seizures are defined as abnormal discharges of electrical impulses from a neuronal network arise in specific loci of cortex in one of the cerebral hemisphere accounting for 60% of all the epilepsies. (1) Patients who have focal epilepsy related to an underlying structural lesion or those with multiple seizure types and developmental delay are particularly likely to require multiple drugs. (2)
Carbamazepine, oxcarbazepine, lamotrigine, phenytoin, levetiracetam, and valproic acid are used as first line drugs. (2) Carbamazepine and Phenytoin are the earliest and most commonly used broad spectrum antiepileptic drugs worldwide for the treatment of focal seizures for over 50 years. (3) NICE guidelines recommend carbamazepine as a first-line treatment for focal seizure, whereas Phenytoin is still used as a first-line drug in low- to middle-income countries in focal seizures. (3, 4) Both Carbamazepine and Phenytoin are metabolised by CYP enzymes and are also inducers of CYP enzymes leading to drug interactions. Both have narrow therapeutic index hence necessitate plasma drug concentration monitoring. (5)
Approximately one-third of patients with epilepsy do not respond to treatment with a single antiepileptic drug, and it becomes necessary to try a combination of drugs to control seizures. (1) An add-on anti epileptic drug is used invariably in patients who do not respond adequately to AED monotherapy or undesirable adverse drug reactions appear. An ideal add-on AED should be safer, have a better pharmacodynamic and pharmacokinetic profile to minimize drug-drug interactions and adverse effect profile. (6)
Levetiracetam (LEV) has better efficacy in focal seizures, lesser adverse drug reactions, better safety profile, less drug interactions, and no requirement of serum level monitoring, making it an attractive first-line or adjunctive therapy for epileptic seizures. In a cochrane review it was shown Levetiracetam as an add-on was four times more effective than placebo and ≈ 30% of adults had significant reduction in the frequency of their focal seizures. (3, 6)
Owing to the dearth of comparative studies on levetiracetam as an add-on to carbamazepine and phenytoin in the treatment of Focal seizures this study was undertaken.
Objectives
The objectives of the present study were to compare the efficacy and safety of levetiracetam as add-on to carbamazepine and phenytoin in treatment of focal seizures.
Materials and Methods
This is a prospective, open label, comparative study conducted from November 2016 to May 2018 at Neurology out-patient department, super-speciality hospital (PMSSY) attached to Bangalore Medical College & Research Institute, Bengaluru. Sample size was 30 with a 1 unit difference in mean (d) and 1.3 as standard deviation (σ) in seizure count between 2 groups with 5% significance level and 80% power and we found 26 and considering dropout cases, we required 30 patients in each group.
After obtaining clearance and approval from the Institutional Ethics Committee (Reference number BMC/PGs/289/2016-17 dated on 03.11.2016), 60 out-patients diagnosed with Focal seizures with or without secondary generalization, who were already on carbamazepine or phenytoin for last 3 months of either gender aged >18 years, < 60 years and willing to give written informed consent with atleast 1 and not >14 focal seizure episodes per month since last 3 months were enrolled and those with history of hepatic, renal, cardiac, metabolic and psychiatric diseases, hypersensitivity to study medication, with other type of seizures as their primary diagnosis, pregnant and lactating mothers were excluded
Patients were treated with levetiracetam 20-50 mg/kg/day tablet as add-on to Carbamazepine 10-30 mg/kg/day or Phenytoin 3-6 mg/kg/day tablet given orally. Initially the dose of Levetiracetam was 500 mg twice daily as add-on to both the groups. The dose of levetiracetam was subsequently titrated depending on the response and tolerability
Follow-up visits were at 4 weeks (visit 2), 8 weeks (visit 3) and 12 weeks (visit 4) after administering Levetiracetam. A deviation of ±2 days for first follow-up and ±1 week for subsequent follow-ups was accepted. At follow-up visits seizure count, responder rate (percentage of patients with ≥ 50% reduction from historical baseline), seizure freedom rate (percentage of patients who did not experience focal seizures during the entire 12-week treatment period), Quality of life, adverse effects and adherence to medication were assessed.
Statistical Analysis
The descriptive data was analysed using Mean ± SD, frequency, percentage. Unpaired t-test was used to test for significance between the 2 groups, repeated measures ANOVA (Analysis of variance) was used for within the group analysis and Chi-square test or Mann-Whitney test was used for qualitative parameters. A p value of < 0.05 was considered as statistically significant
Results
We screened a total of 74 patients and 60 were recruited based on the inclusion and exclusion criteria. The participant flow diagram is given in Figure 1. The commonest reasons for exclusion were failure to give consent and were on other concomitant anti-epileptic medications. The 60 patients with focal seizures already on treatment with Carbamazepine (Group 1) and Phenytoin (Group 2) were given Levetiracetam as an add-on.
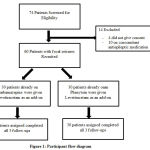 |
Figure 1: Participant flow diagram |
All patients belonged to the age group of 18 to 60 years with an average of 33.38 years. The mean age in carbamazepine group was 32.36 years and in phenytoin group was 34.46 years. Male preponderance (63%) was observed across both the groups. History of febrile seizures was observed in 14 patients, 8 in carbamazepine group and 6 in phenytoin group. Hypertension (n = 7) followed by Diabetes mellitus (n = 4) was the most common Co-morbidity seen in both the groups All the above parameters were matched at the baseline (Table 1).
Table 1: Baseline Characteristics between groups
| Variable | CBZ+LEV (n = 30) | PHT+LEV (n = 30) | p value | |
| Age (years±SD) | 32.36±10.21 | 34.46±11.18 | 0.45* | |
| Gender | Male | 20 (66.6%) | 18 (60%) | 0.79# |
| Female | 10 (33.3%) | 12 (40%) | ||
| History of Presenting illness | Focal seizures with secondary Generalization | 9 (30%) | 10(33.3%) | 1.0# |
| Focal seizures without secondary Generalization | 21 (70%) | 20 (66.6%) | ||
| Family History of seizure | Yes | 5 (16.6%) | 4 (13.3%) | 0.99$ |
| No | 25 (83.3%) | 26 (86.6%) | ||
| Febrile seizure | Yes | 8 (26.6%) | 6 (20%) | 0.76# |
| No | 22 (73.3%) | 24 (80%) | ||
| Co-morbidities | Hypertension | 3 (10%) | 4 (13.3%) | 0.37# |
| Diabetes mellitus | 3 (10%) | 1 (3.3%) | ||
| Hemi paresis | 1 (5%) | 1 (3.3%) | ||
| IHD | 0 (0%) | 2 (6.6%) |
CBZ: Carbamazepine PHT: Phenytoin LEV: Levetiracetam
p<0.05 Statistically significant * (t test) # (Chi square test) $ (Fischer exact test)
Seizure Count
The mean (SD) seizure count at baseline was 1.96 (1.29) per month in carbamazepine group and 2.03 (1.12) in phenytoin group for the past 3 months and it was not statistically significant (p = 0.83). The mean (SD) seizure count at reduced to 0.2 (0.61) per month and 0.07 (0.25) per month at the end of 12 weeks in the carbamazepine and phenytoin groups respectively (Table 2) and was not statistically significant (p = 0.65). However within their groups there was a significant difference (p < 0.001) at all the follow-up visits (Table 3).
Table 2: Comparison of Mean Seizure Count across different time periods
| Visit | Group | N | Mean ± SD | Median | Min | Max | p value# |
| Baseline | CBZ+LEV | 30 | 1.96±1.29 | 2.00 | 1 | 6 | 0.62 |
| PHT+LEV | 30 | 2.03±1.12 | 2.00 | 1 | 5 | ||
| Week 4 | CBZ+LEV | 30 | 0.53±1.22 | 0.00 | 0 | 6 | 0.54 |
| PHT+LEV | 30 | 0.57±0.85 | 0.00 | 0 | 3 | ||
| Week 8 | CBZ+LEV | 30 | 0.33±0.99 | 0.00 | 0 | 5 | 0.49 |
| PHT+LEV | 30 | 0.07±0.25 | 0.00 | 0 | 1 | ||
| Week 12 | CBZ+LEV | 30 | 0.20±0.61 | 0.00 | 0 | 3 | 0.65 |
| PHT+LEV | 30 | 0.07±0.25 | 0.00 | 0 | 1 |
CBZ: Carbamazepine PHT: Phenytoin LEV: Levetiracetam
p<0.05 statistically significant # Mann Whitney U test
Table 3: Differences in Mean seizure count within the groups across different time periods
| Baseline | 4th week | 8th week | 12th week | p value | |
| CBZ+LEV (n = 30) | 1.96±1.29 | 0.53±1.22 | 0.33±0.99 | 0.20±0.61 | < 0.001 |
| PHT+LEV (n = 30) | 2.03±1.12 | 0.57±0.85 | 0.07±0.25 | 0.07±0.25 | < 0.001 |
Friedman test
At the end of 4 weeks, 26 patients in the carbamazepine group had ≥ 50% reduction in seizure count and increased to 27 at the end of the study whereas in the phenytoin group 22 patients responded initially and at end of the study, all the patients had ≥ 50% reduction in seizure count. There were no significant differences in seizure score between groups across different time periods (Table 4).
Table 4: Differences in seizure count between groups across different time periods
| Seizure Score | 4th week follow-up | 8th week follow-up | 12th week follow-up | |||
| CBZ+LEV (n = 30) | PHT+LEV (n = 30) | CBZ+LEV (n = 30) | PHT+LEV (n = 30) | CBZ+LEV (n = 30) | PHT+LEV (n = 30) | |
| ≥ 50% reduction in Seizure count from Baseline | 26 (86.6%) | 22 (73.3%) | 26 (86.6%) | 30 (100.0%) | 27 (90.0%) | 30 (100.0%) |
| p value | 0.196# | 0.112* | 0.237* | |||
| Seizure free rate | 23
(76.6%) |
19 (63.3%) | 26 (86.6%) | 28 (93.3%) | 27 (90.0%) | 28 (93.3%) |
| p value | 0.259# | 0.670* | 1.0* | |||
# Chi square test * Fisher Exact Test
Seizure free rate was observed in 23 and 19 patients at 4th week, 27 and 28 patients at 12th week in carbamazepine and phenytoin groups respectively. 20 patients in carbamazepine group and 17 patients in phenytoin group had seizure freedom from historic baseline.
Adverse Effects
During the study period, we only encountered a few minor side effects associated with both groups which did not require hospitalization or discontinuation of medication. The adverse effects observed were Headache, Giddiness, slurring of speech, sedation and backache (Figure 11). Headache was the most frequently encountered adverse effects and there was no significance difference between the 2 groups.
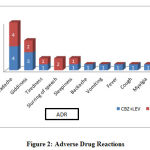 |
Figure 2: Adverse Drug Reactions |
Quality of Life in Epilepsy 10p questionnaire was used to assess QOL. The mean (SD) of QOLIE- 10p score at the baseline was 36.7 and 35.7, reduced to 32.2 and 31.8 in the carbamazepine and phenytoin groups respectively. This difference was statistically significant within the groups (p < 0.0001) (Table 5A) but not between the groups (p = 0.59) (Table 5B).
Table 5A: Quality of Life within the group
| QOL | Baseline | 12th week | P value |
| CBZ+LEV (n = 30) | 36.7±3.8 | 32.2±3.6 | < 0.0001 |
| PHT+LEV (n = 30) | 35.7±3.5 | 31.8±3.1 | < 0.0001 |
| Total (n= 60) | 36.2±3.7 | 32±3.3 | < 0.0001 |
Paired t test
Table 5B: Quality of Life between the groups
| GROUP | N | Mean ± SD | Mean Difference | P value | |
| QOL at Baseline | CBZ+LEV | 30 | 36.7±3.8 | 0.97 | 0.321 |
| PHT+LEV | 30 | 35.7±3.5 | |||
| QOL at 12th week | CBZ+LEV | 30 | 32.2±3.6 | 0.47 | 0.599 |
| PHT+LEV | 30 | 31.8±3.1 |
Unpaired t test
The adherence to medication was evaluated throughout the study and it was found to be satisfactory. At 4th week, 93.3% and 86.6% of patients were adherent to their medications in the carbamazepine and phenytoin groups respectively. With prompt advice and education on the importance of medication adherence to anti-seizure medications, it increased to 100% in both the groups (Table 6).
Table 6: Medication adherence in both the groups at different time periods
| Follow-up | CBZ+LEV
(n = 30) |
PHT+LEV
(n = 30) |
p value |
| 4th week | 28 (93.3%) | 26 (86.6%) | 0.670 |
| 8th week | 30 (100) | 27 (90%) | 0.237 |
| 12th week | 30 (100%) | 30 (100) | 1.0 |
Fisher Exact Test
Discussion
The desired aim in the treatment of the epilepsies is satisfactory seizure control with monotherapy. An ideal anti-epileptic would suppress all seizures without causing any unwanted effects. (2) The medical management of seizure disorders should be based on tailoring every patient’s regimen to his or her seizure type, co-morbidities, lifestyle, and history of tolerability to medication.
Carbamazepine and Phenytoin are the earliest and most commonly used broad spectrum antiepileptic drugs worldwide for the treatment of focal seizures for over 50 years. (3) If monotherapy with a particular anti epileptic fails, or any undesirable adverse drug reactions appear, the next step will be substitution with other anti epileptic drugs. About one third of patients with Focal seizures require an add-on anti epileptic drug. (8) An ideal add-on AED should be safer, have a better pharmacodynamic and pharmacokinetic profile to minimize drug-drug interactions and adverse effect profile. US-FDA has approved newer AEDs – felbamate, tigabine, lamotrigine, pregabalin, gabapentin, topiramate, oxcarbazapine, zonisamide, and levetiracetam for the adjunctive therapy of focal seizures. (8)
Levetiracetam has shown better efficacy in focal seizures, lesser adverse drug reactions, better safety profile, less drug interactions with older anti epileptic drugs and does not require routine plasma monitoring, thus making it an better adjunctive therapy in focal seizures. (3) Levetiracetam is also approved as adjunctive therapy in the treatment of focal seizures, myoclonic seizures in JME and in primary GTCS in patients aged ≥ 6 years according to NICE guidelines.
In the current study the mean age 33 years which is in line with previous study in China by Wu et al (32.8 years). (9) Focal seizures were observed in 63% of males in our study and is in accordance with a study by Wu et al. whereas in SKATE study, little female predominance (57.8%) was observed. (7, 9) Focal seizures are the most common type of seizure after first year of life and its incidence is ≈ 20 cases/1 lakh population between the age group of 1 – 65 years in USA. There is no reported predisposition to gender, race, or ethnicity in focal seizures and the Incidence of focal seizures has been high among elderly, particularly in those with cerebrovascular disease. (10) Hypertension followed by Diabetes mellitus was the most common Co-morbidities. Inadequate control of postictal systemic BP may play an important role in the pathophysiology of syncope and sudden unexpected death in epilepsy (SUDEP). (11)
In the present study, ≥ 50% reduction in seizure count was observed in 90% patients in carbamazepine group and all the patients in phenytoin group at the end of 12th week whereas it ranges from 66-82 % in previous studies. (1, 12, 13) Levetiracetam is a newer antiepileptic drug, acts by binding to synaptic vesicle protein-2A (SV2A) in the brain, causing selective inhibition of high voltage activated N-type Calcium channel and modulates the release of glutamate and GABA, ultimately affecting neural excitability. (7)
In the present study, 3/4th of patients in carbamazepine group had no episode of focal seizure at the end of 4th week which increased to 90% at the end of study, whereas in phenytoin group, only 2/3rd of patients were seizure free which increased to 90% at the respective visits. Seizure freedom from historic baseline that is no seizures after initiating levetiracetam add-on therapy was observed in 66% and 56% of patients in carbamazepine and phenytoin group respectively, however the difference was not statistically significant. These results were similar to previous studies conducted by Tanaka S et al., Hideaki Kanemuraa et al. and Konrad J. Werhahn et al. with a seizure freedom rate between 42 – 54% for Levetiracetam as an add-on therapy from baseline to the end of 12th week. (1, 13, 14) However the seizure freedom rate 10 – 20% in a study conducted by Wu et al. and in the KEEPER trial. (9, 12) In a study conducted by Hideaki K et al. the mean percentage reduction in seizure frequency from baseline was 89%. (13)
These results evidently demonstrate that treatment with Levetiracetam as an add-on to carbamazepine and phenytoin significantly reduces the frequency of focal seizures.
One patient in carbamazepine group showed a mild increase in seizure count and the dose of Levetiracetam was increased at 8th week to 1.5 g/day and subsequently the seizure count reduced. In majority of patients Levetiracetam was better tolerated and showed reduction in seizure count at dose of 1g/day. In a study conducted by Wu et al. responder rate with 3000mg/day LEV (41.3%) was better compared to 1000 mg/day of LEV (28.5%). (9)
Headache, giddiness, slurring of speech, sedation and backache were the most frequently encountered adverse effects in both the groups and were similar to previous studies. (9, 12) None of the patients in both the groups reduced the dose or discontinued Levetiracetam due to an ADR.
Adverse effects result into decreased medication adherence which results into increased chances of seizure episodes and more the chances of seizure episodes hence poorer the quality of life. (15) QOLIE-10p scale was used to assess the quality of life, there was an significant improvement in QOL in both the groups from baseline till the end of study however no significant difference was observed between the groups. Improving patient’s QOL is recognized as an essential component of the management of patients with epilepsy.
Adherence to medication plays an important role in chronic illnesses like epilepsy which can affect seizure recurrence which in turn affects the quality of life. Non-adherence to antiepileptic drugs (AEDs) can result in breakthrough seizures many months or years after a previous episode and can have serious repercussions on an individual’s perceived quality of life. (16) The adherence to medication was evaluated in the current study and it increased from 7 – 14% at 4th week to 100 % at the end of 12th week in both the groups.
Strengths: we tried to assess the efficacy and safety of Levetiracetam as add on to Carbamazepine and Phenytoin among Indian patients, on which the data was scarce and was conducted at neurology department of tertiary centre,
Limitations: the study was conducted for only 12 weeks after which the patients were not followed up. Since Levetiracetam was added to both the groups, patients were not randomised. We assessed only 60 patients with Focal seizures and elderly population was excluded from the study. Further there is a need for a multicentre randomised study involving large number of patients for a longer duration to evaluate Levetiracetam efficacy, seizure recurrence and adverse effects.
Conclusion
The results from our study showed that Levetiracetam is efficacious in significantly reducing the number of seizures as an add-on to either carbamazepine or phenytoin among patients with Focal seizures. Mild adverse effects were observed, quality of life improved in both the groups at the end of the study. However no significant difference was observed between the groups. Hence Levetiracetam is a better add-on in focal seizures as it showed > 90% responder rate, fewer adverse effects and better Quality of life.
Conflict of Interest
No conflict of interest
Funding Source
None
Statement of Informed Consent
Informed consent was taken from all the patients who were part of this study.
References
- Tanaka S, Tanaka T. Levetiracetam add-on therapy in Japanese patients with refractory partial epilepsy. Epileptic disorders. 2013;15(2):132–141.
- Lowenstein DH Seizures and Epilepsy In: Fauci SA, Bruanwald E, Kasper LD, Hauser LS, Longo LD, Jameson LJ et al, editors. Harrison’s principles of internal medicine, 19th ed. Vol 2. New York: The McGraw-Hill companies, Inc; 2015. p. 2542-2559.
- Mbizvo GK, Dixon P, Hutton JL, Marson AG. Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review. Cochrane Epilepsy Group, editor. Cochrane Database of Systematic Reviews [Internet]. 2012 Sep 12 [cited 2018 Oct 16]; Available from: http://doi.wiley.com/10.1002/14651858.CD001901.pub2
- National institute for health and care excellence. Epilepsies: diagnosis and management. January 2012. nice.org.uk/guidance/cg137
- Nevitt SJ, Marson AG, Weston J, Tudur Smith C. Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review. Cochrane Epilepsy Group, editor. Cochrane Database of Systematic Reviews [Internet]. 2017 Feb 27 [cited 2018 Oct 17]; Available from: http://doi.wiley.com/10.1002/14651858.CD001911.pub3
- Swaroop HS, Ananya C, Nithin K, Jayashankar CA, Babu HS, Srinivas BN. Levetiracetam: a review of its use in the treatment of epilepsy. International Journal of Medicine and Biomedical Research. 2013;2(3):166–172.
- Lambrechts DAJE, Sadzot B, van Paesschen W, van Leusden JA, Carpay J, Bourgeois P, et al. Efficacy and safety of levetiracetam in clinical practice: Results of the SKATE TM trial from Belgium and The Netherlands. Seizure. 2006 Sep;15(6):434–42.
- Loring Levine R, Ko DY. New Add-on Therapy for Partial-onset Epilepsy. US Neurology. 2008;4(1):48.
- Wu X-Y, Hong Z, Wu X, Wu L-W, Wang X-F, Zhou D, et al. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in Chinese patients with refractory partial-onset seizures. Epilepsia. 2009 ;50(3):398–405.
- Kumar A, Sharma S. Seizure, Simple Partial. [Updated 2018 May 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500005/
- Morrell MJ, Leppik I, French J, Ferrendelli J, Han J, Magnus L. The KEEPER trial: levetiracetam adjunctive treatment of partial-onset seizures in an open-label community-based study. Epilepsy Res. 2003 May;54(2–3):153–61.
- Kanemura H, Sano F, Ohyama T, Sugita K, Aihara M. Efficacy of levetiracetam as first add-on therapy to carbamazepine and valproate sodium for children with epilepsy. Journal of Pediatric Epilepsy. 2015 Jul 18;3(2):077–83.
- Werhahn KJ, Klimpe S, Balkaya S, Trinka E, Krämer G. The safety and efficacy of add-on levetiracetam in elderly patients with focal epilepsy: A one-year observational study. Seizure. 2011 May;20(4):305–11.
- Verma SK, Bala S, Singh Y, Kohli S, Kalra J, Dhasmana DC, et al. Evaluation of levetiracetam and valproic acid as monotherapy on quality of life in patients of generalized tonic clonic epilepsy. International Journal of Basic & Clinical Pharmacology. 2018 Mar 23;7(4):669.
- Eatock J, Baker GA. Managing patient adherence and quality of life in epilepsy. Neuropsychiatric disease and treatment. 2007;3(1):117.
- Panebianco M, Prabhakar H, Marson AG. Rufinamide add-on therapy for refractory epilepsy. Cochrane Epilepsy Group, editor. Cochrane Database of Systematic Reviews [Internet]. 2018 Apr 25 [cited 2018 Dec 18]; Available from: http://doi.wiley.com/10.1002/14651858.CD011772.pub2








