Nagla El-Nabarawy1* , Ahmed Gouda1 and Ezzeldin Shalaby2
, Ahmed Gouda1 and Ezzeldin Shalaby2
1National Egyptian Center of Environmental and Toxicological Research (NECTR)- Faculty of Medicine – Cairo University, Egypt
2Forensic and Clinical Toxicology Department -Faculty of Medicine, – Cairo University, Egypt
Corresponding Author E-mail: n.a.nabarawy@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1803
Abstract
Redox equilibrium is altered due to elevation of reactive oxygen species (ROS) or inadequate antioxidant defense, therapeutic effects of natural antioxidant such as curcumin (CMN) have been investigated. The aim of this study was to investigate the beneficial effects of curcumin (a natural polyphenol) on oxidative status of lung and liver and assessment of level of interleukin-6 (IL-6) in rats against paraquat toxicity. Forty adult male wistar rats were divided into five groups with eight animals each as followed: Group 1: control, Group 2: rats received olive oil. Group 3: rats received curcumin (CMN) (200 mg/kg body weight in olive oil) orally. Group 4 (model group): rats were given a single oral dose of paraquat (PQ) 50 mg/kg body weight dissolved in distilled water intra-peritoneally (I.P) Group 5: rats received CMN orally daily for 10 days prior to PQ administration with the same previous doses and after PQ. After forty eight hours of PQ administration, rats were sacrificed and lung and liver tissues samples were examined for detection of biochemical parameters and histopathological changes. Significant histopathological changes had resulted from PQ administration in lung and liver tissues in addition to significant increase in malondialdehyde (MDA), and significant decrease of catalase (CAT), superoxide dismutase (SOD) and glutathione reductase (GR). However, treatment with CMN produced increasing antioxidant markers and depletion of MDA compared to the model group. Also there is significant increase in serum IL-6 after PQ administration compared to control group. However, the level of IL-6 significantly decreased in treated group with curcumin compared to the model group. Curcumin possesses remarkable protection of the altered lung and liver tissues in paraquat intoxicated rats and could reduce the damaging effect by increasing antioxidant activity and decreasing lipid peroxidation, oxidative stress and IL-6.
Keywords
Paraquat; curcumin; oxidative stress; interleukin-6; reactive oxygen species
Download this article as:| Copy the following to cite this article: El-Nabarawy N, Gouda A, Shalaby E. Therapeutic Intervention of Curcumin on Interleukin-6 and Oxidative Stress Induced by Paraquat Toxicity of Lung and Liver in Rats. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: El-Nabarawy N, Gouda A, Shalaby E. Therapeutic Intervention of Curcumin on Interleukin-6 and Oxidative Stress Induced by Paraquat Toxicity of Lung and Liver in Rats. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2PPu0zQ |
Introduction
Pesticides, herbicides and chemical fertilizers are used in modern agriculture and these are the most important causes of toxicity to humans and animals. Paraquat is a herbicide used commonly in agriculture for higher quality crop (1). Its toxicity explained by intervening with the electron transfer photosystem intracellularly resulting in hindrance of the oxidized nicotinamide adenine dinucleotide phosphate (NADP+) reduction to reduced nicotinamide adenine dinucleotide phosphate (NADPH). Also PQ ion is reduced to PQ monocation radical which reacts currently with oxygen resulting in formation of reactive oxygen species (ROS) (2). In addition, radical forms of PQ cause lipid perioxidation, injury to membrane, multi-system damage, and cell death. Oxidative stress has been reported that enhanced production in cytokines (such as IL-6 and tumour necrosis factor alpha (TNF-α)). IL-6 plays an important role in regulation of hepatocytes, hematopoietic progenitor cells, cardiovascular system, placenta, nervous and endocrine system (3). Moreover, PQ can cause toxicity for lungs, liver, kidney and brain in human and experimental animals (4). It is generally accepted that PQ can affect multiple organ dysfunctions leading to complications such as acute pulmonary fibrosis, cardiogenic shock, renal and hepatic failure and death (5). There are no antagonists for PQ so its management has remained supportive and directed toward decreasing its absorption or increasing its elimination (6, 7). Curcumin (CMN) also called diferuloyt methane, is the main natural polyphenol (8). Curcuma longa is a medicinal plant used due to its antioxidant, anti-inflammatory (9) antimutagenic, antimicrobial (10,11), anticancer (12,13), antiapoptotic and anti-coagulation (14-16). Also CMN has an effective role against diabetes, allergies, arthritis, Alzheimer’s disease (17) and other chronic diseases (18). It has been reported that the antioxidant activity of CMN equivalent to vitamin C and E (19). In addition CMN reduces lipid peroxidation and injuries by hindrance of superoxide radicals, hydrogen peroxide and nitric oxide radicals in lung and hepatic tissues (20-22). Therefore, the present study was designed to detect the effect of antioxidant activity of CMN against PQ intoxication of lung and liver tissues in rats.
Materials and Methods
Experimental Study
Animals
The study was ethically approved by the Institutional Animal care and use committee (CU-IACUC), Faculty of Medicine, Cairo University (CU-111-F-6-19).
Forty adult male wistar rats (weights ranged from 180-200 gm) were included in this study, kept under standard laboratory conditions, and permitted free access to standard dry pellet diet and water ad libitum. Rats were acclimated for one week before divided into groups.
Experimental design
Rats were caged into five groups (eight rats for each) as followed: Group 1: control group, Group 2: rats were given olive oil (vehicle of CMN) Group 3: (CMN group) rats received only CMN (200 mg/kg body weight in olive oil) orally (23) Group 4: rats received one dose of PQ 50 mg/kg body weight dissolved in distilled water intraperitoneally (I.P) Group 5: rats received CMN orally daily for 10 days prior to PQ administration with the same previous doses and after PQ spaced (57). After two days (48h) from PQ adminstration, rats were sacrificed after I.P. injection of sodium phenobarbital anaesthesia. Liver and lung tissues were separated from each group. Then the specimens prepared and stained according to Bancroft et al, 1996 (24).
Chemicals
Paraquat (PQ) and curcumin (CMN) were purchased from sigma-Aldrich CO, St Louis, MO, USA.
Kits for malondialdehyde (MDA), catalase (CAT), glutathione reductase (GR) and superoxide dismutase (SOD) were purchased from Biodiagnostic, Egypt.
Assessment of oxidative stress biomarkers in liver and lung tissues
In liver and lung tissues lipid peroxidation, MDA level, and antioxidant emzymes activities (CAT, SOD, GR) were estimated by (Boeco S-20 spectrophotometer, Hamburg, Germany) following Okhawa (25), Aebi (26), Nishikimi et al (27), Goldberg and Spooner (28) respectively.
Detection of Interleukin-6
IL-6 was determined in serum of experimental rats using ELISA kit (ELabscience Biotechnology Co. Ltd, USA) following the manufacturer’s instructions.
Statistical analyses
Data was analyzed by SPSS statistical package, version 20 with Excel computer program which was used to tabulate the results and represent them. The significant difference between groups was noted using one-way analysis of variance (ANOVA) test followed by multiple comparison test to show the significance between each of the two groups. P- values < 0.05 were considered significant.
Results
The level of MDA in the lung tissue was significantly elevated to 38.12 + 7.58 nmoL/g in PQ intoxicated group (group 4) compared to the control group (group 1), group 2 and 3, 17.62 + 1.92 nmoL/g, 17.12 + 1.55 nmoL/g and 17.50 + 1.77 nmoL/g respectively. The fifth group, showed significant reduction of the level of MDA to 31.50 + 550 nmoL/g compared to group 4 as shown in table 1.
While enzymatic antioxidant parameters in the lung tissue as CAT showed significant decrease in PQ intoxicated group (group 4) compared to groups 1,2 and 3 (0.29 + 0.09 U/g) versus 0.77 + 0.03 U/g, 0.76 ± 0.02 U/g and 0.75 ± 0.03 U/g respectively. Rats in group 5 which received PQ and CMN showed significant elevation in CAT to 0.44 ± 0.10 U/g compared to model group (group 4) as shown in table (1).
Table 1: Biochemical Markers of oxidative stress measured in lung tissue of the studied groups
| Biochemical Markers | Mean | Std. Deviation | Minimum | Maximum | P value | ||
| MDA
nmol/g |
Group 1 (Control Group) | 17.6250 | 1.92261 | 15.00 | 20.00 | !**4,5 | |
| Group 2 | 17.1250 | 1.55265 | 15.00 | 20.00 | 2**4,5 | ||
| Group 3 (Normal Rats+ CMN) | 17.5000 | 1.77281 | 15.00 | 20.00 | 3**4,5 | ||
| Group 4 (PQ) (Model Group) | 38.1250 | 7.58641 | 28.00 | 50.00 | 4**1,2,3,5 | ||
| Group 5 (PQ+ CMN) | 31.5000 | 5.50325 | 22.00 | 40.00 | 5**1,2,3,4 | ||
| Total | 24.3750 | 9.82067 | 15.00 | 50.00 | |||
| Catalase
U/g |
Group 1 (Control Group) | .7750 | .03423 | .70 | .81 | !**4,5 | |
| Group 2 | .7650 | .02777 | .71 | .80 | 2**4,5 | ||
| Group 3 (Normal Rats+ CMN) | .7588 | .03357 | .71 | .80 | 3**4,5 | ||
| Group 4 (PQ) (Model Group) | .2950 | .09562 | .20 | .51 | 4**1,2,3,5 | ||
| Group 5 (PQ+ CMN) | .4400 | .10515 | .31 | .60 | 5**1,2,3,4 | ||
| Total | .6068 | .21331 | .20 | .81 | |||
| SOD
U/g |
Group 1 (Control Group) | 319.6250 | 1.06066 | 318.00 | 321.00 | !**4,5 | |
| Group 2 | 319.0000 | 1.30931 | 317.00 | 321.00 | 2**4,5 | ||
| Group 3 (Normal Rats+ CMN) | 319.5000 | 1.19523 | 318.00 | 321.00 | 3**4,5 | ||
| Group 4 (PQ) (Model Group) | 309.6250 | 6.96804 | 300.00 | 321.00 | 4**1,2,3,5 | ||
| Group 5 (PQ+ CMN) | 315.7500 | 2.60494 | 312.00 | 320.00 | 5**1,2,3,4 | ||
| Total | 316.7000 | 5.05964 | 300.00 | 321.00 | |||
| GR
U/g |
Group 1 (Control Group) | 79.5250 | 2.65639 | 74.40 | 83.30 | !**4,5 | |
| Group 2 | 79.0500 | 2.19610 | 75.50 | 82.30 | 2**4,5 | ||
| Group 3 (Normal Rats+ CMN) | 79.2250 | 2.16844 | 75.50 | 82.40 | 3**4,5 | ||
| Group 4 (PQ) (Model Group) | 51.8625 | 6.59241 | 40.70 | 60.30 | 4**1,2,3,5 | ||
| Group 5 (PQ+ CMN) | 66.5500 | 6.02068 | 60.00 | 75.10 | 5**1,2,3,4 | ||
| Total | 71.2425 | 11.76814 | 40.70 | 83.30 | |||
** Significant difference p value less than 0.05.
Also, the SOD was significantly decreased to 309.62 ± 6.96 U/g compared to groups 1,2 and 3 (319.62 ± 1.06 U/g, 319.00 ± 1.30 U/g and 319.50 ± 1.19 U/g) respectively. In the treated group (group 5) the SOD was significantly increased in comparison to PQ intoxicated group (group 4) 3.15 ± 2.60 U/g versus 309.62 ± 6.96 U/g as shown in table (1). In the fourth group the level of GR in the lung tissues was significantly reduced in comparison to groups 1,2 and 3 (51.86 ± 6.59 U/g versus 79.52 ± 2.65 U/g, 79.05 ± 2.19 U/g, 79.22 ± 2.16 U/g) respectively as shown in table 1. Also, the treated group (group 5) showed significant increase in the GR to 66.55 ± 6.02 U/g compared to model group (group 4) as shown in table 1. In addition, the level of MDA in the liver tissue in PQ intoxicated group (group 4) was significantly elevated to 35.37 ± 4.06 nmoL/g in comparison to groups 1,2 and 3 (17.87 ± 1.80 nmoL.g, 17.75 ± 1.66 nmoL/g and 17.50 ± 1.60 nmoL/g) respectively. The treated group (group 5) showed significant reduction of the level of MDA to 25.00 ± 3.42 nmoL/g compared to the model group (group 4) as shown in table 2. Also, enzymatic antioxidant parameters in the liver tissue showed that CAT was significantly decreased in PQ intoxicated group (group 4) compaed to groups 1,2 and 3 (0.24 ± 0.06 U/g versus 0.77 ± 0.04 U/g, 0.73 ± 0.06 U/g, 0.75 ± 0.03 U/g) respectively. In the fifth group the level of CAT was significantly increased to 0.53 ± 0.07 U/g in comparison to the model group as shown in table 2. The level of SOD in the liver tissue in PQ intoxicated group (group 4) was significantly reduced compared to groups 1,2 and 3 (305.12 ± 9.65 U/g, versus 319.62 ± 1.40 U/g, 318.75 ± 1.58 U/g, 319.62 ± 1.06 U/g) respectively. While, in the treated group who received PQ and CMN the level of SOD showed significant elevation to 312.87 ± 2.74 U/g compared to PQ intoxicated group as shown in table 2. The level of GR in the fourth group in the liver tissues was significantly decreased to 45.75 ± 10.09 U/g compared to groups 1,2 and 3 (76.86 ± 3.55 U/g, 77.46 ± 2.77 U/g and 76.67 ± 3.27 U/g respectively. In the fifth group the level of GR ws significantly elevated to 62.37 ± 9.09 U/g in comparison the model group as shown in table 2.
Table 2: Biochemical Markers of oxidative stress measured in liver tissue of the studied groups
| Biochemical Markers | Mean | Std. Deviation | Minimum | Maximum | P value | |
| MDA
Nmol/g |
Group 1 (Control Group) | 17.8750 | 1.80772 | 20.00 | 15.00 | !**4,5 |
| Group 2 | 17.7500 | 1.66905 | 20.00 | 15.00 | 2**4,5 | |
| Group 3 (Normal Rats+ CMN) | 17.5000 | 1.60357 | 20.00 | 15.00 | 3**4,5 | |
| Group 4 (PQ) (Model Group) | 35.3750 | 4.06861 | 42.00 | 30.00 | 4**1,2,3,5 | |
| Group 5 (PQ+ CMN) | 25.0000 | 3.42261 | 30.00 | 20.00 | 5**1,2,3,4 | |
| Total | 22.7000 | 7.48400 | 42.00 | 15.00 | ||
| Catalase
U/g |
Group 1 (Control Group) | .7713 | .04390 | .81 | .67 | !**4,5 |
| Group 2 | .7375 | .06882 | .80 | .60 | 2**4,5 | |
| Group 3 (Normal Rats+ CMN) | .7563 | .03249 | .80 | .71 | 3**4,5 | |
| Group 4 (PQ) (Model Group) | .2475 | .06541 | .32 | .12 | 4**1,2,3,5 | |
| Group 5 (PQ+ CMN) | .5337 | .07269 | .63 | .41 | 5**1,2,3,4 | |
| Total | .6093 | .21049 | .81 | .12 | ||
| SOD
U/g |
Group 1 (Control Group) | 319.6250 | 1.40789 | 322.00 | 318.00 | !**4,5 |
| Group 2 | 318.7500 | 1.58114 | 321.00 | 316.00 | 2**4,5 | |
| Group 3 (Normal Rats+ CMN) | 319.6250 | 1.06066 | 321.00 | 318.00 | 3**4,5 | |
| Group 4 (PQ) (Model Group) | 305.1250 | 9.65753 | 314.00 | 285.00 | 4**1,2,3,5 | |
| Group 5 (PQ+ CMN) | 312.8750 | 2.74838 | 316.00 | 309.00 | 5**1,2,3,4 | |
| Total | 315.2000 | 7.18688 | 322.00 | 285.00 | ||
| GR
U/g |
Group 1 (Control Group) | 76.8625 | 3.55766 | 80.20 | 70.30 | !**4,5 |
| Group 2 | 77.4625 | 2.77434 | 80.10 | 71.40 | 2**4,5 | |
| Group 3 (Normal Rats+ CMN) | 76.6750 | 3.27665 | 80.10 | 70.50 | 3**4,5 | |
| Group 4 (PQ) (Model Group) | 45.7500 | 10.09597 | 60.00 | 30.00 | 4**1,2,3,5 | |
| Group 5 (PQ+ CMN) | 62.3750 | 9.09423 | 75.10 | 50.50 | 5**1,2,3,4 | |
| Total | 67.8250 | 14.02304 | 80.20 | 30.00 | ||
** Significant difference p value less than 0.05.
http://biomedpharmajournal.org/wp-content/uploads/2019/12/Vol12No4_The_Nag_fig1.jpg
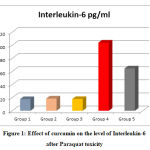 |
Figure 1: Effect of curcumin on the level of Interleukin-6 after Paraquat toxicity |
Administration of PQ 50 mg/kg BW induced a significant increase in serum IL-6 (P<0.001) when compared with group 1 (control group). On the contrary, the treated group with CMN (group 5) the level of IL-6 was significantly decreased (P<0.001) compared with the model group (group 4) the group that received PQ alone as depicted in figure 1.
Histopathological examination of lung and liver tissues
In groups 1, 2, 3 normal histological structure of alveolar wall and bronchial epithelial lining in lung tissue. The lung vasculature showed normal intact basement membrane and endothelial lining as show in Fig. 2A. However, in PQ intoxicated group (Group 4) showed congestion of both peri-alveolar and peri-bronchial blood vessels. The bronchioles showed desquamation of its epithelial lining and hyperactivity of goblet cells with excessive mucous as shown in Fig. 2B. Also, hyperplasia of lymphoid follicles was considered as prominent lesions in the most of examined animals. Marked thickening of pulmonary blood vessels with protrusion of endothelial were seen Fig. 2C. In addition, the treated group with CMN (Group 5) revealed thickening of alveolar wall and congestion of peri-alveolar capillaries Fig.2D. Peri-bronchial and peri-arteriolar oedema and leukocytic infiltration mainly lymphocytes, macrophages and few number of neutrophils were noticed Fig. 2E.
Observation of liver tissue by light microscope revealed normal liver architecture in groups 1, 2, 3 in the form of normal large polygonal cells with prominent round nuclei and eosinophilic cytoplasm, and few spaced hepatic sinusoids arranged with kupffer cells Fig. 3A. While in PQ intoxicated group the hepatic tissue section of rats revealed fatty degeneration of peripheral and mid zonal hepatocytes which characterized by numerous number of empty vacuoles. The hepatic parenchyma showed centro-lobular necrosis Fig. 3B. Some cases in group 4 revealed apoptotic activity in form of deeply eosinophilic apoptotic bodies. Hyperplasia of bile duct and newly formed bile ductules were noticed. Mononuclear infiltration at the portal area mainly lymphocytes and macrophages were also observed Fig. 3C. While, in the treated group who received CMN showed congestion and dilatation of both central veins and hepatic sinusoids Fig. 3D. The hepatic cells showed swelling and granularity of its cytoplasm. Hyperplasia of kupffer cells was seen. The portal area showed dilatation of hepatic artery and portal vein with few number of mononuclear cell infiltration Fig. 3E. Also the hepatic cords showed organization of hepatocytes without necrosis or apoptosis.
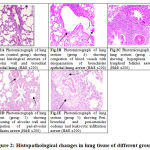 |
Figure 2: Histopathological changes in lung tissue of different groups |
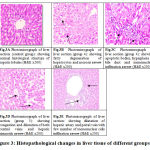 |
Figure 3: Histopathological changes in liver tissue of different groups
|
Discussion
In developing countries, toxicity of PQ remains a major health problem and mortality rate is still tragically high. Generation of ROS and oxidative damage which is stimulated by redox cycle may be due to PQ toxicity (29). This study revealed that MDA level was significantly increased in PQ intoxicated group and significantly decrease in treated group by CMN in lung and liver tissues which was in accordance with previous studies. These studies reported that MDA acts as a marker of lipid peroxidation and oxidative stress (30, 31). Also, Atashpour et al (32) found significant increase in MDA level in PQ group and decrease in PQ treated by salep in comparison to PQ group alone. Our results are compatible with those found by Blanco Ayala et al (33) who showed that PQ induces oxidative stress through modulation of redox cycling which increase in oxidative parametes and leads to reduction of endogenous antioxidant levels. In addition, many studies showed that the PQ promotes ROS via reduction of PQ, inhibition of mitochondrial, and interaction with nitric oxide syntheses (cytosolic) (34- 38).
Furthermore, the present work reported that the level of antioxidant parameters CAT, SOD and GR were significantly reduced in PQ intoxicated group and significantly elevated after treatment by CMN in liver and lung tissues. These results coincided with those of Lee and Steinert (39) who showed that antioxidant enzymes mainly SOD, CAT and GR are the main defence against free radicals (40). Our data is in line with several studies which reported on experimental animals showing significant decrease in CAT, SOD and GR activity as a result of exposure to PQ and organophosphate insecticides (41- 43). In addition Park et al (44) found that PQ accumulates in the lung, liver, kidney and brain and causes its toxicity (45, 46). Furthermore, Huang et al (47) reported that lung cells release cytokines/chemokines in oxidative stress which induces neutrophil recruitment and enhancement of transcription factor such as nuclear transcription factor be (NF-be) and activator protein-1 (AP-1) resulting in increasing the inflammation and lung damage. As one of the pro-inflammatory mediators, IL-6 showed inflammatory activation, differentiation of cells, promotes neutrophil respiratory outbreaks and degranulation to produce oxygen free radicals resulting in organ injuries. Also, liver plays a key role in the metabolism of xenobiotic compounds with biochemical changes occurring in toxic conditions (48). Cytochrome P450 (CYP) have been shown to facilitate formation of ROS during xenobiotic metabolism induces oxidative stress and damage (49- 51). In comparison to PQ intoxicated group, our results showed that CMN can ameliorate PQ toxicity in the form of decreased oxidative parameter (MDA) and increased antioxidant parameters CAT, SOD and GR in liver and lung tissues. These results are compatible with Kim et al (52) who noted that in animals CMN has high ROS scavenging capacity therefore reduces oxidative stress. Also, He et al (53) found that the antioxidant effect of CMN is due to increasing nuclear factor erythroid like-2 (NrF2) which enhances the transcription through antioxidant response elements (ARE) resulting in an increasing antioxidant activities (54-56). Also, CMN can decrease the oxidant-burden with stimulation of lung glutathione which plays a key role in shielding from PQ lung damage (57- 59). It can also enhance glutathione and SOD levels in the liver tissues (59). Moreover, CMN can reduce the iron-induced hepatic damage by reducing LPO, enhance xenobiotic detoxifying enzymes activity, the hepatic total antioxidant capacity (60- 62) and finally CMN can activate the cytoprotective enzyme HO-1 in the liver (63). On the other hand, there is accumulating evidence that CMN may not be so effective and safe. Some studies demonstrated that CMN can inhibit the activity of the drug metabolizing enzymes cytochrome P450, glutathione-S-transferase and UDP-glucuronosyl-transferase (64, 65). The inhibition of these enzymes by CMN may result in an unfavorable increase in the plasma concentration of some drugs and cause toxicity (64). The histopathological changes induced by PQ in the present work of lung tissues were examined precisely and the findings were in agreement with those of other investigators (66,67) who observed sever congestion, edema, prominent peribronchiolar round cell infiltration and connective tissue proliferation. The observed PQ effect in lung tissue was evident in Rezayat et al (68) and Smith and Heath (69) studies. They reported that PQ intoxication produced swelling and fragmentation of the alveolar epithelium followed by acute inflammatory exudate leading to infiltration into the alveolar spaces of fibroblasts to produce intra alveolar fibrosis. While, CMN exerted protective effect against PQ intoxication in lung tissue as shown in Avasarala et al (70) and Tyagi et al (71) studies. They observed that CMN attenuated multiple markers of inflammations and injury including pulmonary edema, neutrophil infiltration and inhibit fibrotic lesion development. They also revealed mild congestion, edema, peribronchiolar round cell infiltration suggesting amelioration after CMN administration.
In addition, PQ intoxicated group showed marked histopathological changes in liver tissues. Similar results were found by Lalruatfela et al (72) who noted that bile duct hyperplasia, granular and degenerative changes in liver parenchyma with infiltration of mononuclear cells, congestion of central veins, focal areas of necrosis around central veins and reticular cells hyperplasia.
The observed PQ effect in liver tissues was reported in Chohan et al (73) and Ahmad et al (74) studies. They showed in PQ intoxicated group excessive infiltration of lymphocytes, macrophages in the liver parenchyma, necrosis in hepatocytes and activation of kupffer cells, inflammatory cells, and inflamed fibrotic bridges between liver lobules (75). Recovery nearly to normal liver histology in PQ + CMN treated group may suggesting that ameliorative role of CMN against PQ induced liver injury. These results are in accordance with those noted by many investigators. Atashpour et al (32) found that CMN showed hepatoprotectived and reduced damage effect of PQ on hepatic tissue in the form of decrease of inflammation around portal vein and in sinusoids, organization of hepatocytes with little necrosis and improved congestion of central veins and sinusoids. These findings coincided with those of Singh et al (76), Salama et al (77) and Baxla et al (78) who showed also, that CMN was a hepatoprotective against CCL4, ethanol, thioacetamide, lead acetate and organophosphate insecticides (37) the mechanism of hepatoprotective effect of CMN may be due to CMN improved mitochondrial function, decreased mitochondrial ROS, down regulation of NF-kB transcription factor, and lower levels of TNF-a (79,80).
However, we need further studies to detect the ameliorative and antioxidant effect of CMN in humans.
Conclusion
Curcumin possesses remarkable protection of the altered lung and liver tissues in paraquat intoxicated rats and could reduce the damaging effect by increasing antioxidant enzymes and decreasing lipid peroxidation, oxidative stress and IL-6.
Source of Funding
The study did not receive any financial support.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Cicchetti F, Drouin-ouellet J, Gross RE. Enviromental toxins and parkinson’s disease: What have we learned from pesticide-induced animal models? Trends Pharmacol Sci 2009; 30: 475-583.
- Vicente J.A, Peixoto F, Lopes M.L, Maderia VM. Differential sensitivities of plant and animal mitochondria to the herbicide paraquat. J. Biochem. Mol. Toxicol. 2001; 15: 322-330.
- Erta M, Quintana A, and Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012; 8(9): 1254-1266.
- Awadalla EA. Efficacy of vitamic C against liver and kidney damage induced by paraquat toxicity. Exp. Toxicol. Pathol. 2012; 64: 431-434.
- Haddad LM, Shannon MW, Winchester JF, et al. Clinical Management of Poisoning and Drug Overdose. Elsevier Saunders 2007; p. 1197.
- Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology 2002; 180: 65-77.
- Vale JA, Meredith TJ, Bucjley BM. Paraquat poisoning clinical features and immediate general management. Hum. Toxicol. 1987; 6: 41-47.
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003; 23, 363-398.
- Lestari ML, Indrayanto G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodal. 2014; 39, 113-204.
- Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002; 4179-4181.
- Reddy RC, Vatsala PG, Keshamouni VG, Padmanaban G, Rangarajan PN. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005; 326, 472-474.
- Vera-Ramirez L, Perez-Lopez P, Varela-Lopez A, Ramirez-Tortosa M, Battino M, Quiles JL. Curcumin and liver disease. Biofactors. 2013; 39, 88-100.
- Wright LE, Frye JB, Gorti B, Timmermann BN, Funk JL. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Curr. Pharm. Des. 2013; 19, 6218-6125.
- Aggarwal BB, Sandaram C, Malani N, Ichikawa H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007; 595; 1-75.
- Eptein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br. J. Nutr. 2010; 103: 1545-1557.
- Noorafshan A, Ashani-Esfahani S. A review of therapeutic effects of curcumin Curr. Pharrm. 2013; 19: 2032-2046.
- Chin D, Huebbe P, Pallauf K, Rimbach G. Neuroprotective properties of curcumin in Alzheimer’s disease-Merits and limitations. Curr. Med. Chem. 2013; 20, 3955-3985.
- Maholova Y, Deneva V, Antonov L, et al. The effect of the water on the curcumin tautomerism: A quantitative approach spectrochim. Acta. A Mol. Biomol. Spectrosc. 2014; 132, 815-820.
- Blumenthal M, Goldberg A, Brinkman J. Herbal Medicine: the expended commission E monography. Newton: Integra Med. Com.; 2000.
- Joe B, Lokesh BR. Role of capsaicin curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim. Biophys. Acta. 1994; 1224, 255-263.
- Xie M, Fan D, Zhao et al. Nano curcumin prepared via supercritical improved anti-bacterial, anti-oxidant and anti-cancer efficacy. Int. J. Pharm. 2015; 496, 732-740.
- Rukkumani R, Akuna K, Varma PS, Menon VP. Curcumin influences hepatic expression patterns of matrix metalloproteinases in liver toxicity. Ital. J. Biochem, 2004; 53, 61-66.
- Ghosh S, Bhattacharyya S, Rashid K, Sil PC. Curcumin protects rat liver from streptozotocin induced diabetic patho. Physiology by counteracting reactive oxygen species and inhibiting the activation of P53 and MAPKS mediated stress response pathways. Toxicology Reports, 2015; 2: 365-376.
- Bancroft JD, Stevans A, Turner DR. Theory and Practice of Histological Techniques, 4th edition, Churchill Living Stone Edinburgh, London, Melbourne, New York, 1996; ISBN-13: 978-0443047602.
- Okhawa H, Ohishi N, Yagi K, Assay for lipid peroxides in animal tissues by thiobarbiuric acid reaction, Anal Biochem. 1979; 95(2), 351-358.
- Aebi H. Catalase, 2nd, H.V. Bergmeyer (Ed.), Methods of Enzymatic Analysis, Vol. 2 Verlag Chemie, Weiheim, 1974, pp. 673-684 ISBN: 978-0-12-091302-2.
- Nishikimi M, Roa NA, Yogi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen, Biochem. Biophys. Res. Commun. 1972; 46(2), 849-854.
- Goldberg DM, Spooner RJ. 3rd, H.V. Bergmeyen (Ed.). Methods of Enzymatic Analysis, vol. 3 Verlog Chemie, Deerfield Beach. Fl, 1983; pp. 258-265.
- Eizadi-Mood N, Sabzghabaee AM, Yaraghi A, et al. Effect of Anti-oxidants on the outcome of therapy in paraquat intoxicated patients. Tropical J of pharmaceutical research. 2011; 10(1): 27-31.
- Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005; 15: 316-328.
- Hong SY, Yang DH, Hwang KY. Associations between laboratory parameters and outcome of paraquat poisoning. Toxicol. Lett. 2000; 118: 53-59.
- Atashpour S, Jahromi HK, Jahromi ZK, Zaret S. Antioxidant effects of aqueous extract of salep on paraquat-induced rat liver injury. World J. Hepatol. 2017; 9(4): 209-216.
- Blanco Ayala T, Anderica-Romero AC, Pedraza-Chaverri J. New insights into antioxidant strategies against paraquat toxicity. Free Radical Research. 2014; 48(6): 623-640.
- Ranjbar A, Pasalar P, Sedighi A. and Abdollahi M. Induction of oxidative stress in paraquat toxicity. Free Radical Research 2014; 48(6): 623-640.
- Gonzalez-polo RA, Radriguez-Martin A, Moran JM, et al. Paraquat-induced apoptotic cell death in cerebellar granule cells. Brain. Res. 2004; 1011(2): 170-6.
- Mitra S, Chakrabarti N, Bhattacharyya A. Differential regional expression patterns of a-synuclein, TNFa, and IL-1B and variable status of dopaminergic neurotoxicity in mouse brain after paraquat treatment. J. Neuroinflammation. 2011; 8: 163.
- Abdel Rheim F, Ragab AA, Hammam F and Hamdy HE. Protective effects of curcumin for oxidative stress and histological alterations induced by pyrethroid insecticide in albino rats. The Egyptian Journal of Aospital Medicine, 2015; 58; 63-73.
- Noeman SA, Hamooda HE. Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats, Diabetol. Metab. Syndr. 2011; 17.
- Lee RF and Steinert S. Use of the single cell gel ecletrophoresis comet assay for detecting DNA damage in aquatic (marine and fresh water) animals, Mutat. Res. 2003; 544: 43-64.
- Ilio D, BelBoccio C, Casaccia R. et al. Selenium level and Gluthathione-dependent enzyme activities in normal and neoplastic human lung tissues. Carcinogen, 1987 ;( 8): 281-284.
- Kale M, Rathore N, John S, Bhatnagar D. Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rats erythrocytes: as possible involvement of reactive oxygen species. Toxicol. Lett. 1999; 105: 197-205.
- Rezayat M, OMiDi M and Ramazani M, et al. Attenation of paraquate toxicity in mice. Medical Journal of the Islamic Republic of Iran 1998; (912), 147-152.
- Lalruatfela PL, Saminathan M, Ingole RS, et al. Toxicopathology of paraquate herbicide in female wistar rats. Asian J of animal and veterinary advances 2014; 9(9): 523-542.
- Park HK, Kim SJ, Kwon DY et al. Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci; 2010; 87(5): 181-6.
- Berisha HI, Pakbaztl H, Absood A and Said SI. Nitric oxide as a mediator of oxidant lung injury due to paraquat. Proc. Natl. Acad. Sci. USA. 1994; 91(16): 7445-9.
- Dinis-Oliveira RJ, Duarte JA, Remiao F, et al. Single high dose dexamethasone treatment decreases the pathological score and increase the survival rate of paraquat-intoxicated rats. Toxicology, 2006; 227(1-2): 73-85.
- Huang J, Ning N, and Zhang W. Effect of Paraquat on IL-6 and TNF-α in macrophages. Experimental and Therapeutic Medicine. 2018; 17(3). 1783-89.
- Shertzer HG, Clay CD, Genter MB, et al. CYP1a2 protects against reactive oxygen production in mouse liver microsomes. Free Radic Biol. Med. 2004; 36(5): 605-17.
- Kumar A, Patel S, Gupta YK and Singh MP. Involvement of endogenous nitric oxide in myeloperoxidase mediated benzo (a) pyrene induced polymorphonuclear leucocytes injury. Mol. Cell. Biochem. 2006; 286(1-2): 43-51.
- Shimada H, Furuno H, Hirai KI, et al. Paraquat detoxicative system in the mouse liver postmitochondrial fraction. Arch. Biochem. Biophys. 2002; 402(1): 149-57.
- Houze P, Baud F, Mouy R, et al. Toxikinetics of paraquat in humans. Hum. Exp. Toxicol. 1990; 9(1): 5-12.
- Kim BH, Lee ES, Choi R, et al. Protective effects of curcumin on renal oxidative stress and lipid metabolism in a rat model of type 2 diabetic nephropathy. Yonsei. Med. J., 2016; 57(3): 664-673.
- He HJ, Wang GY, Gae Y, et al. Curcumin attenuates Nrf2 signaling defect oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes, 2012; 15; 3(5): 94-104.
- Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004; 44, 97-111.
- Hu M, Du O, Vancurova I, et al. Proapoptoic effect of curcumin on human neutrophils: Activation of the P38 mitogen-activated protein kinase pathway. Crit. Care Med. 2005; 33, 2571-2578.
- Literat A, Su F, Norwicki M, et al. Regulation of pro-inflammatory cytokine expression by curcumin in hyaline membrane disease (HMD). Life Sci., 2001; 70, 253-267.
- Venkatesan N. Pulmonary protective effects of curcumin against paraquat toxicity pharmacology let, 2000, 66(2), 21-28.
- Ghosh N., Ghosh R., Mandal V., Mandal S.C. Recent advances in herbal medicine for treatment of liver diseases. Pharm. Biol., 2011; 49, 970-988.
- Kaur G, Tirkey N, Bharrhan S, et al. Inhibition of oxidative stress and cytokine activity by curcumin in amelioration of endotoxin-induced experimental hepatotoxicity in rodents. Clin. Exp. Immunol. 2006; 145, 313-321.
- Rukkumani R, Aruna K, Varma PS, et al. Curcumin influences hepatic expression patterns of matrix metallo-proteins in liver toxicity. Ital. J. Biochem. 2004; 53, 61-66.
- Iqbal M, Sharma SD, Okazaki Y, et al. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddy male mice possible role in protection against chemical carcinogenesis and toxicity. Pharmacol. Toxicol. 2003; 92, 33-38.
- Xie Z, Wu B, Shen G, et al. Curcumin alleviates liver oxidative stress in type 1 diabetic rats. Molecular medicine reports, 2017; 17(1): 7911.
- Cerny D, Lekic N, Vanova K, et al. Hepatoprotective effect of curcumin in lipopolysaccharide/galactosamine model of liver injury in rats. Relationship to HO-1/CO antioxidant system. Fitoterapia. 2011; 82, 786-791.
- Mancuso C, Barone E. Curcumin in clinical practice myth or reality? Trends pharmacol. Sci. 2009; 30: 333-4.
- Thapliyal R, Maru GB. Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo. Food. Chem. Toxicol. 2001; 39: 541-7.
- Lalruatfela PL, Saminathan M, Ingole RS, et al. Toxicopathology of paraquat herbicide in female wistar rats. Asian J of Animal and veterinary advances. 2014; 9(9): 523-542.
- Krall J, Speranza MJ, Lynch RE. Paraquat-resistant hela cells, increased cellular content of glutathione peroxidase Arch. Biochem. Biophys. 1991; 286: 311-315.
- Rezayat M, Omidi M, Ramazani M, Karami H. Attenuation of paraquat toxicity in mice. Medical Journal of the Islamic Republic of Iran. 1998; 12(2): 147-152.
- Smith P and Heath D. The pathology of the lung in paraquat poisoning. J. Clin. Path. 1975; 28(9), 81-93.
- Avasarala S, Zhang F, Liu G, et al. Curcumin modulates the inflammatory response and inhibit subsequent fibrosis in a mouse model of viral induced acute respiratory distress syndrome plos. One. 2013; 8(2) e 57285.
- Tyagi N, Dash D, Singh R. Curcumin inhibits paraquat induced lung inflammation and fibrosis by extracellular matrix modifications in mouse model inflammopharmacology. 2016; (6), 335-345.
- Lalruatfela PL, Saminathan M, Ingole RS, et al. Toxicopathology of paraquat herbicide in female wistar rats. Asian J of Animal and veterinary advances. 2014; 9(9): 523-542.
- Chohan MS, Tahir M, Lone KP, et al. Paraquat induced hepatotoxicity in Albino mice. Pakistan J. Zool. 2010; 42(1): 69-73.
- Ahmad I, Kumar A, Shukla S, et al. The involvement of nitric oxide in maneb- and paraquat-induced oxidative stress in rat polymorphonulclear leukocytes. Free Radic. Res. 2008; 42: 849-862.
- Day BJ, Patel M, Calavetta L, et al. A mechanism of paraquat toxicity involving nitric oxide synthase. Med. Sci. 1999; 96: 12760-12765.
- Singh I, Vertiselvan S, Shankar J, et al. Hepatoprotective activity of aqueous extract of curcuma longa in ethanol induced hepatotoxicity in albino wistar rats. Int. J. Phytopharmacology 2012; 3(3): 226-233.
- Salama SM, Abdulla MA, Alrashdi AS, et al. Hepatoprotective effect of ethanolic extract of curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC complementary and alternative medicine. 2013; 13: 1-17.
- Baxla SL, Dr. Maria AnastasiadouGora RH, Kerketta P, et al. Hepatoprotective effect of curcuma longa against lead induced toxicity in wistar rats, Vet World, 2013; 6(9): 664-667.
- Wang L, Lv Y, Yao H, et al. Curcumin prevents the non-alcoholic fatty hepatitis via mitochondria protection and apoptosis reduction. J. Clin. Exp. Pathol. 2015; 8, 11503-11509.
- Kim KM, Pae HO, Zhung M, et al. Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW264. 7 macrophages. Biomed. Pharmaco Ther, 2008; 62, 630-636.







