Arpita Rai1,3⁎ , Musarrat Siddiqui2, Shama Parveen3, Saba Parveen2, Abdur Rasheed2 and Sher Ali3
, Musarrat Siddiqui2, Shama Parveen3, Saba Parveen2, Abdur Rasheed2 and Sher Ali3
1Department of Oral Medicine and Radiology, Dental Institute, Rajendra Institute of Medical Sciences, Ranchi
2Faculty of Dentistry, Jamia Millia Islamia, New Delhi.110025
3Centre for Interdisciplinary Research in Basic Sciences, Jamia Millia Islamia, New Delhi
Corresponding Author E-mail: arai@jmi.ac.in
DOI : https://dx.doi.org/10.13005/bpj/1835
Abstract
Oral submucous fibrosis (OSMF) is a chronic, potentially malignant condition of the oral mucosa and the habitual chewing of areca nut is believed to be the most potent etiological factor. The role of reactive oxygen species (ROS), epithelial-mesenchymal transition (EMT) and various cytokines and growth factors has been established in recent studies. The components of areca nut particularly, arecoline, flavonoids and copper have been found to affect fibroblasts, endothelial and epithelial cells through various biological pathways which are either down-regulated or up-regulated during different stages of the disease. However, the underlying molecular pathogenesis of OSMF is still partially understood.
Keywords
oral submucous fibrosis; OSF, OSMF; reactive oxygen species; TGF; EMT
Download this article as:| Copy the following to cite this article: Rai A, Siddiqui M, Parveen S, Parveen S, Rasheed A, Ali S. Molecular Pathogenesis of Oral Submucous Fibrosis: A Critical Appraisal. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Rai A, Siddiqui M, Parveen S, Parveen S, Rasheed A, Ali S. Molecular Pathogenesis of Oral Submucous Fibrosis: A Critical Appraisal. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/2ryWtjX |
Introduction
Oral submucous fibrosis (OSMF) is a chronic, progressive, potentially malignant condition of the oral cavity that predominantly affects people of South-East Asian origin [1]. It is characterized by a juxtaepithelial inflammatory reaction followed by fibroelastic change in the lamina propria and epithelial atrophy [2] which leads to stiffness of the oral mucosa, trismus and inability to eat. It affects most parts of the oral cavity and may extend over time to include the pharynx and the upper third of esophagus [2].
Several published reports on OSMF elucidate its geographic distribution together with data on percentage prevalence. Estimates of OSMF in 1996 reveal that 2.5 million people are affected globally, with most cases concentrated in the Indian subcontinent, especially Southern India [3]. In India, OSMF cases have increased dramatically from an estimated 250,000 cases in 1980 to 14 million cases in 2010[4]. Epidemiological studies have reported the prevalence of OSMF to be 6.42 per 1000[5], the male to female ratio, 4.9:1[5] and the malignant transformation of OSMF as 2.3-7.6% in the Indian context [6].
Etiology
The pathogenesis of the disease is considered to be multifactorial. Areca nut chewing, ingestion of chillies, nutritional deficiencies, genetic and immunological processes [7] are the potential etiological factors. The chewing of betel quid which contains areca nut, tobacco and slaked lime, has been recognized as one of the most important risk factors for OSMF [8, 9].
Areca nut is composed of alkaloids, flavonoids and trace elements. Four alkaloids have been identified in areca nut: arecoline, arecaidine, guvacine and guvacoline of which arecoline is the most potent agent [10]. Flavonoid components such as tannins and catechins directly affect collagen metabolism [7, 11].
Globally, 600 million people are estimated to be areca nut chewers [12]. Though, only 1-2% of areca nut users may ensue the disease [13]. This shows a clear genetic susceptibility. The development of OSMF even in the absence of intraoral influences suggests the role of other factors including genetic predisposition and an increased frequency of human leukocyte antigens (HLA) [14].
Polymorphism of genes such as Cytochrome P450 3A, may also contribute to an increased susceptibility to OSMF [15]. Xenobiotic metabolizing enzymes process most of the carcinogenic moieties in 2 broad steps: phase I which is mediated by cytochrome P450 (CYPs) and phase II which is catalyzed by glutathione s-transferases (GSTs) [16]. The expression of CYP enzymes i.e., CYP1A1 and CYP2E1, is altered as a result of polymorphisms which can influence an individual’s genetic susceptibility to cancer [17]. On the other hand, GSTs prevent the initiation of the carcinogenic process by inactivating or detoxifying electrophilic carcinogens. Among the GSTs, polymorphisms in GSTM1 and GSTT1 result in a complete loss of functional activity [16]. Studies have also revealed that polymorphisms at N-acetyltransferase 2 (NAT2) locus can increase the risk of OSMF in men if exposed to areca nut [18].
Histopathological features
OSMF is characterized by juxtaepithelial inflammation together with marked edema, fibroblasts and an inflammatory cell infiltrate consisting of neutrophils and eosinophils [19]. Collagen bundles with early hyalinization and more chronic cell types such as lymphocytes and plasma cells are seen in the later stages of the disease. The epithelial lining frequently becomes thin and hyperkeratotic. Formation of thick collagen bands and hyalinization extending into the submucosal tissues, decreased vascularity and muscle degeneration is seen in advanced stages of the disease [19].
Histopathological evidence shows stage-specific alterations in the extracellular matrix (ECM). At the onset of the disease, tenascin, perlecan, fibronectin and collagen type III are found to be manifested in the lamina propria and submucosa whereas extensive and irregular deposits of elastin are found around muscle fibers in the intermediate stage, along with the above molecules. Collagen type I appears to dominate the ECM in the advanced stages. Their gene expression levels varied with the progression of fibrosis. Difficulty in opening the mouth may be related to loss of various ECM molecules such as elastin and replacement of muscle by collagen type I [20].
OSMF as collagen metabolism disorder
The pathogenesis of OSMF involves disruption of collagen metabolism by components of areca nut. The alkaloids and flavonoid components are assimilated and undergo metabolism as a result of persistent contact between betel quid and oral mucosa. Alkaloids stimulate fibroblasts to produce collagen whereas flavonoids inhibit collagenase. As a result, there is increase in collagen production along with decrease in collagen degradation. In addition, there is significant amount of copper in areca nut which up-regulates lysyl oxidase, an enzyme that is involved in cross-linking of collagen. This process renders collagen fibrils resistant to collagenase egradation [21]. For this reason, OSMF can be regarded as a collagen-metabolic disorder [22].
Collagen production pathway involves three main events which are upregulated in OSMF:
Activation of procollagen genes
Elevation of procollagen proteinase levels: (a) procollagen C-proteinase (PCP) / bone morphogenic protein 1 (BMP1) and (b) procollagen N-proteinase (PNP)
Up-regulation of lysyl oxidase (LOX) activity Collagen degradation pathway involves
Activation of tissue inhibitor of matrix metalloproteinase gene (TIMPs)
Activation of plasminogen activator inhibitor (PAI) gene.
Polymorphisms of collagen-related genes revealed that single nucleotide polymorphisms (SNPs) of transforming growth factor β-1 (TGFβ-1) gene has a consequential association with OSMF [23]. Association of SNP in the matrix metalloproteinase-3 (MMP-3) promoter region with the 5A alleles has an increased risk for developing the disease while SNPs in the MMP-2 and MMP-9 promoter region is not associated with susceptibility to OSMF [24,25].
Molecular pathogenesis of Oral Submucous Fibrosis
There are several biological pathways that are involved in the pathogenesis of OSMF. It is likely that the normal regulatory mechanisms are either down regulated or up regulated during different stages of the disease (figure 1).
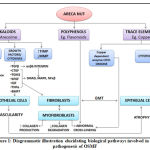 |
Figure 1: Diagrammatic illustration elucidating biological pathways involved in the pathogenesis of OSMF |
Ultimately, the following cell types are affected through these pathways, namely, fibroblasts, endothelial cells and epithelial cells. ROS activation, damage to cellular targets like DNA, protein, lipid after metabolic activation of the areca nut components by phase 1 enzymes (e.g., cytochrome P450s), the cytotoxic effects of areca nut constituents, keratinocyte inflammation and oncogene activation are considered to be the promoting factors [26].
Reactive oxygen species (ROS)
They are generated in considerable amounts during chewing of areca nut by auto-oxidation in saliva or via intracellular metabolic activation [27]. This has been proved both in vitro [28, 29] and in vivo [30]. Receptors, receptor-activated protein kinases and nuclear transcription factors, such as growth factor receptors, Janus kinase (JAK), SRC kinase, RAS signaling, mitogen-activated protein kinases (MAPKs), phosphoinositide3-kinase (PI3K)/protein kinase B (AKT) pathway, and nuclear factor-κβ (NF-κβ) [26] are activated by ROS. Both MAPK and NF-κβ pathways involved in EMT are stimulated by ROS. As a result of that, exposure to areca nut causes alterations in normal keratinocyte morphology, cell cycle arrest and senescence-associated phenotypes. It is observed that both ROS and hypoxia-inducible factor 1α (HIF-1α) are essential for hypoxia-induced TGFβ1 up-regulation [31]. Arecoline is also found to elevate HIF-1α mRNA expression in a dose-dependent manner [32]. Also, HIF-1α has been implicated as a major regulator of autophagy under hypoxic conditions. Alternatively, oxidative stress may activate autophagic reaction. Elevated levels of HIF-1α have been found in OSMF patients, which may contribute to the advancement of the disease, indicating that autophagy is expected to be induced in OSMF [33].
Cytokines and growth factors
Arecoline causes up-regulation of an array of cytokines and growth factors namely, TGFβ, connective tissue growth factor (CTGF), transglutaminase-2 (TGM2), b-fibroblast growth factor (bFGF), insulin-like growth factor (IGF), thrombospondin-1 (THBS1), secreted phosphoprotein-1 (SPP1), tazarotene-induced gene-1 (TIG1) and down-regulation of bone morphogenic protein-7 (BMP7) which is a negative modulator of fibrosis[34]. TGFβ is a strong stimulator of production and deposition of extracellular matrix, increased collagen production and decreased matrix degradation pathways [22, 35]. Loss of adipose tissue in OSMF is due to the ability of TGF β to cause lipodystrophy. Level of TGF β secretion is more during the early stages of the disease [36]. It activates pro-collagen genes resulting in production of more pro-collagen and induces the secretion of PCP and PNP, both of which are required for conversion of pro-collagen to collagen fibrils. It also triggers the gene for TIMP and PAI causing a decrease in collagen degradation [22]. Studies have revealed that keratinocytes secrete TGF β through αvβ6 integrin expression [37]. Same authors have illustrated loss of TGF β expression using the drug tropicamide that blocks αvβ6 integrin confirming that OSMF is an epithelial driven connective tissue disease [37]. Treatment of keratinocytes by catechin, tannin and alkaloids resulted in phosphorylation of SMAD-2, hence it is stated that areca nut mediated activation of p-SMAD-2 involves up-regulation and activation of TGF β [38] (figure 2).
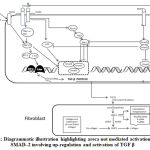 |
Figure 2: Diagrammatic illustration highlighting areca nut mediated activation of p-SMAD–2 involving up-regulation and activation of TGF β |
A recent study has demonstrated activation of TGF-β signaling with sub-cytotoxic dose of areca nut in epithelial cells and has recognized an important role of c-Jun N-terminal kinases (pJNK) in this process [39]. Calcium is released and calmodulin-dependent protein kinase (CAMKII) is activated as a result of action of areca nut on muscarinic acid receptors. It also induces intracellular ROS. CAMKII and ROS together activate JNK that phosphorylates activating transcription factor (ATF2) and c-Jun transcription factors which subsequently induce TGF-β2 promoter. The translated TGF-β protein can now activate the canonical SMAD signaling pathway and auto-induce TGF-β and other targets in epithelial cells.
In OSMF, CTGF acts as a mediator of fibrosis. The coarse particles of areca nut causing physical microtrauma to the oral mucosa results in production of CTGF by fibroblasts at the sites of injury. CTGF has shown to induce collagen synthesis in both TGFβ1-dependent and TGFβ1-independent manner. CTGF and TGFβ1 when acting together produce a prolonged fibrotic response [40]. Deng et al have shown the expression of CTGF in OSMF fibroblasts and endothelial cells in all the OSMF cases in their study [41]. The in vitro data have shown that arecoline stimulated CTGF production in buccal mucosal fibroblasts through generation of ROS, and by the activation of NF-kappa B, JNK and p38 MAPK pathways. Epigallocatechin-3-gallate (EGCG) in turn can completely block TGFβ1 induced CCN2 synthesis by suppressing JNK and p38 in buccal mucosal fibroblasts, which may be useful in controlling OSMF [42].
During the onset of disease, b-FGF is increased in fibroblasts and endothelial cells and in advanced fibrosis, it is found to be more in stroma [43]. A significant association of FGF-2 and FGFR-2 expression with malignant transformation from OSMF to oral squamous cell carcinoma (OSCC) was observed both at phenotypic and molecular level. IGF-1 expression is up-regulated in OSMF both at m-RNA and protein levels and arecoline is accountable for this increase in a dose-dependent manner [44]. It is reported that the homozygous wild genotype tumor necrosis factor α2 (TNF-α2) is associated with an increased risk of OSMF and the mutant allele TNF-α2 is about 7 times more effective in promoter function than the wild allele [45].
BMP7 has been reported to be a negative modulator of ECM production (collagen production and fibrosis) induced by TGF β [46]. It has been shown that the role of BMP7 in prevention or reversal of fibrosis is due to the reduction of pSMAD2 accumulation in the nucleus in renal and pulmonary fibrosis [46, 47]. BMP-7 binds to several receptors (activinA receptor type I and BMP receptors type 1A and 1B) to activate SMAD1 and SMAD5 and thereby suppress renal fibrosis by inhibiting SMAD3-dependent gene transcription as well as SMAD-independent pathways, including p38 and extracellular signal-regulated protein kinase (ERK) [48]. The exact mechanism of its action in OSMF remains to be studied.
Mast cells (MC) produce and store various profibrotic cytokines including TGF β, fibroblast growth factor (FGF), platelet derived growth factor (PDGF), interleukin 1 and 6 (IL-1 & IL-6), and TNF-α. A recent study has shown that altered balance in the subepithelial and deep distribution of tryptase and chymase positive MCs play an important role in the pathogenesis of OSF and its malignant transformation [49].
Regulation of MMPs & TIMPs
MMPs are a group of structurally related zinc-dependent endopeptidases and have the ability to degrade all components of ECM. To achieve balanced collagen metabolism, it is essential to maintain equilibrium between MMPs and TIMPs. In OSMF, this equilibrium is disturbed that it ultimately results in increased deposition of ECM. MMP1 is the main human enzyme that degrades fibrillar collagen and its down-regulation leads to a reduction in collagen degradation. Several studies have reported decreased expression of MMP-1 in OSMF. TIMPs are specific endogenous inhibitors of MMPs. TGFβ activates TIMP gene and their increased expression in OSMF is responsible for inhibition of collagenase and decrease in collagen degradation [50]. Arecoline increases the production of TIMP-1 which influences deposition of ECM and the effect is enhanced when fibroblasts are co-cultured with keratinocytes [51]. Chang et al found that arecoline acted not only as an inhibitor of gelatinolytic activity of MMP-2, but also as a stimulator for TIMP1 activity in OSMF [52]. They concluded that MMPs transcription activity might be associated with genesis of OSMF in younger areca nut chewers. Chaudhary et al assessed the association of SNP in the MMP-3 and MMP-1 promoter region of OSMF and suggested that expression of MMP-3 genotype associated with the 5A alleles and MMP-1 genotype associated with 2G alleles may have an important role in the susceptibility of the patients to develop OSMF [53, 54].
Polyphenols
Other components of areca nut, such as catechin, flavonoids, flavan- 3:4-diols, leucocyanidins, hexa hydroxy flavans and tannins cause collagen fibers to crosslink thereby making them less susceptible to collagenase degradation. This results in decrease in collagen breakdown which ultimately leads to increased fibrosis [55]. Also, the collagenase activity has been shown to be inhibited by flavonoids [56].
Several trace elements
like sodium, magnesium, chlorine calcium, vanadium, manganese, copper and bromine are present in areca nut [57]. High copper content has been previously reported in saliva [58] and serum [59, 60] of areca nut users and OSMF patients. Copper causes up-regulation of LOX enzyme which plays an important role in cross-linking of collagen [61]. Copper along with combination of alkaloids may induce cytotoxicity of epithelial cells which can lead to epithelial atrophy, a hallmark of OSMF. When copper (II) combines with arecoline, it leads to the formation of arecoline-copper complex in which arecoline gets oxidized resulting in reduction of copper (II) to copper (I). Reduced copper (I) in turn donates an electron to oxygen leading to the formation of superoxide radical. This superoxide radical could possibly be responsible for DNA cleavage which could generate an apoptotic response in the cell [62].
Epithelial mesenchymal transition (EMT)
It is a process defined by conversion of epithelial cells into migratory mesenchymal cells and the most common characteristic associated with EMT is the loss of E-cadherin expression [63]. Snail (SNAI1), Slug (SNAI2), zinc finger E-box binding homeobox 1 (ZEB-1), and Twist are E-cadherin transcriptional repressors which are found to be involved in promoting EMT [63]. The expression of ZEB1, which is a well-known transcriptional factor in EMT, as well as the myofibroblast marker a-smooth muscle actin (α-SMA), were found to be significantly increased in OSMF tissues [64]. Twist which functions as a transcription repressor to activate EMT traits by repressing the expression of epithelial marker E-cadherin, is found to be up-regulated in OSMF tissues [65]. S100A4, a member of the calcium-binding proteins, is dramatically elevated in a variety of fibrotic diseases, has been shown to be significantly up-regulated in OSF specimens [66]. Stage-specific embryonic antigen-4 (SSEA-4), a sialyl-glycolipid, is frequently overexpressed in cancer cells and cancer stem cells. Studies have demonstrated a significantly higher expression of SSEA-4 in areca quid chewing-associated OSF tissues [67]. STRO-1, a mesenchymal stem cell marker, confers myofibroblast trans- differentiation in fibroblasts derived from OSMF tissues [68].
Pant et al have proposed a model on the role of areca nut and TGFβ in OSMF progression. Areca nut induces and activates TGF-β in epithelial cells which can act together on the fibroblast cells invoking a possible EMT in the initiation of OSMF. It also induces expression of other pro-fibrotic cytokines (Endothelin and CTGF). These cytokines can further enhance the fibrotic response and aid in conversion of fibroblasts to myofibroblasts expressing γSMA and αSMA markers. Myofibroblasts facilitate matrix accumulation by increasing collagen production, decreasing collagen degradation and promoting abnormal cross-linking [69]. Areca nut and TGFβ can influence expression of cytoskeletal reorganizing protein LIM domain kinase 1 (LIMK1). Collagen maturation and stabilizing enzymes (BMP1 and Procollagen-Lysine, 2-Oxoglutarate 5-Dioxygenase 2 [PLOD2] respectively) can also be induced by areca nut along with TGFβ [69].
It has been documented that the Senescence associated secretory phenotype (SASP) can promote malignant phenotypes and induce an epithelial-to-mesenchyme transition leading to the development of invasive carcinoma and this is mainly due to the SASP component factors interleukin-6 (IL-6), interleukin-8 (IL-8) and growth-related oncogene-α (GRO-α) [70]. Another study has demonstrated that areca nut alkaloids induce senescence in oral fibroblasts and promote increased secretion of TGF-β and MMP-2 that may develop a tissue environment crucial for the progression of OSMF to malignancy [71].
Reduced vascularity
Growth factors and cytokines along with increased amount of ROS and DNA double strand breaks (DDBs), produced as a result of cell injury induced by areca nut, suppress endothelial cell proliferation. The alteration of specific cell cycle regulatory proteins is possibly due to the anti-proliferative and cytotoxic effects of arecoline. Therefore, the resulting endothelial cell damage leads to impaired vascular function, contributing to the pathogenesis of OSMF [72]. Loss of vascularity may lead to atrophy of the epithelium and the resulting hypoxic environment may predispose the tissue to carcinogenesis.
Mechanism of ECM remodeling
TGM-2 plays a central role in cross-linking extracellular collagen and fibronectin, making them more reluctant to breakdown and contributing to fibrosis by ECM accumulation. Studies have shown that TGM-2 expression is up-regulated in OSMF tissues which is mediated by ROS generation [73]. Heat shock protein (HSP) 47/ colligin, a stress protein which acts as a chaperone for collagen, is also up-regulated in OSMF [74]. Cystatin C, a non-glycosylated basic protein, is found to be up-regulated both at mRNA and protein levels in OSMF and arecoline is responsible for this up-regulation in a dose dependent manner [75]. Malondialdehyde (MDA), a lipid peroxidation end-product with the potential to stimulate fibroblasts and increase collagen production. MDA level is elevated as the grading of OSMF progressed [76].
Conclusion
OSMF remains an enigmatic disease with an imprecisely explored molecular pathogenesis. Though the roles of TGFβ, EMT, ROS and MMPs are established, the exact pathogenesis remains unexplored. Elucidation of pathways involved in OSMF may enable development of effective management strategies and preventing its subsequent conversion to OSCC. Finally, creating awareness across the section of society about the consequence of OSMF and avoiding the use of areca nut permanently would go a long way to save the people from oral cancer. Efforts on this line would be rewarding but people would not give up their habits unless use of areca nut is totally banned. For long term major gains, some sacrifice is ardently warranted. This issue needs due deliberation both at the level of policy makers and with people who are involved in dealing with human health care system.
Acknowledgement
Conflict of interest
None
Funding source
This work is not directly funded by any funding source. However, Dr. Arpita Rai has received funding from Ministry of AYUSH (Extra Mural Research Grant)
References
- Rajendran R. Oral submucous fibrosis: etiology, pathogenesis and future research. Bulletin of World Health Organization, 1994;72(6):985-996.
- Pindborg J & Sirsat S. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966;22(6):764-779.
- Aziz Shahid R. Coming to America: Betel Nut and Oral Submucous Fibrosis. J Am Dent Assoc. 2010;141:423-28.
- Philip T, Kumar TD, Rajkumar K, Karthik KR, Priyadharsini N & Kumar A R. Immunohistochemical evaluation of myofibroblasts using alpha-smooth muscle actin in oral submucous fibrosis. SRM J Res Dent Sci. 2014;5;243-7.
- Hazarey V K, Erlewad DM, Mundhe KA & Ughade SN. Oral submucous fibrosis: study of 1000 cases from central India. J Oral Pathol Med. 2007;36:12-17.
- Murti PR, Bhonsle RB, Pindborg JJ, Daftary DK., Gupta PC & Mehta FS Malignant transformation rate in oral submucous fibrosis over a 17-year period. Community Dent Oral Epidemiol. 1985;13:340-341.
- Ahmad MS. Epidemiological and etiological study of oral submucous fibrosis among Gutkha chewers of Patna, Bihar, India. J Indian Soc Pedod Prev Dent. 2006;24:84-8.
- Kwan HW. A statistical study on oral carcinomas in Taiwan with emphasis on the relationship with betel nut chewing: a preliminary report. J Formos Med Assoc. 1976;75:497-505.
- Sinor PN, Gupta PC, Murti PR, Bhonsle RB, Daftary DK, Mehta FS, et al. A case-control study of oral submucous fibrosis with special reference to the etiologic role of areca nut. J Oral Pathol Med. 1990;19:94-8.
- Hardie J. Oral submucous fibrosis: a review with case reports. J Can Dent Assoc. 1987;53:389-93.
- Caniff JP, Harvey W. The etiology of oral submucous fibrosis: the stimulation of collagen synthesis by extracts of areca nut. Int J Oral Surg. 1981;10:163-7.
- Gupta PC, Warnakulasuriya S. Global epidemiology of areca nut usage. Addict Biol. 2002;7:77-83.
- Tilakaratne WM, Ekanayaka RP, Warnakulasuriya S. Oral Submucous Fibrosis: A historical perspective and a review on etiology and pathogenesis. Oral Surg Oral Med Oral Pathol and Oral Radiol. 2016;122(2):178-91.
- Jayanthi V, Probert CS, Sher KS, Mayberry JF. Oral submucosal fibrosis: a preventable disease. Gut. 1992;33(1) 4-6.
- Li N, Hu Q, Jiang C, et al. Novel genetic biomarkers for susceptibility to oral submucous fibrosis: cytochrome P450 3A. Med Hypotheses. 2011;77:834-6.
- Agrawal D, et al. Role of GSTM1 and GSTT1 Polymorphism: Susceptibility to Oral Submucous Fibrosis in the North Indian Population. Oncology 2010;79(3-4):181-6.
- Yaming P, et al. Roles of CYP1A1 and CYP2E1 Gene Polymorphisms in Oral Submucous Fibrosis. Asian Pac J Cancer Prev. 2016;17(7):3335-3340.
- Mukherjee S, et al. Association of XRCC1,XRCC3 and NAT2 polymorphisms with the risk of oral submucous fibrosis among eastern Indian population. J Oral Pathol Med. 2012;41(4):292-302.
- Chiang CP, Hsieh RP, Chen TH, Chang YF, Liu BY. High incidence of autoantibodies in Taiwanese patients with oral submucous fibrosis. J Oral Pathol Med. 2002;31:402-9.
- Utsunomiya H, Tilakaratne WM, Oshiro K, et al. Extracellular matrix remodeling in oral submucous fibrosis: its stage-specific modes revealed by immunohistochemistry and in situ hybridization. J Oral Pathol Med. 2005;34:498-507.
- Arakeri G, Brennan PA: Oral submucous fibrosis: an overview of the etiology, pathogenesis, classification, and principles of management. Br J Oral Maxillofac Surg. 2013;51:587-593.
- Rajalalitha P, Vali S. Molecular pathogenesis of oral submucous fibrosis- a collagen metabolic disorder. J Oral Pathol Med. 2005;34:321-8.
- Rajendran R, Harish RK, Anil S, Vidyadharan R, Banerjee M. Transforming growth factor-β-1 polymorphisms are infrequent but exist at selected loci in oral submucous fibrosis. Indian J Dent Res. 2010;21:413-9.
- Chaudhary AK, Singh M, Bharti AC, et al. Synergistic effect of stromelysin-1 (matrix metalloproteinase-3) promoter (-1171 5A->6A) polymorphism in oral submucous fibrosis and head and neck lesions. BMC Cancer. 2010;10:369.
- Chaudhary AK, Pandya S, Mehrotra R, Singh M, Singh M. Role of functional polymorphism of matrix metalloproteinase-2 (-1306 C/T and -168 G/T) and MMP-9 (-1562 C/T) promoter in oral submucous fibrosis and head and neck squamous cell carcinoma in an Indian population. 2011;16:577-86.
- Chang MC, et al. Areca nut components stimulate ADAM17, IL-1`, PGE2 and 8-isoprostane production in oral keratinocyte: role of reactive oxygen species, EGF and JAK signaling. 2016;7(13):16879-94.
- Jeng JH, Chang MC, Hahn LJ. Role of areca nut in betel quid-associated chemical carcinogenesis: current awareness and future perspectives. Oral Oncol. 2001; 37:477-92.
- Nair UJ, Friesen M, Nair J, Bussachini V, Friesen M, Bartsch H. Formation of reactive oxygen species and 8-OH dG in DNA in vitro with betel quid ingredients. Chem Biol Interact. 1987;63:157-169.
- Liu TY, Chen CL, Chi CW. oxidative damage to DNA induced by areca nut extract. Mutat Res. 1996;367:25-31.
- Nair UJ, Nair J, Friesen MD, Bartsch H, Ohshima H. Ortho- and meta-tyrosine formation from phenylalanine in human saliva as a marker of hydroxyl radical generation during betel quid chewing. 1995;16:1195-1198.
- Tilakaratne WM, Iqbal Z, Teh MT, et al. Upregulation of HIF-1alpha in malignant transformation of oral submucous fibrosis. J Oral Pathol Med. 2008; 37:372-7.
- Ho YC, et al. Regulation of hypoxia-inducible factor-1α in human buccal mucosal fibroblasts stimulated with arecoline. J Formos Med Assoc. 2017;116(6):484-487.
- Li J, et al. Autophagy mediates oral submucous fibrosis. Exp Ther Med. 2016;11(5):1859-1864.
- Khan I, Agarwal P, Thangjam GS, Radhesh R, Rao SG, Kondaiah R. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors. 2011;29:119-27.
- Chang JZ, Yang WH, Deng YT, Chen HM, Kuo MY. EGCG blocks TGFβ1-induced CCN2 by suppressing JNK and p38 in buccal fibroblasts. Clin Oral Investig. 2013;17:455-61.
- Kale AD, Mane DR, Shukla D. Expression of transforming growth factor β and its correlation with lipodystrophy in oral submucous fibrosis: an immunohistochemical study. Med Oral Patol Oral Cir Bucal. 2013;18:e12-8.
- Moutasim KA, Jenei V, Sapienza, K, et al. Betel-derived alkaloid up-regulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosis. J Pathol. 2011;223:366-377.
- Khan I, Kumar N, Pant I, Narra S, Kondaiah P. Activation of TGF-β pathway by areca nut constituents: A possible cause of oral submucous fibrosis. PLoS One. 2012;7:e51806.
- Pant I et al. Role of areca nut induced JNK/ATF2/Jun axis in the activation of TGF-βpathway in precancerous Oral Submucous fibrosis. Rep. 2016;6: 34314.
- Sharma M, et al. CTGF is obligatory for TGFβ1 mediated fibrosis in OMSF. Oral Oncol. 2016;56:e10-1.
- Deng YT, Chen HM, Cheng SJ, Chiang CP, Kuo MY. Arecoline-stimulated connective tissue growth factor production in human buccal mucosal fibroblasts: Modulation by curcumin. Oral Oncol. 2009;45:e99-e1052.
- Chang JZ, Yang WH, Deng YT, Chen HM, Kuo MY. EGCG blocks TGFβ1-induced CCN2 by suppressing JNK and p38 in buccal fibroblasts. Clin Oral Investig. 2013;17:455-61.
- Bishen KA, Radhakrishnan R, Satyamoorthy K. The role of basic fibroblast growth factor in oral submucous fibrosis pathogenesis. J Oral Pathol Med. 2008; 37:402-11.
- Tsai CH, Yang SF, Chen YJ, Chou MY, Chang YC. The upregulation of insulin-like growth factor-1 in oral submucous fibrosis. Oral Oncol. 2005;41:940-6.
- Chiu CJ, Chiang CP, Chang ML, et al. Association between genetic polymorphism of tumor necrosis factor-alpha and risk of oral submucous fibrosis, a pre-cancerous condition of oral cancer. J Dent Res. 2001;80:2055-9.
- Zeisberg M., Hanai J, Sugimoto H, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9. 964-968(2003).
- Izumi N., Mizuguchi S., Inagaki Y., Saika S., Kawada N., Nakajima Y., Inoue K., Suehiro S., Friedman S.L. & Ikeda K. BMP-7 opposes TGF-beta1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L120-L126.
- Meng X. M., Nikolic-Paterson D. J. &Lan H. Y. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325-38.
- Yadav A, et al. Altered Immunohistochemical Expression of Mast Cell Tryptase and Chymase in the Pathogenesis of Oral Submucous Fibrosis and Malignant Transformation of the Overlying Epithelium. PloS One. 2014;9(5):e98719.
- Illeperuma RP, Ryu MH, Kim KY, Tilakaratne WM, Kim J. Relationship of fibrosis and the expression of TGF-β1, MMP-1, and TIMP-1 with epithelial dysplasia in oral submucous fibrosis. Oral Med Pathol. 2010;15:21-28.
- Xia L, Tian-You L, Yi-Jun G, Dong-sheng T, Wen-Hui L. Arecoline and oral keratinocytes may affect the collagen metabolism of fibroblasts. J Oral Pathol Med. 2009;38:422426.
- Chang YC, Yang SF, Tai KW, et al. Increased tissue inhibitor of metalloproteinase-1 expression and inhibition of gelatinase A activity in buccal mucosal fibroblasts by arecoline as possible mechanisms for oral submucous fibrosis. Oral Oncol. 2002;38(2):195-200.
- Chaudhary AK, Singh M, Bharti AC, et al. Synergistic effect stromelysin-1 (matrix metalloproteinase-3) promoter (-1171 5A->6A) polymorphism in oral submucous fibrosis and head and neck lesions. BMC Cancer. 2010;10:369.
- Chaudhary AK, Pandya S, Mehrotra R, et al. Functional polymorphism of the MMP-1 promoter (-1607 1G/2G) in potentially malignant and malignant head and neck lesions in an Indian population. 2010;15(8):684-92.
- Sharan RN, Mehrotra R, Choudhury Y, Asotra K. Association of betel nut with carcinogenesis: revisit with a clinical perspective. PLoS One. 2012;7:e42759.
- Oku N, Matsukawa M, Yamakawa S, et al. Inhibitory effect of green tea polyphenols on membrane-type I matrix metalloproteinase, MT1-MMP. Biol Pharm Bull. 2003;26:1235-8.
- Amudhan MS, Begum VH, Hebbar KB. A review on phytochemical and pharmacological potential of Areca catechu L seed. Int J Pharm Sci Res. 2012;3:4151-4157.
- Raja KB, Hazarey VK, Peters TJ, Warnakulasuriya S. Effect of areca nut on salivary copper concentration in chronic chewers. Biometals. 2007;20:43-7.
- Khanna SS, Karjodkar FR. Circulating immune complexes and trace elements (Copper, Iron and Selenium) as markers in oral precancer and cancer: a randomised, controlled clinical trial. Head Face Med. 2006;2:33.
- Tadakamadla J, Kumar S, Mamatha GP. Evaluation of serum copper and iron levels among oral submucous fibrosis patients. Med Oral Patol Oral Cir Bucal. 2011;16:e870-3.
- Shieh TM, Tu HF, Ku TH, Chang SS, Chang KW, Liu CJ. Association between lysyl oxidase polymorphisms and oral submucous fibrosis in older male areca chewers. J Oral Pathol Med. 2009;38:109-13.
- Khan, I. et al. Epithelial atrophy in oral submucous fibrosis is mediated by copper (II) and arecoline of areca nut. J Cell Mol Med. 2015;19:2397-2412.
- Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48 (56):365-375.
- Chang YC, et al. Arecoline-induced myofibroblast transdifferentiation from human buccal mucosal fibroblasts is mediated by ZEB1. J Cell Mol Med. 2014;18(4):698-708.
- Lee YH, et al. Elevation of Twist expression by arecoline contributes to the pathogenesis of oral submucous fibrosis. J Formos Med Assoc. 2016;115(5):311-7.
- Yu CC, et al. Elevation of S100A4 Expression in Buccal Mucosal Fibroblasts by Arecoline: Involvement in the Pathogenesis of Oral Submucous Fibrosis. PLoS One. 2013;8(1):e55122.
- Yu CC, et al. Aberrant SSEA-4 upregulation mediates myofibroblast activity to promote pre-cancerous oral submucous fibrosis. Sci Rep. 2016;6:37004.
- Yu CC, et al. STRO-1 confers myofibroblast transdifferentiation in fibroblasts derived from oral submucous fibrosis. J Oral Pathol Med. 2018;47(3):299-305.
- Pant, I., Kumar, N., Khan, I., Rao, S. G. &Kondaiah, P. Role of Areca Nut Induced TGF-β and Epithelial-Mesenchymal Interaction in the Pathogenesis of Oral Submucous Fibrosis. PLoS One. 2015;10(16):e0129252.
- Campisi J, et al. Celullar senescence: A link between cancer and age-related degenerative disease? Semin Cancer Biol. 2011;21:354-9.
- Rehman, et al. Areca nut alkaloids induce irreparable DNA damage and senescence in fibroblasts and may create a favourable environment for tumour progression. J Oral Pathol Med. 2016;45(5):365-72.
- Tseng SK, Chang MC, Su CY, et al. Arecoline induced cell cycle arrest, apoptosis, and cytotoxicity to human endothelial cells. Clin Oral Investig. 2012;16:1267-73.
- Lee SS, et al. Elevated transglutaminase-2 expression mediates fibrosis in areca quid chewing-associated oral submucocal fibrosis via reactive oxygen species generation. Clin Oral Investig. 2016;20(5):1029-34.
- Kaur J, et al. Co-expression of colligin and collagen in oral submucous fibrosis: plausible role in pathogenesis. Oral Oncol. 2001;37(3):282-7.
- Chung-Hung T, Shun-Fa Y, Yu-Chao C. The upregulation of cystatin C in oral submucous fibrosis. Oral Oncol. 2007;43:680-5.
- Shetty SR, Babu SG, Kumari S, Rao V, Vijay R, Karikal A. Malondialdehyde Levels in Oral Sub Mucous Fibrosis: A Clinicopathological and Biochemical Study. N Am J Med Sci. 2012;4:125-128.








