Preeti Chaudhary1*, Shamim Ahmad2 and Najam Ali Khan3
1Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology, Meerut, Uttar Pradesh, India
2Department of Pharmacy, Translam Institute of Pharmaceutical Education and Research, Meerut, Uttar Pradesh, India
3Department of Pharmacy, I.F.T.M University, Moradabad, Uttar Pradesh, India
Corresponding Author E-mail: chpreeti03@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1817
Abstract
The main aim and objective of my research work is the isolation of volatile oil from the leaves of plants by GC-MS analysis and screening of in vitro antioxidant potential of hydroalcoholic extracts of some Indigenous plants of Northern India in rodents. To perform this research work the fresh leaves of Prunus persica, Calotropis procera & Canscora decussata were taken. The isolation of volatile oil was done by using Clavenger apparatus. The samples were further analyzed by Gas Chromatography-Mass Spectrometry. In vitro antioxidant potential was carried out by DPPH, Hydrogen peroxide, Hydroxyl radical and Nitric oxide scavenging activity. Ascorbic acid was used as standard drug in this study. The percent inhibition values for antioxidant potential were obtained and tabulated. The present experimental data clearly displayed that the hydroalcoholic extract of above mentioned plant’s leaves exhibited antioxidant potential.
Keywords
In vitro antioxidant potential; Prunus persica; Calotropis procera Canscora decussata; DPPH, GC-MS analysis
Download this article as:| Copy the following to cite this article: Chaudhary P, Ahmad S,Khan N.A. Isolation and Characterization of Volatile Oil by Gc-Ms Analysis and Screening of In Vitro Antioxidant Potential of Hydroalcoholic Extracts of Some Indigenous Plants of Northern India in Rodents. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Chaudhary P, Ahmad S,Khan N.A. Isolation and Characterization of Volatile Oil by Gc-Ms Analysis and Screening of In Vitro Antioxidant Potential of Hydroalcoholic Extracts of Some Indigenous Plants of Northern India in Rodents. Biomed Pharmacol J 2019;12(4).Available from: https://bit.ly/2PgoYMt |
Introduction
Free radicals are chemical species which contain one or more unpaired electrons due to which they are highly unstable and cause damage to other molecules by extracting electrons from them in order to attain stability1. Free radicals are generated as part of the body’s normal metabolic process and play a dual role in our body as both deleterious and beneficial species. Excess production of reactive oxygen species (ROS) or a decrease in antioxidant levels may lead to the tissue damage and different diseases.2 Free radicals are involved in the pathogenesis of liver diseases. They act as messengers for signal transduction and also affect gene expression. Besides, free radicals are also involved in the pathogenesis of several chronic diseases such as inflammation, neurodegenerative diseases, ageing, rheumatoid arthritis, heart diseases, cancer, hypertension, some metabolic diseases like atherosclerosis, diabetes, and so on.3 Reactive oxygen species (ROS) encompass free radicals such as superoxide anion radicals (O2–), hydroxyl radicals (OH–) and non-free radicals such as H2O2, Singlet Oxygen along with several forms of active oxygen are involved in various physicochemical processes in the body and aging.4 Free radicals combine with the cellular macromolecules such as proteins, lipids and DNA leading to a cascade of oxidation and reduction reaction, which cause liver damage.5 Additionally, these reactive metabolites may induce disruption of ionic gradients and also intracellular calcium stores, which results in mitochondrial dysfunction and loss of energy production. Its dysfunction releases excessive amount of oxidants which in turn injures hepatic cells. Activation of some enzymes in the cytochrome P-450 system such as CYP2E1 also leads to oxidative stress.6
Antioxidants or inhibitors of oxidation are compounds which retard or prevent the oxidation and in general prolong the life of the oxidizable matter. Antioxidant plays a major role in protecting our body from disease by reducing the oxidative damage to cellular component caused by ROS.7
Some indigenous plants of northern India were taken to perform this activity. Prunus persica is naturally growing plant belongs to the Rosaceae family. The leaves are insecticidal, sedative, diuretic, demulcent, expectorant, vermicidal and are used in leucoderma, and in piles.8 Leaf paste is used to kill worms in wounds, and fungal infections. The treatment of gastritis, whooping cough, and chronic bronchitis is carried out internally with leaves.9
Calotropis procera is a wild growing plant belongs to the family Asclepiadaceae. It is well known for its medicinal properties. A number of ethanomedicinal uses of the drug are reported. Whole plant either alone or with other herbs was used for the treatment of common diseases such as fever, rheumatism, indigestion, cold, eczema and diarrohea, paste of root bark was locally applied in the treatment of elephantiasis and Root bark powder was used in the treatment of diarrhea and dysentery and it is an excellent substitute for ipecac. Traditionally it was used to treat cholera, extracting guinea worms and indigestion.10
Canscora decussata is popularly known as “Shankhpushpi” and belongs to the family Gentianaceae. It is found throughout India, up to an altitude of 1300 m. It is also found to contain triterpines, alkaloids and xanthones. It is also a natural source of pentaoxygenated, hexaoxygenated and dimeric xanthones.11
Material and Methods
Collection and Authentication of Plant Materials
The leaves of Prunus persica and Calotropis procera were collected from the garden of Aman Vihar Colony, Krishna Nagar, Roorkee Road Meerut and leaves of Canscora decussata were purchased from Khari Baoli street, Chandni chowk, New Delhi in the month of July and then authenticated by Dr. R.S. Saxena, Reader & Head Botany Department, Meerut College, Meerut (U.P.) 25000, and deposited to T.I.P.E.R. for future reference.
Preparation of Leaves Extract
The coarsely powdered leaves (500 g) were extracted with soxhlet apparatus using petroleum ether for about 24 hrs. After defatting, the marc was dried in hot air oven at 500C, packed in soxhlet apparatus, and further extracted with 1 L of 95% (V/V) ethanol and water mixture by percolation method. The solvent were removed from the extracts under reduced pressure by using rotary vacuum evaporator.12
Preliminary Phytochemical Screening
The preliminary phytochemical investigation was carried out for hydroalcoholic extracts of above mentioned plants for the detection of phytoconstituents present. Test for the presence of common phytochemicals were carried out by standard methods.
Isolation & Characterization of Plants
The fresh leaves of Calotropis procera, Canscora decussata & Prunus persica (1 Kg) each were hydrodistilled using an all glass Clavenger apparatus. A pale greenish yellow essential oil (3.45 %), (2.34 %) & (2.67 %) respectively, was obtained. It was dried over anhydrous sodium sulfate and stored at 4ºC in the dark and the samples were further analyzed by Gas Chromatography-Mass Spectrometry.13
Chemicals and Reagents
1,1-Diphenyl-2-picrylhydrazyl (DPPH), ethanol, ascorbic acid, H2O2 solution, EDTA, FeCl3, phosphate buffer, trichloroacetic acid (TCA), and all other chemicals including solvents were of analytical grade and procured from Sisco Research Laboratories Pvt Ltd.
Antioxidant Assay
The antioxidant activity of plants extracts was determined by different in vitro methods such as the DPPH free radical scavenging assay, Hydrogen peroxide scavenging activity, Hydroxyl radical scavenging activity and Nitric oxide scavenging activity. The different extracts were dissolved in distilled water at different concentration. All the assays were carried out in triplicate, and average values were considered.
DPPH radical scavenging activity
The free radical scavenging activity of hydroalcoholic extracts of Prunus persica, Calotropis procera and Canscora decussata leaves were measured by 1,1-diphynyl-2-picryl-hydrazil (DPPH) using the method of Blois.14
Reagents & Apparatus
DPPH (Sigma chemicals, USA), Ethanol (SD-Fine Chemicals Limited, India), Ascorbic acid as a standard (SD-Fine Chemicals Limited, India), Pipette (1-10 ml), UV Spectrophotometer, Test-tubes, Beakers.
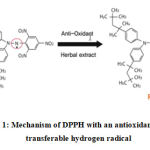 |
Figure 1: Mechanism of DPPH with an antioxidant having transferable hydrogen radical |
Preparation of reagents
Preparation of DPPH Solution (0.004%w/v)
In order to prepare 0.004% (w/v) DPPH solution 4 mg of DPPH was dissolved in 100 ml of 95% (V/V) ethanol and water mixture in a dark room.
Preparation of Standard Ascorbic Acid Solution
2 mg ascorbic acid was dissolved in 2.5 ml of distilled water, so the concentration of the solution is 800 µg/ml. This is called stock solution. Then serial dilution was prepared in order to prepare different concentrated solution (10 µg/ml, 25 µg/ml, 50 µg/ml, 75 µg/ml, 100 µg/ml, 125 µg/ml).
Preparation of Stock Solution
4 mg of extracts of different plants (Prunus persica, Calotropis procera and Canscora decussata) was dissolved in 10 ml of ethanol and water mixture in order to prepare 400 µg/ml solutions and then serial dilutions was performed to prepare required concentrated solutions.
Preparation of Control
3 ml DPPH solution was used as a negative control. The blank for this solution was ethanol.
Procedure
2ml of hydroalcoholic solution of plant extracts at different concentration was taken in different test tubes.
3ml of hydroalcoholic solution of DPPH was added into the test tube.
Then incubated at room temperature (25 ± 2ºC) for 30 minutes in a dark place to complete the reaction.
Then the absorbance of the solution was measured at 517 nm using UV-Visible spectrophotometer against blank.
A typical blank solution contained all reagents except plants extracts or standard solution.
Ascorbic acid was used as the reference material.
The percentage of inhibition was calculated by comparing the absorbance values of the control and test samples from the following formula-

All the tests were performed in triplicate and the graph was plotted with the mean values. % inhibition were plotted against concentration and from the graph IC50 was calculated.
Hydrogen peroxide scavenging activity
Hydrogen peroxide acts as a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of essential thiol (-SH) groups. Hydrogen peroxide can easily cross cell membranes rapidly, once it reaches inside the cell, H2O2 can probably react with Fe2+, and possibly Cu2+ ions to form hydroxyl radical and this may be the reason for origination of many of its toxic effects. It is therefore biologically beneficial for cells to control the amount of hydrogen peroxide that is allowed to accumulate.15
Scavenging activity of Hydrogen peroxide (H2O2) by the plant extracts was determined by the method of Ruch.16
Procedure
Plant extracts (4 ml) prepared in distilled water at various concentrations was mixed with 0.6 ml of 4 mM H2O2 solution prepared in phosphate buffer (0.1 M, pH 7.4) and incubated for 10 min.
The absorbance of the solution was measured at 230 nm.
Ascorbic acid was taken as a positive control compound.
The percentage of inhibition was calculated by comparing the absorbance values of the control and test samples.

All the tests were performed in triplicate and the graph was plotted with the mean values. % inhibition was plotted against concentration and from the graph IC50 was calculated.
Hydroxyl radical scavenging activity
The scavenging activity for hydroxyl radical was measured according to the modified method of Halliwell.17
Stock solution of EDTA (1 mM), FeCl3 (10 mM), ascorbic acid (1 mM), H2O2 (10 mM), deoxyribose (10 mM), were prepared in distilled deionized water.
The assay was performed by adding 0.1 ml EDTA, 0.01 ml of FeCl3, 0.1 ml H2O2, 0.36 ml of deoxyribose, 1.0 ml of the extracts of different plants at different concentration (10-125μg/ml) dissolved in distilled water, 0.33 ml of phosphate buffer (pH-7.4), 0.1 ml of ascorbic acid in sequence.
The mixture was then incubated at 37˚C for 1 hour.
A 1.0 ml portion of the incubated mixture was mixed with 1.0 ml of 10% TCA & 1.0 ml of 0.5% TBA to develop the pink chromogen measured at 532nm.
The hydroxyl radical scavenging activity of the extracts is reported as % inhibition of deoxyribose degradation is calculated by using following equation-

Nitric oxide scavenging activity
Principle
Nitric oxide radical scavenging activity was determined according to the method reported by Garrat.18 In aqueous solution, sodium nitroprusside spontaneously generates nitric oxide at physiological pH, which interacts with oxygen to produce nitrite ions, which were determined by the use of the Griess Illosvoy reaction.19 Nitric oxide (NO) acts as a powerful pleiotropic mediator of physiological process such as smooth muscle relaxant, neuronal signaling, inhibition of platelet aggregation and regulation of cell mediated toxicity. It also acts as a diffusible free radical which plays many roles as an effectors molecule in diverse biological systems including neuronal messenger, vasodilatation and antimicrobial and antitumor activities.20 Although nitric oxide and superoxide radicals are engaged in host defense, over production of these two radicals contributes to the pathogenesis of some inflammatory diseases.17 Moreover in the other pathological conditions, nitric oxide reacts with superoxide anion and form potentially cytotoxic molecules, peroxynitrite. Nitric oxide inhibitors have been shown to have beneficial effects on some aspect of inflammation and tissue damage seen in inflammatory diseases.
Method
2 mL of 10 mM sodium nitroprusside in 0.5 mL phosphate buffer saline (pH 7.4) was mixed with 0.5 mL of extracts of Prunus persica, Calotropis procera and Canscora decussata and the reference compound in different concentrations (10-125μg/ml).
The mixture was incubated at 25oC for 150 min.
From the incubated mixture 0.5 mL was taken out and added into 1.0 mL sulfanilic acid reagent (33% in 20% glacial acetic acid) and incubated at room temperature for 5 min.
Finally, 1.0 mL naphthylethylene diamine dihydrochloride (0.1% w/v) was mixed and incubated at room temperature for 30 min.
The absorbance of the chromophore formed was measured at 546 nm by using spectrophotometer.
Ascorbic acid was used as a positive control compound.
All the tests were performed in triplicate and the results averaged.
The percentage inhibition of nitric oxide generated was measured by comparing the absorbance values of control and test samples using following equation-

Statistical Analysis
All the data are expressed as mean ± SEM of six animals from each group. The data were evaluated by one-way ANOVA followed by using Graph Pad Prism software. A probability of less than 5% (p<0.05) was considered statistically significant.
Results and Disscussion
The leaves of Prunus persica and Calotropis procera were powdered (500 g) were extracted with Soxhlet apparatus using petroleum ether for about 12 hrs. After defatting, the marc was air dried and packed in soxhlet apparatus, and further extracted with 95% ethanol. The solvent were removed from the extracts under reduced pressure by using rotary vacuum evaporator. The percentage (%) yield of Prunus persica, Calotropis procera and Canscora decussata was found to be 7.0 %, 8.5 % and 6.5% respectively. The preliminary phytochemical screening confirmed the presence of alkaloids, glycosides, saponins, flavonoids, phenols and proteins in the hydroalcoholic leaves extract of Prunus persica. After phytochemical screening of the hydroalcoholic extract of Calotropis procera, it was found that the extract contain alkaloids, glycosides, saponins, phytosterols, flavonoids and the hydroalcoholic extract of Canscora decussata showed the presence of alkaloids, carbohydrates, glycosides, saponins, flavonoids, phenols and proteins.
Characterization of Isolated volatile oil by GC-MS Analysis
The analysis of samples of plants occurs by using GC-MS technique. As elucidated by GC-MS spectrum, the various chemical constituents, their retention times and percentage compositions are given in their respective tables. There were about fourteen different compounds isolated in the Prunus persica and about seventeen compounds in Calotropis procera leaves volatile oil which were recorded in the Table1 and Table2 respectively. The compounds which were identified in the Canscora decussata leaves volatile oil were given in the Table-3.
 |
Figure 2: GC-MS Chromatogram of Phytochemicals Present in Volatile oil of Prunus Persica Leaves
|
Table 1: Chemical Composition of volatile oil of the leaves of Prunus persica.
| S. No. | Chemical Constituents | Chemical Nature | RT (min) | Area (%) |
| 1 | m-xylene | Aromatic hydrocarbon | 3.92 | 3.76 |
| 2 | 1,2,4-Trimethylcyclohexane | Aliphatic cyclic compound | 4.13 | 0.23 |
| 3 | o-xylol | Phenolic Compound | 4.59 | 82.86 |
| 4 | 1-Ethyl-3-methyl cyclohexane | Aliphatic cyclic compound | 4.69 | 8.98 |
| 5 | m-xylol | Phenolic Compound | 4.82 | 0.69 |
| 6 | Isopropyl benzene | Aromatic hydrocarbon | 4.99 | 1.13 |
| 7 | m-Tolualdehyde | Aromatic aldehyde | 8.44 | 1.17 |
| 8 | Querceitin | Flavonoid | 21.62 | 1.52 |
| 9 | 6,10,14-Trimethyl-2-pentadecanone | Nor-diterpenic ketone | 28.07 | 0.08 |
| 10 | β-Sitosterol | Phytosterol | 30.02 | 0.94 |
| 11 | Phytol | Diterpenol | 31.45 | 8.61 |
| 12 | 1,2,3,4,5-Penta-methyl cyclopentane | Aliphatic cyclic ketone | 31.72 | 0.30 |
| 13 | 1-Docosene | Aliphatic alkene | 32.37 | 0.13 |
| 14 | n-Eicosane | Aliphatic hydrocarbon | 37.38 | 0.07 |
The volatile oil of the leaves of Prunus persica consists of mainly fourteen compounds ranging from terpenes, fatty acids, essential oils, alkaloids, phenolic compounds, flavonoids, esters, vitamins and others. The predominant constituents were m-xylene, 1, 2, 4-Trimethylcyclohexane, o-xylol, 1-Ethyl-3-methyl cyclohexane, m-xylol, Isopropyl benzene, m-Tolualdehyde, Quercetin, 6, 10, 14-Trimethyl-2-pentadecanone, β-Sitosterol, Phytol, 1, 2, 3, 4, 5-Penta-methyl cyclopentane, 1-Docosene and n-Eicosane.
 |
Figure 3: GC-MS Chromatogram of Phytochemicals Present in Volatile oil of Leaves of Calotropis Procera
|
Table 2: Chemical Composition of volatile oil of the leaves of Calotropis procera.
| S. No. | Chemical Constituents | Chemical Nature | RT (min) | Area (%) |
| 1 | m-xylene | Aromatic hydrocarbon | 3.90 | 3.21 |
| 2 | 1,2,4-Trimethylcyclohexane | Aliphatic cyclic compound | 4.13 | 0.22 |
| 3 | o-xylol | Phenolic Compound | 4.53 | 88.09 |
| 4 | p-xylol | Phenolic Compound | 4.80 | 0.77 |
| 5 | Isopropyl benzene | Aromatic hydrocarbon | 4.97 | 1.14 |
| 6 | m-Tolualdehyde | Aromatic aldehyde | 8.43 | 1.53 |
| 7 | Rutin | Flavonoid | 11.23 | 0.88 |
| 8 | Quercetin | Flavonoid | 19.82 | 1.21 |
| 9 | 6,10,14-Trimethyl pentadecanone-2-one | Nor-diterpenic ketone | 28.07 | 0.17 |
| 10 | n-Eicosane | Aliphatic hydrocarbon | 29.08 | 0.06 |
| 11 | (E)-S-Eicosene | Aliphatic alkene | 29.16 | 0.45 |
| 12 | 3,6,6-Trimethyl-2- norpinanol | Bicyclic monoterpenes | 31.12 | 0.23 |
| 13 | 3-Pentadecyl valerate | Organic acid ester | 31.30 | 0.49 |
| 14 | n-Hexdec-2-en-1-ol | Aliphatic alcohol | 31.43 | 3.00 |
| 15 | 1,2,3,4,5-Pentametyl cyclopentane | Aliphatic cyclic compound | 31.72 | 0.16 |
| 16 | Tetratriacontyl hepta flurobutyrate | Aliphatic fluoro compound | 32.60 | 0.49 |
| 17 | Kaempferol | Flavonoid | 32.30 | 1.52 |
The volatile oil of Calotropis procera consists of mainly seventeen compounds. The predominant constituents were m-xylene, 1, 2, 4-Trimethylcyclohexane, o-xylol, p-xylol, Isopropyl benzene, m-Tolualdehyde, Rutin, Quercetin, 6,10,14-Trimethyl pentadecanone-2-one, n-Eicosane, (E)-S-Eicosene, 3,6,6-Trimethyl-2- norpinanol, 3-Pentadecyl valerate, n-Hexdec-2-en-1-ol, 1,2,3,4,5-Pentametyl cyclopentane, Tetratriacontyl hepta flurobutyrate, Kaempferol.
 |
Figure 4: GC-MS Chromatogram of Phytochemicals of Volatile oil of Canscora decussate
|
Table 3: Chemical Composition of Volatile oil of the leaves of Canscora decussata.
| S. No. | Chemical Constituents | Chemical Nature | RT (min) | Area (%) |
| 1 | m-xylene | Aromatic hydrocarbon | 3.96 | 5.41 |
| 2 | o-xylene | Aromatic hydrocarbon | 4.71 | 89.60 |
| 3 | m-xylol | Phenolic Compound | 4.90 | 0.66 |
| 4 | 1,2,3-Trimethyl benzene | Aromatic hydrocarbon | 5.05 | 1.27 |
| 5 | m-Tolualdehyde | Aromatic aldehyde | 8.43 | 2.10 |
| 6 | Quercetin | Flavonoid | 19.88 | 1.16 |
| 7 | 6,10,14-Trimethyl-2-pentadecanone | Nor-diterpenic ketone | 28.07 | 0.04 |
| 8 | Isobutyl octyl phthalate | Aromatic ester | 28.44 | 0.07 |
| 9 | Ethyl palmitate | Fatty ester | 30.09 | 0.10 |
| 10 | Methyl linolenate | Fatty ester | 31.29 | 0.11 |
| 11 | Phytol | Diterpenol | 31.43 | 0.41 |
| 12 | Ethyl linoleate | Fatty ester | 31.90 | 0.04 |
| 13 | Ethyl linolenate | Fatty ester | 31.97 | 0.11 |
| 14 | Dotriacontyl penta-fluropropionate | Fluro-fatty ester | 32.36 | 0.08 |
| 15 | Mangiferin | Phenolic Compound | 35.72 | 2.53 |
The analysis of volatile oil of Canscora decussata by GC-MS resulted in the identification of fifteen components. These components were m-xylene, o-xylene, m-xylol, 1, 2, 3-Trimethyl benzene, m-Tolualdehyde, Quercetin, 6, 10, 14-Trimethyl-2-pentadecanone, Isobutyl octyl phthalate, Ethyl palmitate, Methyl linolenate, Phytol, Ethyl linoleate, Ethyl linolenate, Dotriacontyl penta-fluropropionate and mangiferin.
In Vitro Antioxidant Activity
DPPH Radical Scavenging Activity
The leaves extracts of Prunus persica, Calotropis procera and Canscora decussata demonstrated H-donor activity. The DPPH radical scavenging activity was detected and compared with Ascorbic acid. Figure 5 shows the % inhibition values of Ascorbic acid and hydroalcoholic extracts of Prunus persica, Calotropis procera and Canscora decussata which were 90.00 %, 82.73 %, 86.36 % and 83.64 % respectively. For the screening of antioxidant potential of plant extracts, DPPH assay is one of the most widely used methods. DPPH is a stable, nitrogen-centered free radical which produces violet color in hydroalcoholic solution. It was reduced to a yellow coloured product, diphenyl-picryl hydrazine, with the addition of the extracts in a concentration-dependent manner. The IC50 value of Ascorbic acid was 9.74 μg/ml, HAEPP was 57.66 μg/ml, HAECP was 46.82 μg/ml and HAECD was 66.45 μg/ml.
Table 4: DPPH Radical Scavenging Activity
| Conc (μg/ml) | Ascorbic acid absorbance | Ascorbic acid % inhibition | HAEPP absorbance | HAEPP % inhibition | HAECP absorbance | HAECP % inhibition | HAECD absorbance | HAECD % inhibition |
| 10 | 0.57±0.01 | 48.18 | 0.97±0.04 | 11.82 | 0.88±0.04 | 20.00 | 1.01±0.02 | 8.18 |
| 25 | 0.48±0.02 | 56.36 | 0.72±0.03 | 34.55 | 0.63±0.06 | 42.73 | 0.86±0.03 | 21.82 |
| 50 | 0.38±0.02 | 65.45 | 0.56±0.01 | 49.09 | 0.50±0.02 | 54.55 | 0.61±0.01 | 44.55 |
| 75 | 0.29±0.03 | 73.64 | 0.38±0.02 | 65.45 | 0.31±0.03 | 71.82 | 0.46±0.03 | 58.18 |
| 100 | 0.21±0.01 | 80.91 | 0.22±0.02 | 80.00 | 0.19±0.04 | 82.73 | 0.28±0.03 | 74.55 |
| 125 | 0.11±0.02 | 90.00 | 0.19±0.02 | 82.73 | 0.15±0.02 | 86.36 | 0.18±0.04 | 83.64 |
| Absorbance of Blank Solution was 1.10. | ||||||||
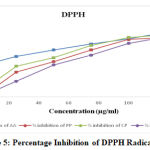 |
Figure 5: Percentage Inhibition of DPPH Radicals
|
Hydrogen Peroxide Scavenging Activity
The hydrogen peroxide scavenging activity of hydroalcoholic leaves extract of plants (Prunus persica, Calotropis procera and Canscora decussata) was evaluated and compared with Ascorbic acid and the results are given in Table 5. The percentage inhibition (% inhibition) at various concentrations (10-125 µg/ml) of hydroalcoholic extracts of plants as well as standard Ascorbic acid were calculated and plotted in Figure 6 using Microsoft Office Excel 2010. The IC50 values are calculated from graph and were found to be 16.75 µg/ml (Ascorbic acid), 40.95 µg/ml (HAEPP), 27.7 µg/ml (HAECP) and 51.94 µg/ml (HAECD).
Table 5: Hydrogen Peroxide Radical Scavenging Activity
| Conc (μg/ml) | Ascorbic acid absorbance | Ascorbic acid % inhibition | HAEPP absorbance | HAEPP % inhibition | HAECP absorbance | HAECP % inhibition | HAECD absorbance | HAECD % inhibition |
| 10 | 0.88±0.03 | 35.77 | 1.04±0.01 | 24.09 | 0.91±0.03 | 33.58 | 1.12±0.04 | 18.25 |
| 25 | 0.53±0.01 | 61.31 | 0.67±0.02 | 51.09 | 0.61±0.02 | 55.47 | 0.71±0.03 | 48.18 |
| 50 | 0.41±0.01 | 70.07 | 0.52±0.01 | 62.04 | 0.49±0.04 | 64.23 | 0.6±0.02 | 56.20 |
| 75 | 0.35±0.02 | 74.45 | 0.46±0.02 | 66.42 | 0.41±0.02 | 70.07 | 0.52±0.01 | 62.04 |
| 100 | 0.25±0.02 | 81.75 | 0.36±0.02 | 73.72 | 0.32±0.03 | 76.64 | 0.41±0.02 | 70.07 |
| 125 | 0.13±0.01 | 90.51 | 0.24±0.01 | 82.48 | 0.19±0.03 | 86.13 | 0.32±0.03 | 76.64 |
| Absorbance of Blank Solution was 1.37. | ||||||||
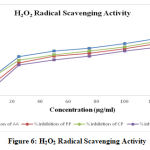 |
Figure 6: H2O2 Radical Scavenging Activity
|
Hydroxyl Radical Scavenging Activity
Hydroxyl radical scavenging activity was quantified by measuring the inhibition of the degradation of deoxyribose by the free radicals generated by the Fenton reaction. The oxygen derived hydroxyl radicals along with the added transition metal ion (Fe2+) causes the degradation of deoxyribose into malondialdehyde which produces a pink chromogen with thiobarbituric acid. The IC50 values are calculated from graph and were found to be 18.43 µg/ml (Ascorbic acid), 43.14 µg/ml (HAEPP), 34.64 µg/ml (HAECP) and 51.71 µg/ml (HAECD).
Table 6: Hydroxyl Radical Scavenging Activity
| Conc (μg/ml) | Ascorbic acid absorbance | Ascorbic acid % inhibition | HAEPP absorbance | HAEPP % inhibition | HAECP absorbance | HAECP % inhibition | HAECD absorbance | HAECD % inhibition |
| 10 | 0.82±0.03 | 36.92 | 1.02±0.01 | 21.54 | 0.93±0.02 | 28.46 | 1.1±0.03 | 15.38 |
| 25 | 0.55±0.01 | 57.69 | 0.64±0.02 | 50.77 | 0.61±0.03 | 53.08 | 0.69±0.02 | 46.92 |
| 50 | 0.39±0.01 | 70.00 | 0.5±0.01 | 61.54 | 0.47±0.03 | 63.85 | 0.53±0.02 | 59.23 |
| 75 | 0.31±0.03 | 76.15 | 0.44±0.02 | 66.15 | 0.41±0.01 | 68.46 | 0.48±0.01 | 63.08 |
| 100 | 0.28±0.02 | 78.46 | 0.35±0.02 | 73.08 | 0.32±0.02 | 75.38 | 0.38±0.01 | 70.77 |
| 125 | 0.11±0.01 | 91.54 | 0.21±0.01 | 83.85 | 0.18±0.02 | 86.15 | 0.27±0.03 | 79.23 |
| Absorbance of Blank Solution was 1.30. | ||||||||
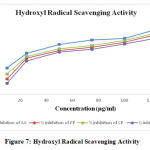 |
Figure 7: Hydroxyl Radical Scavenging Activity
|
Nitric Oxide Scavenging Activity
Hydroalcoholic extracts of Prunus persica, Calotropis procera and Canscora decussata effectively reduced the generation of nitric oxide from sodium nitroprusside. Figure 8 shows the % inhibition values of Ascorbic acid, HAEPP, HAECP and HAECD which were 74.81 %, 67.41 %, 70.37 % and 64.44 % respectively. Scavenging activity of nitric oxide radical is based on the generation of nitric oxide. Sodium nitroprusside in buffered saline, which reacts with oxygen to produce nitrite ions that can be measured by using Griess reagent. The absorbance of the chromophore is measured at 546 nm in the presence of the fractions. The plant extracts decrease the amount of nitrite generated from the decomposition of sodium nitroprusside in vitro. The plants extracts order of decreasing the amount of nitrite were as follows-
HAECP> HAEPP> HAECD
The IC50 values of Ascorbic acid was 63.19 μg/ml, HAEPP was found to be 84.07 μg/ml, HAECP was found to be 77.36 μg/ml and IC50 value of HAECD was found to be 91.27 μg/ml.
Table 7: Nitric Oxide Scavenging Activity
| Conc (μg/ml) | Ascorbic acid absorbance | Ascorbic acid % inhibition | HAEPP absorbance | HAEPP % inhibition | HAECP absorbance | HAECP % inhibition | HAECD absorbance | HAECD % inhibition |
| 10 | 0.94±0.07 | 30.37 | 1.10±0.04 | 18.52 | 1.02±0.04 | 24.44 | 1.13±0.03 | 16.30 |
| 25 | 0.88±0.06 | 34.81 | 0.93±0.03 | 31.11 | 0.91±0.05 | 32.59 | 0.97±0.03 | 28.15 |
| 50 | 0.73±0.03 | 45.93 | 0.86±0.05 | 36.30 | 0.82±0.03 | 39.26 | 0.88±0.04 | 34.81 |
| 75 | 0.62±0.03 | 54.07 | 0.74±0.02 | 45.19 | 0.71±0.04 | 47.41 | 0.78±0.02 | 42.22 |
| 100 | 0.51±0.04 | 62.22 | 0.61±0.03 | 54.81 | 0.59±0.04 | 56.30 | 0.65±0.02 | 51.85 |
| 125 | 0.34±0.02 | 74.81 | 0.44±0.03 | 67.41 | 0.40±0.02 | 70.37 | 0.48±0.04 | 64.44 |
| Absorbance of Blank Solution was 1.35. | ||||||||
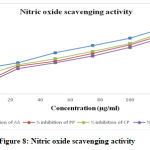 |
Figure 8: Nitric oxide scavenging activity
|
Discussion
The DPPH test showed the ability of the test compounds to act as a free radical scavenger. DPPH assay method is based on the ability of 1, 1-diphenyl-2-picrylhydrazyl (DPPH), a stable free radical, to decolorize in the presence of antioxidants. DPPH, a protonated radical, has characteristic absorbance maximal at 517 nm, which decreases with the scavenging of the proton radical. This property has been widely used to evaluate the free radical scavenging effect of natural antioxidants. When DPPH radical is scavenged, the color of the reaction mixture changes from purple to yellow with decreasing of absorbance at wavelength 517nm. In this analysis, the scavenging activity of hydroalcoholic extracts was similar to that of Ascorbic acid. The DPPH radical scavenging activity of ascorbic acid and hydroalcoholic extracts, increased in a dose-dependent manner. At a concentration of 125 μg/ml hydroalcoholic extracts of Prunus persica, Calotropis procera, Canscora decussata and standard Ascorbic acid showed 82.73 %, 86.36 %, 83.64 % and 90.00 % antioxidant activity respectively by DPPH radicals scavenging assay. Hydrogen peroxide (H2O2) is a byproduct of respiration and is made in all living cells. It is harmful and must be removed as soon as it is produced in the cell. The generation of even low levels of H2O2 in biological systems may be important. Cells make the enzyme catalase to remove hydrogen peroxide. Different plant materials show different amounts of catalase activity. Hydrogen peroxide scavenging activity depends upon the phenolic content of the extract, which can donate electrons to H2O2 and thereby neutralizing it in to water. The hydroalcoholic extracts of Prunus persica, Calotropis procera, Canscora decussata were capable of scavenging H2O2 in a dose dependent manner. At a concentration of 125 μg, Prunus persica, Calotropis procera, Canscora decussata and standard Ascorbic acid showed 82.48 %, 86.13 %, 76.64 % and 90.51% scavenging activity. Thus, the present study demonstrated the significant antioxidant activity of the extracts tested.
In hydroxyl radical scavenging activity model, at a concentration of 125 μg Prunus persica, Calotropis procera, Canscora decussata and standard Ascorbic acid showed 83.85 %, 86.15 %, 79.23 % and 91. 54 % antioxidant activity respectively.
The hydroalcoholic extracts of plants effectively reduced the generation of nitric oxide from sodium nitroprusside. At a concentration of 125 μg, Prunus persica, Calotropis procera and Canscora decussata and standard Ascorbic acid showed 67.41 %, 70.37 %, 64.44 % and 74.81 % antioxidant activity respectively. The results indicate that the hydroalcoholic extracts of all the plants leaves taken for this study has significant antioxidant activity. This may be probably due to the higher contents of flavanoids and phenolic compounds.
Conclusion
On the basis of the results obtained in the present study, it was concluded that the hydroalcoholic leaves extracts of the plants possess significant antioxidant activity. Presence of adequate amount of phenol and flavonoid compounds may account for this fact. So these findings of present study suggest that Prunus persica, Calotropis procera and Canscora decussata have a potential source of natural antioxidant. Out of all the mentioned plants Calotropis procera at the dose of 400 mg/kg showed the best result for in vitro antioxidant activity. Further studies are warranted for the characterization of antioxidant compounds by other methods, and also in vivo studies are needed for understanding their mechanism of action as antioxidants.
Acknowledgement
The authors are gratefully acknowledged and thank Dr. Sayeed Ahmad, Associate Professor at Jamia Hamdard for the academic support and the facilities provided to carry out the research work at his Institute. We also express our gratitude to Dr. Mohammed Ali, Former professor at Jamia Hamdard for encouraging and helping in the characterization of the compounds present in the plants. He supported us at various phases of our work. Thank you so much sir.
Conflict of Interest
No conflict of interest.
References
- Leong C N, Tako M, Hanashiro I, Tamaki H., 2008. Antioxidant flavonoids glycosides from the leaves of Ficus pumila L. Food Chem. 109; 415-20.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J., 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 39: 44-84.
- Zhou, D., Ruan J., 2010. Antioxidant and hepatoprotective activity of ethanol extract of Arachniodes exills ching. Journal of ethnopharmacology. 129, 232-237.
- Finkel, T., Holbrook, N.J., 2000. Oxidant, oxidative stress and the biology of ageing. Nature. 408, 239–247.
- Miller, N.J., Rice-Evans, C., 1997. Factors influencing the antioxidant activity determined by the ABTS+ radical cation assay. Free Rad. Res. 26, 195–199.
- Patel Chirag J, Tyagi S, Halligudi N, Yadav J, Pathak S, Singh Satya P, Pandey A, Kamboj D.S, Shankar P., 2013. Antioxidant activity of herbal plants: A recent review. Journal of Drug Discovery and Therapeutics. Vol 1 (8), pp 01-08.
- Huda AW, Munira MA, Fitrya SD, Salmah M. Antioxidant activity of Aquilaria malaccensis (Thymelaeaceae) leaves. Pharm Res 2009;1: 270-3.
- Nadkarni AK., 1996. Indian Materia Medica. Mumbai, Popular Prakashan, 3, 1236.
- Kirtikar, K.R., Basu, B.D., 2005. Indian medicinal plants. Published by international book distributors, Vol. 3, pp. 340-345.
- Chaudhary P, Ahmad S and Khan NA., 2017. A review on medicinal utility of Calotropis procera. World Journal of Pharmaceutical and Medical Research, Vol. 3, Issue 1, pp. 335-342.
- Sethiya N. K, Trivedi A, Patel M. B, Mishra S. H, 2010. Comparative pharmacognostical investigation on four ethanobotanicals traditionally used as Shankhpushpi. India. J Adv Pharm Tech Res, Vol. 1, No. 4, pp. 388-395.
- Sundaram, R. and Murugesan, G., 2011. Hepatoprotective and antioxidant activity of a mangrove plant Lumnitzeraracemosa, Asian Pacific Journal of Tropical Biomedicine, pp. 348-352.
- Shamim Ahmad, Mohd. Ali, Shahid H. Ansari., 2012. Volatile oil composition and antimicrobial activity of Curcuma oligantha var. lutea rhizomes. Int. J. Res. Ayur. Pharm. Vol. 3(5): 742-745.
- Blois, M.S. 1958. Antioxidant determinations by the use of a stable free radical, Nature, 181: 1199- 1200.
- Nagulendran K, Velavan S, Mahesh R, Begum V.H., 2007. In Vitro Antioxidant Activity and Total Polyphenolic Content of Cyperus Rotundus Rhizomes, Vol. 4(3): 440-449. 42.
- Ruch R.J, Cheng S.J, Klaunig J.F., 1989. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea, Carcinogenesis, Vol. 10: 1003–1008.
- Halliwell B, Gutteridge J M C., 1993. Free Radicals in Biology and Medicine, Oxford, Clarendon, 419.
- Garrat D C., 1964. The Quantitative Analysis of Drugs, Japan, Chapman and Hall, 456.
- Green, L. C.; Wagner, D. A.; Glogowski, J.; Skipper, P. L.; Wishnok, J. S.; Tannenbaum, S. R., 1982. Analysis of nitrate, nitrite, and [15N] nitrate in biological samples. Anal. Biochem. 126, 131- 138.
- Guo X, Wang W P, Ko J K and Cho C H., 1999. Gasteroenterology, 117: 884.
- Jamuna, S., Paulsamy S and Karthika K., 2012. Screening of in vitro antioxidant activity of methanolic leaf and root extracts of Hypochaeris radicata L. (Asteraceae). Journal of Applied Pharmaceutical Science. Vol 02 (07), 149-154
- Sharma, U.S, Kumar A., 2019. In vitro antioxidant activity of Rubus ellipticus fruits. Journal of Advanced Pharmaceutical Technology & Research. Vol 2(1), 47-50.
- Moukette, B.M, Pieme, C.A, Njimou, J.R, Nya Biapa, C.P, Marco, B and Ngogang J.Y., 2015. In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biological Research. Vol. 48:15, 1-17.
- Alam, M.N, Bristi N.J, Rafiquzzaman, M., 2013. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal. Vol. 21, 143–152.
- Olamide E. Adebiyi, Funsho O. Olayemi, Tan Ning-Hua, Zeng Guang-Zhi., 2017. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef University Journal of Basic and Applied Sciences. Vol. 6, 10–14.
- Badami, S and Channabasavaraj, K.P., 2007. In Vitro Antioxidant Activity of Thirteen Medicinal Plants of India’s Western Ghats. Pharmaceutical Biology. Vol. 45, No. 5, pp. 392–396.
- Shang H.M, Zhou H.Z, Yang J.Y, Li R, Song H, Wu H.X., 2018. In vitro and in vivo antioxidant activities of inulin. Plos One. Vol 13(2), 1-12.
- Geetha, R.V, Roy, A, Sitalakshmi T., 2013. In Vitro Antioxidant and free Radical Scavenging activity of the Ethanolic extract of Aesculus hippocastanum. Int. J. Drug Dev. & Res. Vol. 5 (3): 403-407.
- Emrobowansan M. I, Patrick J. M and Voster M., 2017. A Report on the In Vitro Antioxidant Properties of Vachellia karroo Leaf Extract: A Plant Widely Grazed by Goats in the Central Eastern Cape of South Africa. Sustainability, Vol. 9, Issue 164, pp- 2-9.
- Rahman M.M, Islam M.B, Biswas M and Khurshid Alam A. H. M., 2015. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes, Vol. 8:621, pp. 2-9.
- Sahu K.G, Khadabadi S.S and Bhide S.S., 2009. Evaluation of in vitro antioxidant activity of Amorphophallus campanulatus (roxb.) Ex blume decne. Int. J. Chem. Sci. Vol. 7(3), pp. 1553-1562.
- Abramovic H, Grobin B, Ulrih N.P, and Blaz C., 2018. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. Hindawi Journal of Chemistry. Volume 2018, Article ID 4608405, pp. 1-9.
- Al-Trad B, Al-Qudah M. A, Al-Zoubi M, Al-Masri A, Muhaidat R, Qar J, Alomari G, Alrabadi N.I., 2018. In vitro antioxidant activity of the butanolic extract from the stem of Ephedra Alte. Biomedical and Pharmacology Journal, Vol. 11.







