Ng Kit Yeng1, Rumaizi Shaari1, Muhammad Luqman Nordin1 and Jasni Sabri2
1Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia
2Department of Veterinary Paraclinical Science, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia
Corresponding Author E-mail: rumaizi@umk.edu.my
DOI : https://dx.doi.org/10.13005/bpj/1816
Abstract
This study was conducted to investigate the wound healing effect of the ethanol extract of Acalypha indica in different parts of the body. Two set of cutaneous wounds were created on an individual rat. A set of wound consisted of a 5mm wound on dorsum and a 5mm wound on hind limb. The wound on left side was treated while the right side acted as control. Three rats were euthanized every 3 days, which was on day 3, 6, 9, 12 and 15 after wounding. The body weight of the rats were recorded and skin samples were obtained for histopathology. Parameters such as epithelialization, angiogenesis, number of PMNLs and macrophages, fibroblast and collagen deposition were used in evaluating the wound healing effect. A semi quantitative scoring system was used to grade the parameters. Results showed increasing of the body weight of the rats indicated that rats were not affected by the wound induction. Gross findings revealed that the percentage of wound contraction was higher in treated wound, indicating that the wound healing process was improved by the application of the extract. Histological findings showed that the period of epithelialization was shorter in the treated wound. The fibroblastic activity, collagen deposition and angiogenic activity were also much higher in the treated wound. In conclusion, A. indica extract has good wound healing effect and plausibly can be further commercialised for wound healing treatment.
Keywords
Acalypha Indica; Cutaneous Wound; Histology; Wound Healing
Download this article as:| Copy the following to cite this article: Yeng N. K, Shaari R, Nordin M. L, Sabri J.Investigation of Wound Healing Effect of Acalypha Indica Extract in Sprague Dawley Rats. Biomed Pharmacol J 2019;12(4). |
| Copy the following to cite this URL: Yeng N. K, Shaari R, Nordin M. L, Sabri J.Investigation of Wound Healing Effect of Acalypha Indica Extract in Sprague Dawley Rats. Biomed Pharmacol J 2019;12(4). Available from: https://bit.ly/33vVRJU |
Introduction
Skin is the largest organ of the body which serves as the physical barrier against the harmful pathogen, radiation and others. Trauma such as fighting, biting, thermal burn, automobile accident and others could lead to the break of the continuity of the skin and cause a wound 1, 2. Cutaneous wound is a common problem encountered in the small animal practice. Breakage of the skin opens the entrance for the pathogen and lead to severe wound infection and systemic infection in the worst scenario 3, 4. Therefore, cutaneous wound should be managed immediately without delay. Healing and restoring of normal functions of body associated with wound would prevent the pain and further infections. Wound management includes disinfection, debridement, lavage, drainage and dressing and involves various type of the commercial products and topical agents. Examples of commercial products used to enhance wound healing are hydrocolloid, hydrogels collages and alginate while examples of topical wound products are topical antimicrobial, topical antiseptic and topical debridement 5. Application of these commercial products on the wound to prevent wound infections and thus to ensure a smooth wound healing process 5. However the chemical products are not the solely agent used in wound management. Nowadays there is increasing demand of herbal medicine and natural products as topical agent.
Alternative medicine is gaining popularity since there is decreasing in new antibiotic and emergence of antimicrobial resistant organism. Besides that, patients with chronic wound require long term wound dressing which is time consuming and increasing the financial burden of the owners 6. Alternative medicine in wound management incorporates moist wound healing, which does not require long term dressing 6. Some of the herbs possess antimicrobial properties which is able to eliminate bacteria and prevent wound infection.
Acalypha indica, which is also known as “pokok kucing galak” locally, is a least concerned medicinal plant in Malaysia. It has catkin type of inflorescence, which is attractive to cats. This plant is widely distributed and found growing in the waste places. It is also considered as weed in cultivated areas. Traditionally, people believe that this plant can be used externally and internally to cure scabies and skin diseases 7. The plant also known to exhibits antiinflammatory and analgesic properties 8, 9. 10 et al. (2009) have also reported the plant possess bactericidal activity which indirectly correlated with wound healing activity. The leaves can be used in deworming purpose and applied externally on the wound to relieve inflammation 10. However there is no meticulous study and still lack of empirical submission regarding wound healing effect of this plant. Therefore, this study was conducted to investigate the effect of the extract of Acalypha Indica in wound healing process at the extremities as well as the dorsum in Sprague Dawley rats.
Methodology
Preparation of the plant’s extraction
The plants of Acalypha indica were collected in the state of Kelantan. The leaves were washed thoroughly with distilled water to remove dust and dirt. The leaves were dried by oven and grinded into powder form. Then 100g of the powder was weighed and soaked in 1 litre of 50% ethanol for 48 hours and filtered. The solvent containing extract was evaporated under reduced pressure in a rotary evaporator and controlled heating bath at 40°C. The yield obtained were kept in oven until used for analysis.
Experimental Animals
The investigation was conducted after obtaining the approval from “Animal Ethic’s Committee” through Final Year Project Research Committee 2016 of Faculty of Veterinary Medicine, University Malaysia Kelantan.
Fifteen Sprague Dawley rats were subjected in this studies. One and half month olds female rats weighed from 160-200g were selected. The rats were purchased from Animal Research and Service Center (ARASC), Universiti Sains Malaysia (USM). Rats were acclimatized for 4 days before the experiment and fed with commercial diet and water, ad-libitum. The rats were divided into 5 groups with 3 rats in a group. The investigations were conducted for 15 days. Two sets of wounds at dorsal interscapular and distal hind limb on the same rat, respectively, were created. The rats were anesthetized with combination of Xylazine (10mg/kg) and Ketamine (80mg/kg) intraperitoneally prior to the wound incision. The untreated rats became the control group. The extract (100%) were given topically daily from the day operation until the end of the study. Rats were kept individually and wound cleaning was done daily to prevent wound infection.
Incision wound creation
Rats were placed on ventral recumbency. Wounds were created at the dorsal interscapular region to prevent self-inflicting while wound at dorsal surface of distal hindlimb was chosen because it is far from the heart and the skin on this location is fix. Wounds at the dorsum were created 2 cm caudal to the ear and about 1 cm apart. The wounds at the hind limb were created at the lateral right and left limb and 1cm above the tarsal joint. Hair on the dorsum region and lateral surface of distal hind limb was shaved and prepared surgically. Center of the shaved area was demarcated with a disposable skin biopsy punch. Five millimetre skin biopsy punch was used to create the wounds. A full thickness skin was removed by scalpel blade and forceps. Hemostasis was achieved by applied gauze to the wound. Set of wound at left side was treated with extract of Acalypha indica while wound at the right ride was not treated. Treatment was given immediately after the wound was created. Figure 1 shows illustration of wound creation.
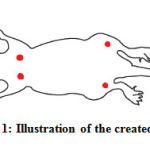 |
Figure 1: Illustration of the created wound |
Evaluation of Wound Healing Process
Macroscopic Evaluation of The Wound
Wound contraction was measured every 3 consecutive days on day 3rd, 6th, 9th, 12th and 15th. The photos of the wound were taken as well. The following formula was used to calculate the percentage of wound contraction:-

Microscopic Evaluation of The Wound
Samples were also collected every 3 days after the creation of wound for histology evaluations. Group 1 were sacrified on day 3, group 2 were scarified on day 6, group 3 were sacrified on day 9, group 4 were sacrified on day 12 and group 5 were sacrified on day 15. The rats were euthanized using pentobarbital with concentration of 150mg/kg intraperitoneally. The wounded tissues with 0.5 cm of peripheral unwounded skin were harvested with a scalpel blade. The samples were fixed in formalin, processed, blocked with paraffin, sectioned into 4 m of tissue and stained with H&E. Modified Abramov’s histologic scoring system was used to evaluate the wound healing process. Epithelialization, angiogenesis, fibroblast, collagen and number of polymorphonuclear cells (PMNLs) and tissue macrophage were evaluated according to this scoring.
Statistical analysis
Comparison between treated and control wound were using independant T-test. All statistical analyses were carried out using the SPSS version 19.0 data analysis system. p-Values < 0.05 were considered to be statistically significant for all comparisons.
Results
Body weight of the rats
There was no changes of the body weight in group 1, while the body weight of the rats from group 2, 3, 4 and 5 were increasing (Table 1).
Table 1: Average weight of the rats before experiment and end of each experiment
| Group (n=3) | Body weight before experiment (Mean± S.D)(g) | Body Weight before euthanasia (Mean± S.D)(g) | Percentage in increase of weight (%) |
| 1 | 155.3 ±13.3 | 155.3 ±13.3 | 0 |
| 2 | 180.0 ±17.8 | 187.7 ± 20.7 | 3.9 |
| 3 | 169.0 ±5.3 | 199.3±8.1 | 17.9 |
| 4 | 173.0±7.9 | 205.0±9.5 | 18.5 |
| 5 | 171.0±4.6 | 212.7±2.1 | 24.0 |
Macroscopic Evaluation of Wound
The macroscopic evaluation of wound for all group were shows in Figure 2. Clot formation was seen forming faster in treated wound of dorsum and the size of the treated wound at the limb were slightly smaller than the untreated wound in group 1. In group 2, all wound were getting smaller indicating the ongoing healing process. Size of the treated wound at the dorsum was smaller than the untreated one. The treated wound of the limb was smaller than the control as well. The wounds at the limbs were smaller than the wounds at the dorsum. This showed that the wounds at the limbs healed faster than those at the dorsum.
In comparison with the control wounds, some of the treated wounds at the dorsum and limb healed completely in group 3. Irregular scar can be seen at the wound site. There was no untreated wound close completely in group 3. Most treated wounds closed completely in group 4. Some of the control wounds on group 4 closed while wounded tissue can be seen in few of the controls. In group 5 all the untreated wound closed. Scar tissue can be seen at all wound site. Throughout the wound healing process, there was no sign of infection such as severe inflammation and pus formation. All the wound decreased in size indicating that the healing process of the wound were going smoothly. Based on the gross morphology, it was shown that the treated wounds of both dorsum and limbs healed faster than the untreated wounds which indicating the effectiveness of the extract.
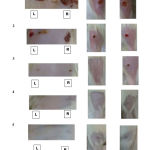 |
Figure 2 : Wound areas of the rats after 15 days of treatment with Acalypha Indica extract. L and R : Left and Right area of the rat’s body respectively. |
Microscopic Evaluation Of The Wound
Histological findings consisted of the parameters such as the epithelialization, amount of fibroblast, collagen deposition, angiogenesis and inflammatory cells. Data presented was based on a semi-quantititive scoring system and were collected at time of euthanasia of the animal. Figure 3 shows histological changes of the wound healing process over time.
From the histological findings, it was known that the treated wound, included the wound on dorsum and limbs had undergone the wound healing process in a slightly faster rate. Table 2 shows that the treated wounds completed the epithelialization in group 3, which was slight faster than the controls. More blood vessel were detected in the angiogenesis phase in the treated wound as well. There was an interesting findings in the angiogenesis score shown in Table 3. High number of blood vessel was seen in the treated wound of group 5 which was not seen in other groups. Number of fibroblast was increasing gradually throughout the wound healing, but it was slight higher in the treated wound in the starting phase of wound healing. It could be said that the treated wound entered proliferation much earlier than those control wound. Table 4 shows scores of fibroblast over time. In general, there is no changes amongst treated and untreated wound regardless at either dorsum and limb parts. Table 5 shows collagen deposition scores over time. Collagen deposition was increasing throughout the whole process and there was no much different between treated and control group. Number of the inflammatory cells, included PMNLs and macrophages were similar in both treated group and control group as well (Table 6). The inflammatory cells were found highest in number in the beginning of the wound healing. The numbers decreased and totally vanished after group 3 (Table 7). All values are expressed as mean ±SD (n=3 rats ).
Table 2: Epithelialization scores over time
| Group | Epithelialization (Mean±SD) | |||
| Dorsum | Limb | |||
| Treated | Control | Treated | Control | |
| 1 | 0.7±0.6 | 0.0±0.0 | 0.7±0.6 | 0.33±0.6 |
| 2 | 2.0±0.0 | 0.7±0.6 | 2.3±0.6 | 1.0±1.0 |
| 3 | 3.0±0.0 | 2.3±0.6 | 3.0±0.0 | 2.0±0.0 |
| 4 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 |
| 5 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 |
Table 3: Angiogenesis scores over time
| Group | Angiogenesis (Mean±SD) | |||
| Dorsum | Limbs | |||
| Treated | Control | Treated | Control | |
| 1 | 2.0±0.0 | 1.7±0.6 | 2.3±0.6 | 1.0±0.0 |
| 2 | 2.7±0.6 | 1.0±0.7 | 2.3±0.6 | 1.6±0.6 |
| 3 | 2.3±0.6 | 2.7±0.6 | 2.7±0.6 | 2.0±0.0 |
| 4 | 1.3±0.6 | 2.0±1.0 | 0.7±0.6 | 1.7±0.6 |
| 5 | 2.7±0.6 | 1.0±0.0 | 1.0±1.0 | 1.0±0.0 |
Table 4: Scores of fibroblast over time
| Group | Fibroblast (Mean±SD) | |||
| Dorsum | Limb | |||
| Treated | Control | Treated | Control | |
| 1 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 | 1.0±0.0 |
| 2 | 2.7±0.6 | 2.3±0.6 | 2.3±0.6 | 2.0±0.0 |
| 3 | 2.3±0.6 | 2.3±0.6 | 2.3±0.6 | 2.3±1.2 |
| 4 | 2.7±0.6 | 3.0±0.0 | 2.7±0.6 | 3.0±0.0 |
| 5 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 |
Table 5: Collagen deposition scores over time
| Group | Collagen deposition (Mean±SD) | |||
| Dorsum | Limb | |||
| Treated | Control | Treated | Control | |
| 1 | 0.6±0.6 | 0.3±0.6 | 0.3±0.6 | 0.0±0.0 |
| 2 | 2.7±0.6 | 2.7±0.6 | 2.7±0.6 | 2.7±0.6 |
| 3 | 2.7±0.6 | 2.7±0.6 | 3.0±0.0 | 2.3±0.6 |
| 4 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 |
| 5 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 |
Table 6: Score of PMNLs over time
| Group | Neutrophil (Mean±SD) | |||
| Dorsum | Limb | |||
| Treated | Control | Treated | Control | |
| 1 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 | 3.0±0.0 |
| 2 | 2.3±0.6 | 2.3±0.6 | 2.3±0.6 | 2.0±0.0 |
| 3 | 0.3±0.6 | 0.3±0.6 | 0.6±0.6 | 0.3±0.6 |
| 4 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| 5 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
Table 7: Scores of tissue macrophages over time
| Group | Tissue macrophages (Mean±SD) | |||
| Dorsum | Limb | |||
| Treated | Control | Treated | Control | |
| 1 | 1.3±0.6 | 1.0±0.0 | 1.3±0.6 | 0.6±0.6 |
| 2 | 1.3±0.6 | 0.6±0.6 | 1.0±1.0 | 0.6±0.6 |
| 3 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| 4 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| 5 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
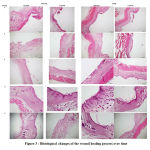 |
Figure 3 : Histological changes of the wound healing process over time |
Discussion
The body weight of the rats were recorded at different interval of time and the results revealed that the rats gained weight gradually after wound induction. One of the biggest worries in this experiment was the pain experienced by the rats after wound induction. Four wounds with size 5mm were made on an individual rats after acclimatization of 4 days. Analgesic was not given during the experiment so that the natural behavior of the rats was not deteriorated. This might induce great degree of stress in the rats, consequently affect the daily activities and normal behavior of the rats. However, the body weight of the rats increased gradually throughout the experiment. This indicated that the procedure of wound induction did not burden the rats. Therefore, the rats were able to perform the daily activities such as eating and drinking and express the normal behavior. Increasing in the body weight indicated that the rats were not affected much by the pain sensation. As the healing of the wound progress, percentage of increase in the body weight became higher. Less pain was experienced by the rat since the wound was recovered gradually. In addition, there was an experiment proved the analgesic effect of the methanolic extract of Acalypha indica. The extract was able to inhibit the writhing reflexes by 51.1% and 57.2% at doses of 200mg/kg and 400mg/kg. The dose of the extract in the experiment was not identified but due to the findings of body weight and the ability of the rats in expressing normal behavior after wound induction, it can be proposed that the extract exhibited analgesic activity which relieve pain and stress in the rats.
In term of wound healing effect, gross findings revealed that extract was able to improve wound healing by increasing the percentage of wound contraction. This findings was same with the findings reported by 11. Wound contraction is essential to close the full thickness wound and this process require collagen and compaction of fibroblast. High amount collagen and fibroblast facilitate in wound contraction. The experiment done by 11 et. al. 2012 showed that the Acalypha indica extract increased the production of collagen and fibroblastic activity of the wound through examining the content of collagen and histopathology. Although there was no quantification of collagen in this experiment, histopathology of the treated wound on day 12 did show higher number of fibroblast and dense deposition of collagen. Besides fibroblast and collagen, the phytochemical constituents of Acalypha indica assist in wound healing as well. Dried leaves and plant of this plant contain tannin, which is able to protect underlying tissue and promote wound healing 12. There was a study conducted to investigate the effect of tannin extracts from immature fruits of Terminalia chebula Fructus Retz. on cutaneous wound healing in rats. This study proved that tannin is useful in cicatrisation of wound through chelation of free radicals and reactive species of oxygen, promoting contraction of the wounds and increase formation of capillary vessels and fibroblast.
Throughout the whole process of wound healing, there was no evidence of delay in wound healing and signs of infection. This could be due to the daily wound management and individual housing of the house. In addition, this was also attributed to the antimicrobial activity of the extract. Aqueous and ethanol extract of this plant were reported to exhibit potent antimicrobial activity against gram positive bacteria such as Staphylococcos aureus, Staphylococcus enteriditis and Bacillus subtilis 7. The methanolic extract of this plant was effective against the gram positive bacteria and gram negative bacteria such as Escherichia coli and Salmonella typhi 13. The ability of inhibition of bacteria is important to ensure a smooth wound healing process.
In comparison the healing rate between limb and dorsum, the limb healed faster than the dorsum. This was the unexpected finding in this experiment. Limbs were expected to be heal slower than the dorsum because the limbs were further from the heart, which was the main blood supply in the rats. However the process of wound healing is not only affected by the blood supply, thickness of the skin is one of the factors as well. The skin of the limb is thinner than the skin of the dorsum in rat. Therefore, the wound of the limb require less time in complete the whole healing process.
Histopathology of the wounds revealed that the treated wound completed the epithelialization slightly earlier than the control wound. Similar findings were reported by 11. Higher number of blood vessels were detected on day 6 and day 15. 14 et al. (2002) and 15 et. al. (2015) also reported the higher angiogenic activity of the aqueous extract of Acalypha indica. Shorter period of epithelialization and the high angiogenic activity contributed in improve the wound healing process, thus the wound treated by the extract heal faster.
Conclusion
The present experiment proved the effectiveness of the extract of Acalypha indica in promoting the wound healing process. There is no significant different of wound healing in different parts of rat’s body. The antimicrobial and analgesic properties of this plant were believed indirectly involved in wound healing activity. All of the findings indicated this medicinal plant is potential to be an alternative medicine in wound management in the future.
Acknowledgements
The authors would like to thank to the Faculty of Veterinary Medicine Universiti Malaysia Kelantan for providing the facilities and supports for completion of this study. The authors also are grateful to all the faculty staff that were involved indirectly in the study.
Funding
This research was funded by Faculty of Veterinary Medicine, Universiti Malaysia Kelantan.
Availability of Data and Materials
The datasets generated and/or analysed during the current study are not publicly available due it is part of a big study (data) but are available from the corresponding author on reasonable request.
Authors’ Contributions
Ng Kit Yeng involved in all aspects of the study including concept, design, data collection, interpretation of data, statistical analysis and drafted of manuscript. Rumaizi Shaari, Muhammad Luqman Nordin and Jasni Sabri involved in obtaining funding, and supervised the experiment and manuscript preparation. Rumaizi Shaari and Muhammad Luqman Nordin contributed to the statistical analysis and evaluated of the manuscript. All authors have read and approved the final manuscript.
Consent for Publication
Not applicable.
Competing Interests
The authors declared that they have no competing interests.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Refrences
- Mathias, E., and Srinivas Murthy, M. (2017). Pediatric thermal burns and treatment: a review of progress and future prospects. Medicines, 4(4), 91.
- Tiwari, V. K. (2012). Burn wound: How it differs from other wounds?. Indian journal of plastic surgery: official publication of the Association of Plastic Surgeons of India, 45(2), 364.
- Ki, V., and Rotstein, C. (2008). Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Canadian Journal of Infectious Diseases and Medical Microbiology, 19(2), 173-184.
- Ribet, D., and Cossart, P. (2015). How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes and Infection, 17(3), 173-183.
- Krahwinkel D.J. and Boothe H.W. (2006): Topical and Systemic Medication for Wounds, Veterinary Clinic Small Animal 36 739-757.
- Dorai, A. A. (2012). Wound care with traditional, complementary and alternative medicine. Indian journal of plastic surgery: official publication of the Association of Plastic Surgeons of India, 45(2), 418.
- Somchit M.N., Rashid R.A, Zuraini A., Zakaria Z.A., Sulaiman M.R., Arifah A.K. and Mutalib A.R. (2010): In Vitro Antimicrobial Activity of Leaves of Acalypha indica Linn. African Journal of Microbiology Research Vol. 4(20) pp. 2133-2136.
- Rahman M.A., Rahmatullah M. and Bachar S.C. (2010) Anagelsic and anti-inflammatory activity of methanolic extract of Acalypha indica Linn, Pak. J. Pharm. Sci., Vol.23, No.3, July 2010, pp.256-258.
- Ranju, G., Niranjan, S., Kumar, P.S., Kumar, P.V., Kumar, P.S., 2011. In vitro anthelmintic activity of Acalypha indica leaves extracts. International Journal of Research in Ayurveda and Pharmacology 2, 247–249.
- Raja R.V., Ramanathan T. And Savitha S. (2009) Studies on wound healing Property of Coastal Medicinal Plant, Journal of Bioscience Technology Vol (1) 2009, 39-44.
- Ganeshkumar M., Ponrasu T., Krithika R. Iyappan K. Gayathri V.S. and Suguna L. (2012): Topical application of Acalypha indica accelerates rat cutaneous wound healing by up-regulating the expression of Type I and III collagen, Journal of Ethnopharmacology 142 14-22.
- Su, X., Liu, X., Wang, S., Li, B., Pan, T., Liu, D., Wang, F., Diao, Y. and Li, K., 2017. Wound healing promoting effect of total tannins from Entada phaseoloides (L.) Merr. in rats. Burns, 43(4), pp.830-838.
- Hussain A.Z. and Kumaresan S. (2013) GC-MS analysis and antibacterial evaluation of Acalypha indica. Asian Journal of Plant Science and Research, 3(6): 46-49
- Reddy, J. S., Rao, P. R., & Reddy, M. S. (2002). Wound healing effects of Heliotropium indicum,Plumbago zeylanicum and Acalypha indica in rats. Journal of ethnopharmacology, 79(2), 249 251.
- Kumarasamyraja D. and Swamivelmanickam M. (2015): Evaluation of In Vivo and In Vitro Wound healing Activity of Aqueous extract of Acalypha Indica, International Research Journal of Pharmacy 6(1)








